Abstract
Background:
The preventive role of non-steroid anti-inflammatory drugs (NSAIDs) and aspirin, in particular, on colorectal cancer is well established. More recently, it has been suggested that aspirin may also have a therapeutic role. Aim of the present observational population-based study was to assess the therapeutic effect on overall survival of aspirin/NSAIDs as adjuvant treatment used after the diagnosis of colorectal cancer patients.
Methods:
Data concerning prescriptions were obtained from PHARMO record linkage systems and all patients diagnosed with colorectal cancer (1998–2007) were selected from the Eindhoven Cancer Registry (population-based cancer registry). Aspirin/NSAID use was classified as none, prediagnosis and postdiagnosis and only postdiagnosis. Patients were defined as non-user of aspirin/NSAIDs from the date of diagnosis of the colorectal cancer to the date of first use of aspirin or NSAIDs and user from first use to the end of follow-up. Poisson regression was performed with user status as time-varying exposure.
Results:
In total, 1176 (26%) patients were non-users, 2086 (47%) were prediagnosis and postdiagnosis users and 1219 (27%) were only postdiagnosis users (total n=4481). Compared with non-users, a survival gain was observed for aspirin users; the adjusted rate ratio (RR) was 0.77 (95% confidence interval (CI) 0.63–0.95; P=0.015). Stratified for colon and rectal, the survival gain was only present in colon cancer (adjusted RR 0.65 (95%CI 0.50–0.84; P=0.001)). For frequent users survival gain was larger (adjusted RR 0.61 (95%CI 0.46–0.81; P=0.001). In rectal cancer, aspirin use was not associated with survival (adjusted RR 1.10 (95%CI 0.79–1.54; P=0.6). The NSAIDs use was associated with decreased survival (adjusted RR 1.93 (95%CI 1.70–2.20; P<0.001).
Conclusion:
Aspirin use initiated or continued after diagnosis of colon cancer is associated with a lower risk of overall mortality. These findings strongly support initiation of a placebo-controlled trial that investigates the role of aspirin as adjuvant treatment in colon cancer patients.
Keywords: colorectal cancer, aspirin, NSAIDs, survival, population based
With 1 000 000 new cases and 600 000 deaths worldwide each year, colorectal cancer is one of the most common cancers in developed countries (Weitz et al, 2005). Aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) are potentially effective chemopreventive agents for a number of cancers, but most clearly for colorectal cancer. Several randomised and observational studies have unequivocally shown that regular use of aspirin or NSAIDs reduces the risk of a colorectal malignancy (Giovannucci et al, 1994, 1995; Baron et al, 2003; Sandler et al, 2003; Chan et al, 2007; Rothwell et al, 2010, 2011). In a recent study by Chan et al (2009) reported a risk reduction of 21% for regular aspirin users as compared with non-users for overall mortality. In their study, patients diagnosed with stage I–III colorectal cancer were included; use of aspirin was assessed using a biannual questionnaire among the cohort of health professionals. The effect was larger when aspirin was initiated after the diagnosis of colorectal cancer. However, widespread use of NSAIDs or aspirin for colorectal cancer prevention is not advised as mentioned in a recent international consensus statement, where it was concluded that the data on the risk-benefit profile for cancer prevention are insufficient and as such no definitive recommendation could be made (Cuzick et al, 2009).
Aspirin inhibits cyclooxygenase (COX), induces apoptosis through p38 activation (Schwenger et al, 1997) and inhibits nuclear factor-kappaB. All of these mechanisms have been hypothesised to inhibit adenomatous polyp formation. Experimental data showed that COX-2 expression is associated with angiogenesis, tumour invasiveness and metastatic potential of colon cancer cells and that these traits can be reversed by selective COX-2 inhibitors (Tsujii et al, 1997, 1998; Chen et al, 2001). Nevertheless, it is not clear if aspirin use can influence the prognosis of patients already diagnosed with colorectal cancer. In animal models, aspirin or other NSAIDs, with activity against COX-2, have shown to inhibit tumour growth and metastases as well as prolonged survival (Tomozawa et al, 1999; Williams et al, 2001; Yao et al, 2003, 2005). A recent study in breast cancer patients (Nurses’ Health Study) showed a decreased risk of distant recurrence and breast cancer death in patients who used aspirin (Holmes et al, 2010). For colorectal cancer (stage I–III) patients who regularly used aspirin after their colorectal cancer had an increased survival (hazard ratio 0.71) (Chan et al, 2009).
The aim of this study was to assess the therapeutic effect of aspirin or non-aspirin NSAIDs use after the diagnosis of colorectal cancer on overall survival. A large, patient-centric data network including multiple, linked databases designed for safety and outcomes research in the Netherlands were linked to data from a regional cancer registry (van Herk-Sukel et al, 2010). The new combined database offers a unique opportunity to assess survival according to aspirin or other non-aspirin NSAIDs use before and after the diagnosis of colorectal cancer.
Patients and methods
Patients
The central patient database of the PHARMO record linkage system is linked to more than 10 different databases and has recently been linked to the database from the Eindhoven Cancer Registry (ECR). Detailed information concerning this new database is described elsewhere (van Herk-Sukel et al, 2010). From the ECR, all patients diagnosed with colorectal cancer between 1998 and 2007 were selected. This southern region of the Netherlands is served by 10 hospitals each serving a population between 150 000 and 250 000 people. All patients were included, there were no exclusion criteria. Of these patients prescriptions of aspirin and NSAIDs were selected from the PHARMO databases (see Supplementary Table 1). The largest proportion of patients used aspirin 80 mg per day (95%), 5% of the patients used aspirin 30 mg per day. The date of dispensing and date of diagnosis were compared to assess whether the aspirin/NSAIDs were prescribed before and/or after diagnosis. Non-users were defined as patients who never used prescribed aspirin/NSAIDs. Patients, who ever used a prescribed aspirin or NSAID, were classified as user if they had a prescription for aspirin or NSAIDs for at least 14 days, patients who had a prescription for less than 14 days were defined as non-users (3.5% of all patients). Frequent users were defined as patients that had a least three prescriptions. Date of diagnosis was compared to the date of prescription; users were subsequently classified as prediagnosis and postdiagnosis use and solely prediagnosis or solely postdiagnosis use. Low-dose aspirin (80 mg) is always on prescription in the Netherlands, so misclassification is lower with aspirin than with NSAIDs.
Statistical analysis
Definition of user and follow-up time
As information concerning the prescriptions in hospital was unknown, follow-up time for the general analysis was defined as 30 days from diagnosis (T0) of colorectal cancer to the last contact date or time of death (patients who died within 30 days were excluded from the survival analysis, 2.4% for colon cancer and 1.0% for rectal cancer). If the follow-up would have started at the date of diagnosis, immortal time bias would have been introduced, as our analysis rely on drug dispensing from pharmacies that requires a patient to be discharged from the hospital. Time to first dispensing was, consequently, calculated as the time from 30 days after diagnosis to the date of the first prescription.
Frequent users were defined as patients who had three or more consecutive refills of aspirin (within 9 months). As the prescribed refills are for 3 months each, follow-up for analyses with frequent user as exposure started 9 months after T0 until to the last contact or time of death. Patients who had less than three refills were classified as non-users. Additionally, we performed a sensitivity analysis, where patients that initiated the frequent use of aspirin with a longer time interval after the diagnosis (3 years) were coded as non-users.
Prediagnosis and postdiagnosis users were defined as those patients that initiated their use before diagnosis and continued to use until at least 3 months after diagnosis. Consequently, the follow-up for this group started at 3 months after T0 and ended at the date of last contact of time of death.
Survival analysis
Vital status was identified through linkage of cancer registry data with the municipal population registries, which record information on their inhabitant’s vital status. For the survival analysis with time-dependent exposure, patients were defined as non-users from T0 to the date of first use of aspirin or NSAIDs and user from first use to the end of follow-up period to prevent immortal time bias (Suissa, 2008). Poisson regression models were used to model the effect of aspirin/NSAID use on overall survival, where death from any cause was coded as event. For the analysis of prediagnosis and postdiagnosis users, a Poisson regression survival model was used without the time-varying covariate. Patients with solely prediagnosis use were analysed separately and defined as user or non-user prediagnosis; analyses were performed using a multivariable Cox proportional hazard model. Multivariable models were build to adjust for sex, age, comorbidity, year of incidence, grade, stage, surgery, adjuvant chemotherapy for colon cancer patients and preoperative radiotherapy for rectal cancer patients. We had information concerning the presence or absence of the next comorbidities: lung diseases, cardiovascular diseases, GI tract, urinary tract, diabetes, nervous system, diseases of the muscles and joints and a group of other comorbidities. All comorbidities were grouped in no comorbidities or ⩾1 comorbidity for the analysis.
Analyses were stratified for colon and rectal cancer patients and for aspirin or NSAID use vs non-users; a small percentage of patients received prescriptions for both (11%), they were included in the category of the medication most prescribed.
Results
In total, 4481 patients were diagnosed with colorectal cancer in the period 1998–2007 and included in the present study. Table 1 shows the characteristics of this population. Almost two-third of the patients were diagnosed with colon cancer (n=2793; 62%) and about one third with rectal cancer (n=1688; 38%). Median age at diagnosis was 69 years (range 22–99). A large proportion of the patients underwent surgery (91%). One in four patients (26%) had never used any prescribed aspirin/NSAID (non-users), 47% before and after the diagnosis of colorectal cancer and 27% only postdiagnosis.
Table 1. Characteristics of the cohort.
| Variable | Number | Percentage |
|---|---|---|
| Sex | ||
| Male | 2534 | 57 |
| Female | 1947 | 43 |
| Localisation | ||
| Colon | 2793 | 62 |
| Rectal | 1688 | 38 |
| Age (years) | ||
| <65 | 1745 | 39 |
| 65–74 | 1407 | 31 |
| ⩾75 | 1329 | 30 |
| Grade | ||
| I | 502 | 11 |
| II | 2580 | 58 |
| III | 682 | 15 |
| Unknown | 717 | 16 |
| Stage | ||
| I | 978 | 22 |
| II | 1419 | 32 |
| III | 1163 | 26 |
| IV | 722 | 16 |
| Unknown | 199 | 4 |
| Surgery | ||
| No | 400 | 9 |
| Yes | 4081 | 91 |
| Postoperative chemotherapy (colon) | ||
| No | 1862 | 72 |
| Yes | 713 | 28 |
| Radiotherapy (rectal) | ||
| No | 623 | 37 |
| Yes | 1065 | 63 |
| Prescribed aspirin or NSAIDs | ||
| None | 1176 | 26 |
| Prediagnosis and postdiagnosis | 2086 | 47 |
| Only postdiagnosis | 1219 | 27 |
| Postdiagnosis | ||
| Aspirin | 275 | 23 |
| NSAIDs | 944 | 77 |
Abbreviation: NSAID=non-steroid anti-inflammatory drug.
Table 2 shows the association of tumour characteristics with aspirin/NSAIDs use, stratified for colon and rectal cancer patients. Patients with postdiagnosis use (with or without prediagnosis use) were more often diagnosed with stage I colon cancer and less often with stage IV disease (P<0.001). For rectal cancer, the associations were less prominent for stage (P=0.1).
Table 2. Aspirin or NSAIDs use and patient and tumour characteristics.
| Aspirin or NSAIDs use | ||||
|---|---|---|---|---|
| None | Prediagnosis and postdiagnosis | Only postdiagnosis | P -value | |
| Colon cancer | ||||
| Sex | ||||
| Male | 389 (52.0) | 707 (53.8) | 383 (52.5) | 0.02 |
| Female | 359 (48.0) | 608 (46.2) | 347 (47.5) | |
| Age | ||||
| <65 years | 237 (31.7) | 451 (34.3) | 292 (40.0) | <0.001 |
| 65–74 years | 215 (28.7) | 421 (32.0) | 246 (33.7) | |
| ⩾75 years | 296 (39.6) | 443 (33.7) | 192 (26.3) | |
| Grade | ||||
| I | 87 (11.6) | 176 (13.4) | 96 (13.2) | <0.001 |
| II | 436 (58.3) | 772 (58.7) | 443 (60.7) | |
| III | 127 (17.0) | 229 (17.4) | 116 (15.9) | |
| Unknown | 98 (13.1) | 138 (10.5) | 75 (10.3) | |
| Stage | ||||
| I | 111 (14.8) | 225 (17.1) | 131 (17.9) | <0.001 |
| II | 274 (36.6) | 476 (36.2) | 271 (37.1) | |
| III | 170 (22.7) | 359 (27.3) | 201 (27.5) | |
| IV | 150 (20.1) | 208 (15.8) | 109 (14.9) | |
| Unknown | 43 (5.7) | 47 (3.6) | 18 (2.5) | |
| Rectal cancer | ||||
| Sex | ||||
| Male | 284 (66.4) | 460 (59.7) | 311 (63.6) | 0.1 |
| Female | 144 (33.6) | 311 (40.3) | 178 (36.4) | |
| Age | ||||
| <65 years | 170 (39.7) | 334 (43.3) | 261 (53.4) | <0.001 |
| 65–74 years | 125 (29.2) | 252 (32.7) | 148 (30.3) | |
| ⩾75 years | 133 (31.1) | 185 (23.0) | 80 (16.3) | |
| Grade | ||||
| I | 37 (8.6) | 72 (9.3) | 34 (6.9) | 0.002 |
| II | 240 (56.1) | 401 (52.0) | 288 (58.9) | |
| III | 46 (10.8) | 87 (11.3) | 77 (15.8) | |
| Unknown | 105 (24.5) | 211 (27.4) | 90 (18.4) | |
| Stage | ||||
| I | 112 (26.2) | 252 (32.7) | 147 (30.1) | 0.1 |
| II | 106 (24.8) | 176 (22.8) | 116 (23.7) | |
| III | 111 (25.9) | 185 (24.0) | 137 (28.0) | |
| IV | 70 (16.4) | 117 (15.2) | 68 (13.9) | |
| Unknown | 29 (6.8) | 41 (5.3) | 21 (4.3) | |
Abbreviation: NSAID=non-steroid anti-inflammatory drug.
Survival analysis with time-varying covariate
Table 3 shows the univariate survival analysis of patient and tumour characteristics. Age (P<0.001), comorbidity (P<0.001), year (P<0.001 for colon and P=0.005 for rectal), grade (P<0.001 for colon and P=0.001 for rectal), stage (P<0.001) and surgery (P<0.001) were associated with survival in colon and rectal cancer patients. Sex was not associated with survival (P=0.09 for colon and P=0.3 for rectal cancer). Chemotherapy for colon cancer patients was not associated with survival (P=0.5); radiotherapy for rectal cancer patients was, on the contrary, associated with survival (P=0.004).
Table 3. Univariate survival analysis of patient and tumour characteristics, stratified by the type of cancer.
| Rate ratio (95%CI) | P -value | |
|---|---|---|
| Colon cancer | ||
| Sex | ||
| Male | Reference | 0.09 |
| Female | 0.85 (0.71–1.02) | |
| Age | ||
| Continuous | 1.06 (1.05–1.07) | <0.001 |
| Comorbidity | ||
| No | Reference | <0.001 |
| Yes | 1.41 (1.17–1.70) | |
| Incidence year | ||
| Continuous | 1.07 (1.04–1.11) | <0.001 |
| Grade | ||
| I | Reference | <0.001 |
| II | 0.87 (0.65–1.15) | |
| III | 1.33 (0.96–1.85) | |
| Unknown | 1.65 (1.17–2.32) | |
| Stage | ||
| I | Reference | <0.001 |
| II | 1.26 (0.91–1.74) | |
| III | 1.76 (1.26–2.47) | |
| IV | 13.80 (10.07–18.91) | |
| Unknown | 6.30 (4.10–9.69) | |
| Chemotherapy | ||
| No | Reference | 0.5 |
| Yes | 1.08 (0.86–1.34) | |
| Surgery | ||
| No | Reference | <0.001 |
| Yes | 0.05 (0.04–0.06) | |
| Rectal cancer | ||
| Sex | ||
| Male | Reference | 0.3 |
| Female | 1.14 (0.89–1.48) | |
| Age | ||
| Continuous | 1.08 (1.07–1.09) | <0.001 |
| Comorbidity | ||
| No | Reference | <0.001 |
| Yes | 1.94 (1.52–2.48) | |
| Incidence year | ||
| Continuous | 1.06 (1.02–1.11) | 0.005 |
| Grade | ||
| I | Reference | 0.001 |
| II | 1.41 (0.85–2.33) | |
| III | 2.59 (1.48–4.53) | |
| Unknown | 1.72 (1.00–2.97) | |
| Stage | ||
| I | Reference | <0.001 |
| II | 2.01 (1.34–3.03) | |
| III | 1.85 (1.24–2.76) | |
| IV | 13.73 (9.33–20.20) | |
| Unknown | 9.66 (5.94–15.70) | |
| Radiotherapy | ||
| No | Reference | 0.004 |
| Yes | 0.70 (0.55–0.89) | |
| Surgery | ||
| No | Reference | <0.001 |
| Yes | 0.08 (0.06–0.10) | |
Table 4 shows the results comparing mortality between users of aspirin postdiagnosis vs non-user. For all patients with colorectal cancer, aspirin use after diagnosis was associated with a significant reduction of the overall mortality rate ratio (RR) 0.75 (95% confidence interval (CI) 0.62–0.92; P=0.005). Adjusted for sex, age, comorbidity, year of incidence, grade, stage and treatment, the multivariable RR for aspirin users compared with non-users was 0.77 (95%CI 0.63–0.95; P=0.015). Frequent use of aspirin postdiagnosis was also associated with a improved survival with a multivariable RR of 0.70 (95%CI 0.57–0.88; P=0.002). Multivariable analysis of the prediagnosis and postdiagnosis users also showed a significant association with survival (RR 0.88 (95%CI 0.83–0.94; P<0.001)).
Table 4. Time-dependent survival analysis (overall survival), stratified by colon or rectal cancer, for non-users and users of aspirin.
| User/non-user | Patients ( n) | Person-years | Deaths | Rate ratio | P -value | Adjusted rate ratio a | P -value | |
|---|---|---|---|---|---|---|---|---|
| Colorectal cancer | ||||||||
| Aspirin postdiagnosisb | Non-user | 1176 | 5059 | 610 | Reference | 0.005 | Reference | 0.015 |
| User aspirin | 275 | 1258 | 114 | 0.75 (0.62–0.92) | 0.77 (0.63–0.95) | |||
| Aspirin frequent users postdiagnosisb | Non-user | 1451 | 5172 | 628 | Reference | 0.001 | Reference | 0.002 |
| Frequent user | 234 | 1145 | 96 | 0.69 (0.56–0.86) | 0.70 (0.57–0.88) | |||
| Aspirin prediagnosis and postdiagnosis | Non-user | 1309 | 5184 | 627 | Reference | 0.5 | Reference | <0.001 |
| User | 819 | 3303 | 383 | 0.96 (0.84–1.09) | 0.88 (0.83–0.94) | |||
| Colon cancer | ||||||||
| Aspirin postdiagnosisb | Non-user | 748 | 3112 | 397 | Reference | <0.001 | Reference | 0.001 |
| User aspirin | 172 | 823 | 65 | 0.62 (0.48–0.80) | 0.65 (0.50–0.84) | |||
| Aspirin frequent users postdiagnosisb | Non-user | 772 | 3194 | 406 | Reference | <0.001 | Reference | 0.001 |
| Frequent user | 148 | 741 | 56 | 0.59 (0.45–0.79) | 0.61 (0.46–0.81) | |||
| Aspirin prediagnosis and postdiagnosis | Non-user | 843 | 3261 | 407 | Reference | 0.3 | Reference | 0.003 |
| User | 533 | 2173 | 249 | 0.92 (0.78–1.08) | 0.89 (0.82–0.96) | |||
| Rectal cancer | ||||||||
| Aspirin postdiagnosisb | Non-user | 428 | 1947 | 213 | Reference | 0.9 | Reference | 0.6 |
| User aspirin | 103 | 435 | 49 | 1.03 (0.75–1.40) | 1.10 (0.79–1.54) | |||
| Aspirin frequent users postdiagnosisb | Non-user | 445 | 1978 | 222 | Reference | 0.5 | Reference | 0.7 |
| Frequent user | 86 | 404 | 40 | 0.88 (0.63–1.23) | 0.94 (0.65–1.34) | |||
| Aspirin prediagnosis and postdiagnosis | Non-user | 466 | 1923 | 220 | Reference | 0.7 | Reference | 0.01 |
| User | 286 | 1130 | 134 | 1.04 (0.84–1.28) | 0.87 (0.78–0.97) | |||
Adjusted for sex, age, comorbidity, year of incidence, grade, stage, chemotherapy (colon cancer), radiotherapy (rectal cancer) and surgery (all variables with P<0.05 in univariate analysis were entered in the multivariable analysis, together with sex and treatment). Median follow-up: 3.5 (0–12) years.
Postdiagnosis users are patients that used aspirin solely postdiagnosis and not prediagnosis.
The bold and italic entries indicate P<0.05.
Stratified analysis for colon and rectal cancer showed that the survival gain was only present in patients with colon cancer. A significant reduction of mortality was shown in aspirin users (independent of the frequency) as compared with non-users with a RR of 0.62 (95%CI 0.46–0.80; P<0.001). After adjusting for potential confounders, the survival gain was still present with a RR of 0.65 (95%CI 0.50–0.84; P=0.001) for users of aspirin as compared with non-users. For frequent users of aspirin the survival gain was somewhat larger with an adjusted RR of 0.61 (95%CI 0.46–0.81; P=0.001) for users as compared with non-users. A sensitivity analysis, where patients that initiated frequent aspirin use after 3 years were coded as non-users, showed an adjusted RR of 0.60 (95%CI 0.44–0.81; P=0.001). Stratified analyses for chemotherapy or no chemotherapy showed a significant survival gain in patients who received no chemotherapy (adjusted RR 0.67 (95%CI 0.51–0.89; P=0.006), however, no significant survival gain in those patients that received chemotherapy (adjusted RR 0.51 (95%CI 0.23–1.13; P=0.1). For elderly patients (75 and older) who received no chemotherapy, the survival gain was especially large (adjusted RR 0.52 (95%CI 0.35–0.78; P=0.001)). The survival gain for patients that used aspirin prediagnosis and postdiagnosis was not significant in univariate analysis (RR 0.92 (95%CI 0.78–1.08; P=0.3)), however, adjusted for potential confounders the overall mortality was significantly reduced in users (RR 0.89 (95%CI 0.82–0.96; P=0.003)).
In rectal cancer patients, no significant survival gain for aspirin users as compared with non-users was observed. In all patients that used aspirin, the adjusted RR was 1.10 (95%CI 0.79–1.54; P=0.6). For frequent users of aspirin with rectal cancer there was also no survival gain (adjusted RR 0.94 (95%CI 0.65–1.34; P=0.7). In multivariable analysis there was a significant survival gain for users prediagnosis and postdiagnosis (RR 0.87 (95%CI 0.78–0.97; P=0.01)).
Table 5 shows the results of the time-dependent survival analysis for the NSAIDs. In all colorectal patients, users of NSAIDs had a decreased survival as compared with non-users; the adjusted RR was 1.93 (95%CI 1.70–2.20; P<0.001). Stratified for colon and rectal cancer, the same pattern was shown in both patient groups with an adjusted RR for colon cancer of 1.79 (95%CI 1.52–2.11; P<0.001) and 2.27 (95%CI 1.84–2.82; P<0.001) for rectal cancer in users as compared to non-users.
Table 5. Time-dependent survival analysis (overall survival), stratified by colon or rectal cancer, for non-users and NSAIDs users.
| User/non-user | Patients ( n) | Person-years | Deaths | Rate ratio | P -value | Adjusted Rate ratio a | P -value | |
|---|---|---|---|---|---|---|---|---|
| Colorectal cancer | ||||||||
| NSAIDs postdiagnosisb | Non-user | 1176 | 6362 | 610 | Reference | <0.001 | Reference | <0.001 |
| User NSAIDs | 944 | 3556 | 440 | 1.29 (1.14–1.46) | 1.93 (1.70–2.20) | |||
| Colon cancer | ||||||||
| NSAIDs postdiagnosisb | Non-user | 748 | 3903 | 397 | Reference | 0.003 | Reference | <0.001 |
| User NSAIDs | 558 | 2049 | 263 | 1.26 (1.08–1.47) | 1.79 (1.52–2.11) | |||
| Rectal cancer | ||||||||
| NSAIDs postdiagnosisb | Non-user | 428 | 2458 | 213 | Reference | 0.003 | Reference | <0.001 |
| User NSAIDs | 386 | 1507 | 177 | 1.36 (1.11–1.65) | 2.27 (1.84–2.82) | |||
Abbreviation: NSAID=non-steroid anti-inflammatory drug.
Adjusted for sex, age, comorbidity, year of incidence, grade, stage, chemotherapy (colon cancer), radiotherapy (rectal cancer) and surgery (all variables with P<0.05 in univariate analysis were entered in the multivariable analysis, together with sex and treatment). Median follow-up: 3.5 (0–12) years.
Postdiagnosis users are patients that used aspirin solely postdiagnosis and not prediagnosis.
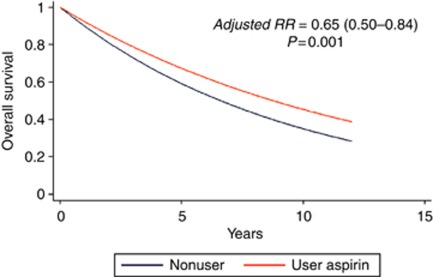
Figure 1 shows the overall survival curve for aspirin users in colon cancer patients.
Figure 1.
Survival curve for overall survival in colon cancer patients according to non-user/user of aspirin.
Supplementary Table 1 shows the analysis of solely prediagnosis users. For patients with colon cancer, prediagnosis users had a significantly worse survival than patients without prescribed aspirin or NSAIDs (adjusted HR NSAIDs 1.3 (95%CI 1.1–1.5; P=0.007) and aspirin HR 1.5 (95%CI 1.2–1.8; P<0.001)). For prediagnosis users with rectal cancer there was no significant association between prediagnosis use of NSAIDs and survival (HR 1.1 (95%CI 0.8–1.4; P=0.7)), however, there was a significant worse survival for patients who used prescribed aspirin prediagnosis (HR 1.4 (95%CI 1.0–2.0; P=0.03)).
Discussion
Aspirin may have an important role as a cancer treatment agent in the adjuvant setting (Neugut, 2009). This is the largest observational study that assessed the value of ‘adjuvant’ use of aspirin/NSAIDs. In this study, we observed that aspirin use initiated or continued after the diagnosis of colon cancer was associated with a markedly increased overall survival. The effect was present in several subgroups and dependent on the frequency of prescription.
Our results add important new observations. First, using a time-varying covariate in a survival model, we show for the first time the therapeutic effect of aspirin in colon cancer patients. Second, owing to the large number of patients, we were able to stratify for age. Elderly patients that received no chemotherapy had the most benefit of aspirin in terms of overall survival. Furthermore, the therapeutic effect of aspirin was larger with increased number of prescriptions. Third, the effect of overall survival was specific for aspirin. Use of non-aspirin NSAIDs in our analysis showed the opposite effect.
These results are in line with other reports that investigated the role of aspirin or NSAIDs in colorectal cancer therapy. The recent study of Chan et al (2009) showed a similar risk reduction as compared to our study; where the present study included more patients and was no biased by recall. In another study by Coghill et al (2011), regular NSAID use before the diagnosis of colorectal cancer was associated with a significant lower colorectal cancer-specific death rate. In that study aspirin use seem to be more relevant than ibuprofen for survival. In our study, surprisingly, the effect on overall survival was only present in colon cancer patients that used aspirin. In rectal cancer patients and in patients using non-aspirin NSAIDs, no effect on survival was seen. On the contrary, non-aspirin NSAID use was associated with a worse survival. We cannot rule out that ‘over the counter’ use of popular pain medication, such as voltaren and ibuprofen, confounded these results. However, low-dose aspirin is always on prescription in the Netherlands and the risk of bias concerning ‘over the counter use’ is neglectable. Interestingly, in our study, the observed survival benefit was largest among patients that initiated aspirin use after the diagnosis of colon cancer. In patients that started aspirin before their colon cancer and continued thereafter, the beneficial effect on overall survival was less profound. The results of our study and others are also in line with the recent observation that long-term use of aspirin in cardiovascular prevention trials is associated with a reduction of death from colorectal cancer that is even greater than the reduction in incidence (Rothwell et al, 2010). An earlier study of patients with stage III colon cancer enrolled in an adjuvant chemotherapy trial showed that aspirin use was associated with a lower risk of disease recurrence and death (Fuchs et al, 2005). In contrast to the studies of Chan et al (2009) and Coghill et al (2011), patients who used aspirin or NSAIDs solely before diagnosis had a worse survival than non-users. Chan et al showed no association between aspirin use before cancer diagnosis and overall mortality (HR 0.93; 95%CI 0.77–1.11); however, Coghill et al showed a significant association between prediagnosis NSAID use and colorectal cancer-specific survival (HR 0.79; 95%CI 0.65–0.97). In the present study, we found a negative association between overall survival and prediagnosis use, which could be associated with the overall worse general health of these patients or could be biased by ‘over the counter’ use of NSAIDs. This ‘over the counter’ use could also have biased the negative association between survival and use of NSAIDs postdiagnosis.
In the Netherlands low-dose aspirin was not prescribed for the purpose of cancer prevention or treatment during the study period. Therefore, it is highly likely that virtually all patients received aspirin to prevent morbidity and mortality from cardiovascular disease. However, the effect of aspirin on overall mortality in cardio-prevention trials is small and not significant in a recent meta-analysis (HR 0.95 (95%CI 0.88–1.02; P=0.1) (Baigent et al, 2009). The survival gain in our analysis remained present after further adjustment for comorbidity and after excluding stage IV patients. Colon cancer patients who used aspirin prediagnosis and postdiagnosis had less benefit of aspirin than postdiagnosis users, which is biologically plausible considering that tumours that developed in prediagnosis users were not prevented by aspirin use (Chan et al, 2009; Neugut, 2009). However, the immortal time bias could have biased the results in this specific subgroup, as patients that were on aspirin could not have died in that period (Suissa, 2008).
The mechanism by which aspirin exerts its activity is not completely understood. An inverse association between the incidence of various cancers and the use of aspirin or other NSAIDs has been supported by epidemiological studies, animal models and plant studies (Elwood et al, 2009). The NSAID’s cancer protective activity has been attributed to direct inhibition of the COX family of enzymes involved in prostaglandin synthesis. The COX-2 enzyme is strongly and rapidly induced in response to mediators of inflammation, growth factors, cytokines and endotoxins; and its expression correlates with increased cell proliferation and tumour promotion (Herschman, 1996). Aspirin can decrease the production of potentially neoplastic prostaglandins arising from COX-2-mediated catalysis of arachidonic acid (Vane, 1971). However, aspirin has a much broader range of downstream effectors, such as NF-KB, insulin-like growth factor I and many others, as well as the inhibition of Wnt signalling and stem cell growth possibly as the result of enhanced beta-catenin phosphorylation (Dihlmann et al, 2001, 2003; Wang et al, 2006).
Cancer is a heterogeneous disease encompassing differentiated cell types but also less committed stem-like cells. Recent evidence suggests that a subpopulation of tumour cells with distinct features such as self-renewal and the ability to differentiate into multiple lineages is responsible for tumour initiation, invasive growth and possibly dissemination to distant organ sites. The acquired ability of a cell to resist to apoptosis is a hallmark of almost all types of cancer. Recently, it has been shown that IL-4 expression is essential for the resistance to DNA damage-induced apoptosis of colon cancer stem cells (CSCs) (Todaro et al, 2007). The CSCs are also resistant to the cytotoxic effect of chemotherapy. It is plausible that aspirin may not only act as a preventive agent in CRC onset by modulating the Wnt pathway in CSCs but also as adjuvant treatment by increasing CSC’s sensitivity to conventional chemotherapy regimens.
This observational study may suffer from several forms of bias. First, despite the biological plausibility of our results, confounding by indication can be a problem. Most of the patients used the low-dose aspirin for cardiovascular disease prevention. Theoretically, the effect of aspirin in our cohort may be considered as a side effect of the drug in these patients, and that makes observational data more suitable for these analyses (Vandenbroucke, 2008). Second, we have only data concerning the prescribed drugs, and do not know the use of other aspirin or NSAIDs. However, by linkage of two validated databases, our study does not suffer from recall bias, which may exist if aspirin use is assessed by questionnaires. Third, unfortunately we did not have any information concerning COX-2 expression of the tumours, which seems to be associated with recurrence and survival for colorectal cancer patients or concerning the cancer-specific survival.
Our findings could have profound clinical implications. In this study, we demonstrate the therapeutic effect of a well-tolerated drug that cost mere pennies per day. Especially in the increasing elderly population with colon cancer, many patients are considered unfit for adjuvant chemotherapy. Age and comorbidity are the strongest predictors of a medical oncologist not recommending adjuvant chemotherapy (Keating et al, 2008). The demonstration of the therapeutic anti-cancer effect of aspirin would be a major clinical advance. However, the formal demonstration will still need a placebo-controlled randomised trial, which will be initiated in the Netherlands.
Acknowledgments
All the co-authors have read and approved the final manuscript and have directly participated in the planning, execution or analysis of the study.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
The authors declare no conflict of interest.
Supplementary Material
References
- Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A (2009) Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 373: 1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, Keown-Eyssen G, Summers RW, Rothstein R, Burke CA, Snover DC, Church TR, Allen JI, Beach M, Beck GJ, Bond JH, Byers T, Greenberg ER, Mandel JS, Marcon N, Mott LA, Pearson L, Saibil F, van Stolk RU (2003) A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med 348: 891–899 [DOI] [PubMed] [Google Scholar]
- Chan AT, Ogino S, Fuchs CS (2007) Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med 356: 2131–2142 [DOI] [PubMed] [Google Scholar]
- Chan AT, Ogino S, Fuchs CS (2009) Aspirin use and survival after diagnosis of colorectal cancer. JAMA 302: 649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WS, Wei SJ, Liu JM, Hsiao M, Kou-Lin J, Yang WK (2001) Tumor invasiveness and liver metastasis of colon cancer cells correlated with cyclooxygenase-2 (COX-2) expression and inhibited by a COX-2-selective inhibitor, etodolac. Int J Cancer 91: 894–899 [DOI] [PubMed] [Google Scholar]
- Coghill AE, Newcomb PA, Campbell PT, Burnett-Hartman AN, Adams SV, Poole EM, Potter JD, Ulrich CM (2011) Prediagnostic non-steroidal anti-inflammatory drug use and survival after diagnosis of colorectal cancer. Gut 60: 491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, Jankowski J, La VC, Meyskens F, Senn HJ, Thun M (2009) Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol 10: 501–507 [DOI] [PubMed] [Google Scholar]
- Dihlmann S, Klein S, Doeberitz Mv MK (2003) Reduction of beta-catenin/T-cell transcription factor signaling by aspirin and indomethacin is caused by an increased stabilization of phosphorylated beta-catenin. Mol Cancer Ther 2: 509–516 [PubMed] [Google Scholar]
- Dihlmann S, Siermann A, von Knebel DM (2001) The nonsteroidal anti-inflammatory drugs aspirin and indomethacin attenuate beta-catenin/TCF-4 signaling. Oncogene 20: 645–653 [DOI] [PubMed] [Google Scholar]
- Elwood PC, Gallagher AM, Duthie GG, Mur LA, Morgan G (2009) Aspirin, salicylates, and cancer. Lancet 373: 1301–1309 [DOI] [PubMed] [Google Scholar]
- Fuchs CS, Meyerhardt JA, Heseltine DL, Niedzwiecki D, Hollis D, Chan AT, Saltz LB, Schilsky R, Mayer RJ. Influence of regular aspirin use on survival for patients with stage III colon cancer: Findings from Intergroup trial CALGB 89803. ASCO (2005) Ref type: Abstract
- Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC, Speizer FE (1995) Aspirin and the risk of colorectal cancer in women. N Engl J Med 333: 609–614 [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC (1994) Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med 121: 241–246 [DOI] [PubMed] [Google Scholar]
- Herschman HR (1996) Prostaglandin synthase 2. Biochim Biophys Acta 1299: 125–140 [DOI] [PubMed] [Google Scholar]
- Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE (2010) Aspirin intake and survival after breast cancer. J Clin Oncol 28: 1467–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating NL, Landrum MB, Klabunde CN, Fletcher RH, Rogers SO, Doucette WR, Tisnado D, Clauser S, Kahn KL (2008) Adjuvant chemotherapy for stage III colon cancer: do physicians agree about the importance of patient age and comorbidity? J Clin Oncol 26: 2532–2537 [DOI] [PubMed] [Google Scholar]
- Neugut AI (2009) Aspirin as adjuvant therapy for colorectal cancer: a promising new twist for an old drug. JAMA 302: 688–689 [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW (2011) Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377: 31–41 [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, Meade TW (2010) Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 376: 1741–1750 [DOI] [PubMed] [Google Scholar]
- Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, Petrelli N, Pipas JM, Karp DD, Loprinzi CL, Steinbach G, Schilsky R (2003) A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med 348: 883–890 [DOI] [PubMed] [Google Scholar]
- Schwenger P, Bellosta P, Vietor I, Basilico C, Skolnik EY, Vilcek J (1997) Sodium salicylate induces apoptosis via p38 mitogen-activated protein kinase but inhibits tumor necrosis factor-induced c-Jun N-terminal kinase/stress-activated protein kinase activation. Proc Natl Acad Sci USA 94: 2869–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suissa S (2008) Immortal time bias in pharmaco-epidemiology. Am J Epidemiol 167: 492–499 [DOI] [PubMed] [Google Scholar]
- Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, Stassi G (2007) Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell 1: 389–402 [DOI] [PubMed] [Google Scholar]
- Tomozawa S, Nagawa H, Tsuno N, Hatano K, Osada T, Kitayama J, Sunami E, Nita ME, Ishihara S, Yano H, Tsuruo T, Shibata Y, Muto T (1999) Inhibition of haematogenous metastasis of colon cancer in mice by a selective COX-2 inhibitor, JTE-522. Br J Cancer 81: 1274–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujii M, Kawano S, DuBois RN (1997) Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA 94: 3336–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN (1998) Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93: 705–716 [DOI] [PubMed] [Google Scholar]
- van Herk-Sukel MP, van de Poll-Franse LV, Lemmens VE, Vreugdenhil G, Pruijt JF, Coebergh JW, Herings RM (2010) New opportunities for drug outcomes research in cancer patients: the linkage of the Eindhoven Cancer Registry and the PHARMO Record Linkage System. Eur J Cancer 46: 395–404 [DOI] [PubMed] [Google Scholar]
- Vandenbroucke JP (2008) Observational research, randomised trials, and two views of medical science. PLoS Med 5: e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane JR (1971) Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 231: 232–235 [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen X, Zhu W, Zhang H, Hu S, Cong X (2006) Growth inhibition of mesenchymal stem cells by aspirin: involvement of the WNT/beta-catenin signal pathway. Clin Exp Pharmacol Physiol 33: 696–701 [DOI] [PubMed] [Google Scholar]
- Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW (2005) Colorectal cancer. Lancet 365: 153–165 [DOI] [PubMed] [Google Scholar]
- Williams CS, Sheng H, Brockman JA, Armandla R, Shao J, Washington MK, Elkahloun AG, DuBois RN (2001) A cyclooxygenase-2 inhibitor (SC-58125) blocks growth of established human colon cancer xenografts. Neoplasia 3: 428–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M, Kargman S, Lam EC, Kelly CR, Zheng Y, Luk P, Kwong E, Evans JF, Wolfe MM (2003) Inhibition of cyclooxygenase-2 by rofecoxib attenuates the growth and metastatic potential of colorectal carcinoma in mice. Cancer Res 63: 586–592 [PubMed] [Google Scholar]
- Yao M, Zhou W, Sangha S, Albert A, Chang AJ, Liu TC, Wolfe MM (2005) Effects of nonselective cyclooxygenase inhibition with low-dose ibuprofen on tumor growth, angiogenesis, metastasis, and survival in a mouse model of colorectal cancer. Clin Cancer Res 11: 1618–1628 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.