Abstract
The cellular and molecular processes that control vascular injury responses following PCI involve a complex interplay among vascular cells and progenitor cells that control arterial remodeling, neoinitimal proliferation and reendothelialization. Drug eluting stents (DES) improve the efficacy of peructaneous coronary intervention (PCI) by modulating vascular inflammation and preventing neointimal proliferation and restenosis. Although positive effects of DES reduce inflammation and restenosis, negative effects delay reendothelialization and impair endothelial function. Delayed reendothelialization and impaired endothelial function may be linked to stent thrombosis and adverse clinical outcomes following DES use. Compared with BMS, DES may also differentially modulate mobilization, homing and differentiation of vascular progenitor cells involved reendothelialization and neointimal proliferation. The effects of DES on vascular inflammation and repair directly impact clinical outcomes with these devices and dictate requirements for extended duration dual antiplatelet therapy.
Keywords: stent thrombosis, restenosis, inflammation, reendothelialization
Introduction
Drug-eluting stents (DES) substantially reduce angiographic and clinical restenosis by 70% across broad patient and lesion subsets, and decrease repeat target lesion interventions. The prototypical antiproliferative DES agents sirolimus (CYPHER stent, Johnson and Johnson), paclitaxel (Taxus Stent, Boston Scientific), zotarolimus (Endeavor stent, Medtronic), and everolimus (Xience stent, Abbott and Boston Scientific) have potent anti-mitotic actions that strongly inhibit smooth muscle proliferation and matrix production,1–3 and thus reduce neointimal formation and restenosis. Despite efficacy in reducing neointimal proliferation and restenosis, DES failure and restenosis still occurs, and is more frequent in the settings of diabetes mellitus and during treatment of restenotic lesions, bypass grafts, and bifurcations.4–6 In addition to restenosis, concern has arisen about the potential for late thromboses or very late thromboses after DES stent implantation, and this concern has led to extended-duration dual anti-platelet therapy.7–9 Mechanisms of stent thrombosis may vary depending on the timing of the event.10 Acute stent thrombosis (within 24h of implantation) and early stent thrombosis (within 30 days) are likely related to mechanical issues with the stent, inadequate platelet inhibition, or prothrombotic patient risk factors. In contrast, late stent thrombosis (up to 1 year), and very late stent thrombosis (after 1 year), have been attributed to delayed reendothelialization and inhibition of vascular repair. The potential for delayed reendothelialization and inhibition of vascular repair is particularly important following implantation of DES because the antiproliferative agents used to prevent smooth muscle cell proliferation also delay reendothelialization in the stented segment.11, 12 Angioscopic13 and pathologic11, 12, 14, 15 evidence suggests that there is delayed arterial healing with DES compared to BMS as DES-treated arteries have more histologic evidence of incomplete reendothelialization, chronic inflammatory cell infiltration, fibrin deposition, and platelet activation. It is important to recognize that inflammatory and thrombotic pathways share commons signaling pathways and that inflammatory responses promote activation of the clotting cascade and stimulate platelet activation (reviewed 16). Experimental studies also suggest that delayed arterial healing and DES-associated inflammation is greatest at sites of overlapping DES with placement of multiple stents.17 The finding of increased inflammation in areas of stent overlap suggest a possible molecular mechanism to explain higher stent thrombosis rates that are associated with overlapping stents.
In addition to antiproliferative drug-associated delayed healing with DES, stent-induced or polymer-induced inflammation has also been identified as a possible contributor to stent thrombosis, especially because late and very late stent thrombosis occurs long after antiproliferative dugs have been eluted from the polymer.18–20 Inflammatory responses to drug, stent, or polymer may result from non-specific innate immune responses which have a predominance of monocyte/macrophage infiltrates, or may be related to antigen-specific adaptive immune hypersensitivity responses typified by infiltration of eosinophils, B-cells, and T-cells (reviewed 21). Several studies have also implicated DES-polymer-induced inflammation in the pathobiology of restenosis and stent thrombosis.18, 19 Currently, the four stent platforms approved for use by the United States Food and Drug Administration utilize different non-erodable polymeric coatings for drug delivery, and experimental animal studies suggest that biological compatibility, immunogenicity, and thrombogenicity may vary among specific polymeric compounds.22 The next generations of DES are attempting to reduce the possibility of polymer-induced inflammation, delayed arterial healing, restenosis, and stent thrombosis through use of polymers that have better biocompatibility and/or are biodegradable.
Aside from delayed arterial healing, emerging evidence suggests that compared to BMS, DES impair endothelial function in arterial segments distal to the stented site.23, 24 Even 6 months following implantation of DES, artery segments distal to the DES show abnormal vasoreactivity. 25,26,27 DES-associated abnormalities in endothelial function could be related to delayed vascular repair and not the DES drug itself, because the kinetics of DES are such that the drugs are completely eluted within months after implantation.28–31 It possible however that in certain circumstances, drug accumulation in the arterial wall32 and the liphophilic core of stented atheroma results in prolonged drug retention/release and ongoing vascular dysfunction. The mechanism of DES-associated endothelial dysfunction is not established, and recent studies have demonstrated that there is variability in the severity of DES-associated endothelial dysfunction among specific DES agents.33–35 It is unclear if DES-associated vascular dysfunction influences clinical outcomes following DES implantation. One small study demonstrated impaired endothelial function in patients presenting with in-stent restenosis compared with matched controls,36 however this association will require validation in larger prospective investigations.
Based on the biology of DES and the potential for delayed reendothelialization and repair, concern was raised about the possibility for increased rates of late and very late stent thrombosis in DES trials, and excess mortality in DES- vs. BMS-treated patients (reviewed7). Because of the insufficient power of individual trials to assess the low-incidence events of late and very late stent thrombosis, multiple meta-analyses were performed to evaluate the risk of stent thrombosis in patients treated with DES vs. BMS using the standardized Academic Research Consortium (ARC)-definition of sent thrombosis 37–41. These meta-analyses and subsequent analyses of stent registry data 42–45 demonstrated nearly equivalent risk of stent thrombosis (approximately 0.5%) in patients treated with DES or BMS. A small increase in the risk of late and very late stent thrombosis on the order of 1–2% cannot be excluded however because available data have insufficient power to evaluate this very rare event.
Analyses of stent thrombosis and outcomes with DES are further complicated by significant differences in stent structure, drug delivery polymers, and anti-proliferative drugs among the rapidly expanding panel of DES. In addition, complex biology controls vascular repair following PCI. Understanding the common and differential molecular pathways that regulate reendothelialization vs. restenosis will provide a biological context for rational use of DES, and will enable development of new DES technologies that can inhibit neointimal proliferation and preserve or even promote endothelial repair. In the following sections, we will highlight key cellular and molecular pathways that regulate vascular injury and repair in the setting of percutaneous coronary revascularization, and we will discuss the role of DES in modulating vascular repair processes.
Role of Inflammation in Restenosis and Vascular Repair
Stent placement leads to mechanical injury that induces substantial local inflammation which stimulates vascular smooth muscle cell proliferation and extracellular matrix deposition, resulting in neointimal thickening and restenosis.46, 47 Vascular inflammation following PCI involves complex interactions between multiple vascular cell types and under normal circumstances, the cellular and molecular processes that control vascular injury responses direct repair and vascular healing. In pathological conditions, dysregulation of vascular repair results in persistent vascular inflammation, neointimal proliferation, and restenotic obstruction of the stent lumen.
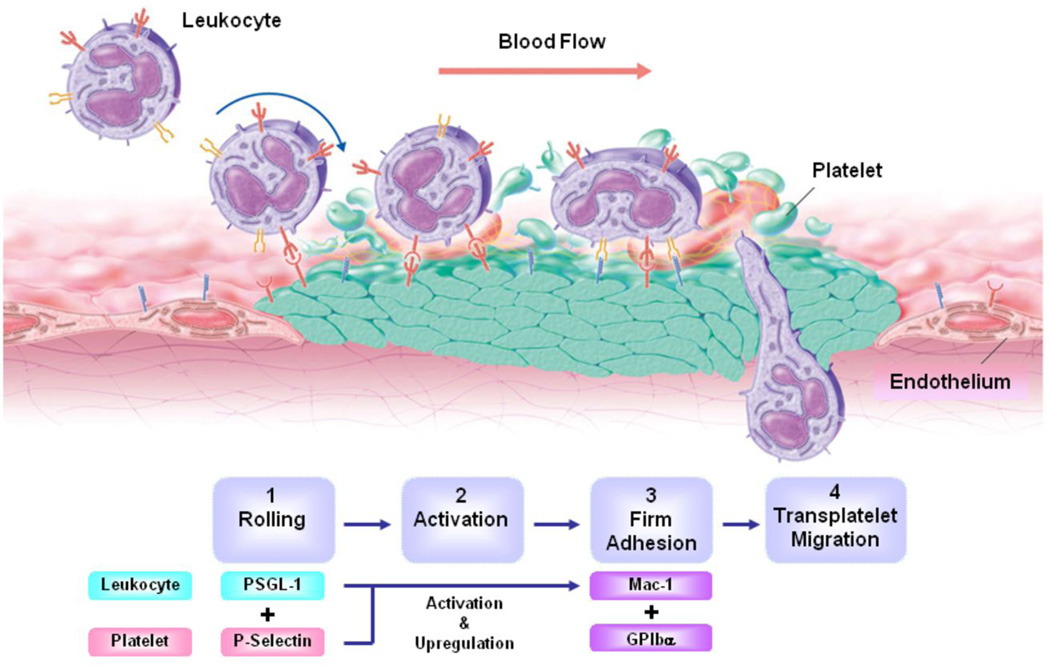
Immediately following PCI, platelets, neutrophils and monocytes play a central role in the initial inflammatory response.47, 48 Platelets and fibrin deposit on the de-endothelialized vessel wall and recruit leukocytes to the injured vessel segment through a cascade of cell adhesion molecules that direct leukocyte attachment and transmigration across surface-adherent platelets. 49 The initial tethering and rolling of leukocytes on platelets is mediated through binding of the leukocyte receptor P-selectin glycoprotein ligand-1 (PSGL-1) to platelet P-selectin.50–52 Rolling leukocytes stop and firmly attach to adherent platelets when the leukocyte integrin Mac-1(CD11b/CD18) binds to platelet glycoprotein Ibα 53 or to fibrinogen bound to the platelet glycoprotein IIb/IIIa (Fig. 1).54 A direct role for Mac-1 in leukocyte adhesion following mechanical injury has been demonstrated in several experimental studies where Mac-1 targeting reduces neointimal thickening after experimental angioplasty.55,56. Clinical studies of patients undergoing PCI further support the premise that Mac-1 and platelet-mediated leukocyte adhesion (also termed secondary capture) plays an important role in vascular inflammation and restenosis following coronary stenting. We have previously shown that compared with circulating neutrophils, Mac-1 surface expression is significantly increased in the neutrophils obtained from the coronary sinus of patients who underwent PCI within the preceding 48 hours, and that high levels of Mac-1 expression are associated with angiographic late lumen loss and increased risk of restenosis.57–60 Increased Mac-1 expression also correlates with increased expression of Pselectin on the surface platelets obtained from the coronary sinus following PCI.57–60
Figure 1. Transplatelet leukocyte migration.
At the site of stent implantation following PCI, endothelial cells are denuded and the subendothelial matrix is exposed to flowing blood. Platelets and fibrinogen immediately adhere surface of the injured vessel. A multistep cascade of platelet and leukocyte adhesion molecules direct leukocyte adhesion to the adherent platelets in a process termed “secondary capture”. Leukocyte capture and rolling are mediated by interaction between platelet P-selectin and leukocyte PSGL-1. Arrest and firm adhesion are mediated by platelet glycoprotien Ibα and leukocyte Mac-1. Chemokines stimulate transmigration into the extraluminal tissue.
Role of Bone Marrow-derived Stem Cells in Restenosis and Vascular Repair
Emerging research is demonstrating that bone marrow-derived progenitor cells play an important role in vascular inflammation responses and in vascular repair. Endothelial progenitor cells (EPCs) mobilized from bone marrow into peripheral blood promote endothelial regeneration and postnatal neovascularization.61, 62 In contrast to the potential protective effects of EPCs, it has been hypothesized that smooth muscle progenitor cells (SMPCs) which are also mobilized from bone marrow, migrate to the sites of vascular injury where they contribute to smooth muscle cell expansion and neointimal proliferation. 63–65, 66, 67
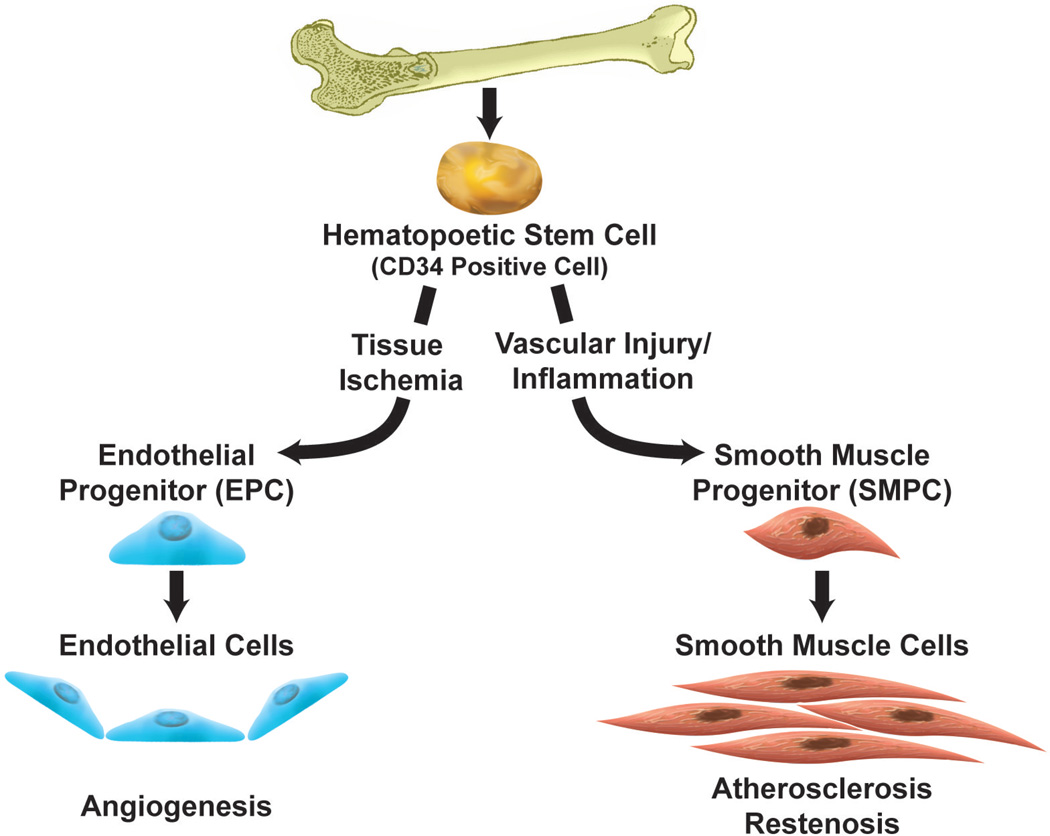
The precise function of EPCs and SMPCs once they home to sites of vascular inflammation is controversial. Previously, CD34-positive cells were believed to be committed population of EPCs, however further study demonstrated that the CD34 surface antigen actually identifies undifferentiated bone marrow-derived stem cells that have the ability differentiate into EPC and SMPCs. Transdifferentiation of CD34 positive cells into EPC or SMPC lineages depends on the local environment; ischemic conditions signal differentiation toward EPC phenotypes in order to promote reendothelialization, 61, 66 and inflammatory conditions signal differentiation toward SMPC phenotypes that promote neointimal proliferation63(Fig. 2).
Figure 2. Differentiation of bone marrow-derived stem cells.
Previously, CD34-positive cells were believed to be committed population of EPCs, however further study demonstrated that the CD34 surface antigen actually identifies undifferentiated bone marrow-derived stem cells that have the ability differentiate into EPC and SMPCs. Ischemic conditions signal differentiation toward EPC phenotypes in order to promote reendothelialization. Inflammatory conditions signal differentiation toward SMPC phenotypes that promote neointimal proliferation.
Several studies have implicated CD34 positive progenitors cells in vascular injury responses following PCI. Circulating CD34 positive cells are increased in the days following acute myocardial infarction, and characterization of these circulating cells suggests that they have an EPC-like phenotype raising the possibility that CD34-positive EPC-like cells are mobilized to promote angiogenesis in the ischemic myocardium. In contrast to ischemia-mediated mobilization, SMPC-like CD34-positive cells increase following PCI in patients with chronic coronary artery disease presumably in response to inflammatory mediators produced at sites of stent implantation.67 In this setting, elevated levels of circulating CD34-positive cells are associated with increased rates of restenosis, suggesting possible involvement regulation of neointimal formation.68
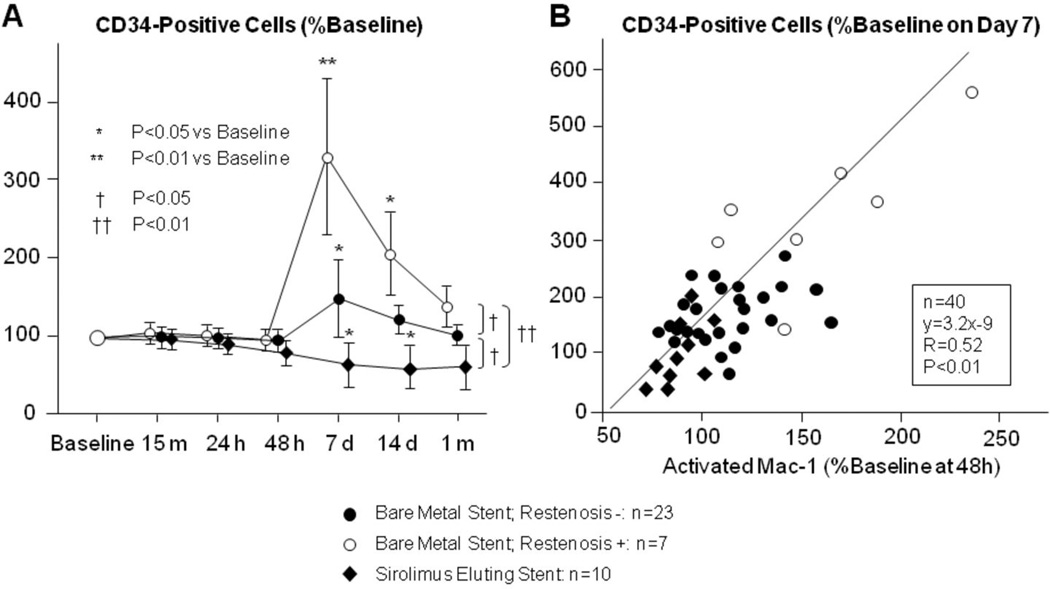
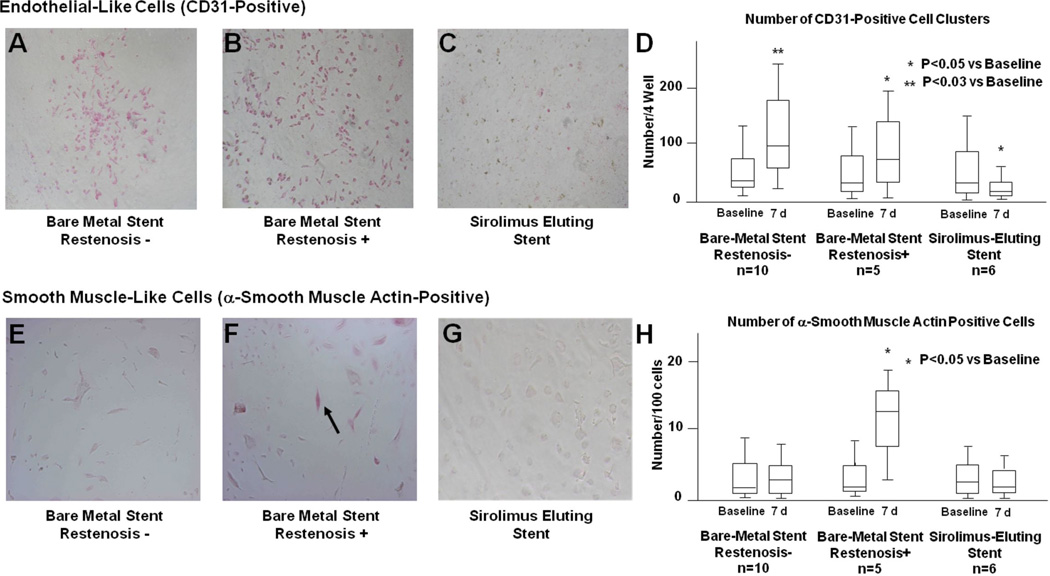
We have also demonstrated that molecular signals generated at sites of local arterial inflammation promote the mobilization of CD34-positive stem cells. 69 In our study, the number of CD34-positive cells in the peripheral blood increased day 7–14 following PCI, and patients who received BMS had significantly more CD34-postive cells than those who received DES (Fig. 3A).69, 70, 72 G-CSF and Mac-1 levels were significantly reduced in patients who underwent implantation of DES compared to those who received BMS, suggesting the anti-proliferative effect of stent drug influenced inflammatory cell activation in this setting (Fig. 3-B).69 This observation is consistent with our hypothesis that inflammatory signals generated at sites of coronary injury mobilize bone marrow-derived progenitor cells involved in vascular repair. To further elucidate the role of CD34-positive cells in vascular injury and repair following PCI, we isolated circulating CD34-positive progenitor cells from patients who received DES and BMS and performed in vitro differentiation assays (Fig. 4).69 In most patients, a proportion of the cultured CD34-positive cells differentiated into both CD31-positive endothelial-like cells and into α-actin-positive cells with features suggestive of smooth muscle cell lineage. Several other observations were made: First, the number of differentiated colonies that formed from the CD34-postive cells correlated with the extent of restenosis during angiographic follow up. Second, patients with more angiographic restenosis appeared to have more CD34-postive cells that differentiated into α-actin containing SMPC-like cells. Third, implantation of SES resulted in reduced differentiation of CD34-positive cells into CD31-positive cells, and reduced differentiation into α-actin-positive cells with smooth muscle cell feature. This finding is consistent with in vitro data demonstrating that sirolimus inhibits differentiation of human bone marrow-derived stem cells into endothelial or smooth muscle cells.71, 72
Figure 3. CD34-positive cell counts and CD34-positive cell Mac-1 expression following PCI.
(A) Circulating CD34 positive cells increase following PCI. The highest levels of CD34-positve cells were seen in the peripheral blood of patients who received BMS that went on to have restenosis at 6 month angiographic follow-up. Implantation of DES was associated with a significant reduction in the number of circulating CD34 positive cells. (B) Neutrophil Mac-1 expression correlates with mobilization of CD34-positive cells. Forty eight hours after PCI, neutrophils were harvested from the coronary sinus of patients who had coronary stents implanted. Neutrophil Mac-1 expression was quantified by flow cytometry. Neutrophil Mac-1 expression at 48 hours correlated with circulating levels of CD34-positive cells 7 days after PCI, demonstrating that higher levels of local vascular inflammation are associated with increased systemic CD34-postive progenitor cell mobilization. Data are expressed as percent change of the baseline values. (adapted with permission: Inoue T, Circulation. 2007;115(5):553-561).
Figure 4. Differentiation of patient-derived CD34-positve stem cells into endothelial-like and smooth muscle-like cells following PCI.
Circulating CD34-positve stem cells were isolated from peripheral blood of patients 7 days after implantation of BMS or SES. Immunohistochemical staining for CD31(A–D). (A) BMS without restenosis, (B) BMS with restenosis, (C) SES, (D) quantification of CD31-positive cell clusters. Patients that received BMS had similar differentiation of CD34-positve stem cells into CD31-positive endothelial-like cells regardless of whether they went on to have restenosis at 6 month angiographic follow-up. Patients that received SES had a significant reduction in the differentiation of CD34-positve stem cells into CD31-positive endothelial-like cells compared to patients that received BMS. Actin staining (E–H). (E) BMS without restenosis, (F) BMS with restenosis, (G) SES, (H) quantification of actin positive cells. Patients that received BMS and went on to have restenosis at 6 month angiographic follow-up had increased numbers of CD34-positve stem cells that differentiated into actin-positive smooth muscle-like cells. Patients that received SES had a significant reduction in the differentiation of CD34-positve stem cells into actin-positive smooth muscle-like cells compared to patients that received BMS. Arrow denotes representative actin-positive cell. (adapted with permission: Inoue T, Circulation. 2007;115(5):553-561).
Several lines of evidence support the premise that PCI induces local inflammatory signals that mobilize bone marrow-derived CD34-postive stem cells, and that these cells have the ability to differentiate along endothelial or smooth muscle cell lines. In the setting of vascular injury, there appears to be a balance between endothelial-like stem cell responses that favor reendothelialization and smooth muscle-like stem cell responses that promote restenosis (Fig. 2). Furthermore, it appears that compared with BMS, SES implantation attenuates production of local inflammatory signals that promote stem cell mobilization and differentiation into smooth muscle like cells that contribute to neointimal proliferation. In the future, targeted pharmacologic therapies might be able to promote reparative progenitor cell responses and/or inhibit responses that result in excess neointimal proliferation.
Local Vascular Inflammation Signals Stem Cell Recruitment
As described above, inflammatory and hematopoietic cytokines produced locally at sites of vascular inflammation direct mobilization of stem cells from the bone marrow. Vascular-derived molecules involved in stem cell mobilization include GCSF, MMP-9, and stromal cell-derived factor-1.
G-CSF, a potent hematopoietic cytokine produced by endothelium and immune cells, is expressed at sites of vascular injury.73 G-CSF promotes stem cell proliferation and mobilization and it has been hypothesized that following PCI and/or myocardial infarction, G-CSF signals production and homing of reparative stem cells that promote angiogenesis and myocardial repair. Despite its experimental effects on stem mobilization, clinical evaluation of systemic G-CSF therapy following myocardial infarction failed to show benefit in limiting infarct size or in improving left ventricular function.74, 75, 77 It is possible that the non-selective mobilization of both EPCs and SMPCs by G-CSF may limit its therapeutic value for treating restenosis and promoting vascular repair.
Neutrophil-derived MMP-9 is another inflammatory mediator that has a role in stem cell mobilization.76 MMP-9 is secreted locally in response to inflammatory inputs including ligand binding to the leukocyte integrin Mac-1.77 MMP-9 is required for G-CSF and chemokine-induced mobilization of hematopoietic stem cells from the bone marrow,78, 79 and provides a mechanism through which inflamed vascular beds generate systemic signals that promote bone marrow-derived stem cell mobilization and vascular repair.
Stromal cell-derived factor-1 (SDF-1) is a member of the CXC group of chemokines that plays a role in stem cell plasticity and engraftment.80 SDF-1 is expressed by smooth muscle cells at sites of atherosclerosis and vascular inflammation. SDF-1 signals the bone marrow to mobilize Sca-1+ lineage progenitor cells that home to sites of vascular injury where the progenitor cells adopt smooth muscle cell phenotypes. In experimental models, SDF-1 directly regulates neointimal smooth muscle cell content, and inhibition of SDF-1 function decreases neointimal formation.80 Because of its function in stimulating neointimal formation, therapies targeting SDF-1 function could potentially inhibit restenosis following PCI.
Modulating Vascular Injury and Repair: New Frontiers in DES technology
Current generation DES agents prevent restenosis by inhibiting smooth muscle cell proliferation. In developing the next generation of DES agents it may be possible to harness differential drug effects on smooth muscle cell proliferation vs. reendothelialization in a manner that could accelerate repair. Vascular endothelial growth factor has attracted attention as a DES agent that could promote endothelial regeneration and angiogenesis.81 Proof of concept investigations have demonstrated that VEGF-gene–eluting stents accelerate reendothelialization and reduce in-stent neointimal area in animal models.82 Another new strategy to promote vascular repair following PCI involves the use of antibodies83 or peptides84 that bind membrane receptors on circulating endothelial progenitor cells. This strategy promotes capture of these cells in order to accelerate healing.83 CD34 antibody-coated stents have been implanted in human coronary arteries in the multicenter Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth (HEALING) II pilot trial and in later follow up studies.85, 86 The long term safety and efficacy of this pro-healing stent technology awaits further evaluation in randomized trials.
In addition to DES technology itself, adjunctive systemic medications may also influence the stem cell homing and the balance between reendothelialization and neointimal proliferation. Interestingly, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) were recently shown to promote EPC proliferation in vitro87 and increase the number of circulating EPCs in patients with coronary artery disease.88 Despite initial optimism that statins might favorably influence arterial healing following DES implantation, enthusiasm has tempered following release of data that high doses of statins started before PCI and continued thereafter increased EPC mobilization but did not increase circulating CD34+ cells and did not improve the angiographic outcome after implantation of a bioengineered EPC-capture stent89.
Thiazolidinediones which are used to treat diabetes, function by activating peroxisome proliferator activating receptor (PPAR) transcription factors. Several of these agents increase the number of EPCs in both circulating blood and bone marrow, and reduce EPC apoptosis in a phosphatidylinositol 3-kinase dependent manner.90 Although there are several potential vasculoprotective actions of statins and thiazilidinediones, further clinical investigation will be required to determine if these medications will positively influence vascular repair resulting in reduced rates of restenosis and enhanced reendothelialization following PCI.
Summary
PCI results in mechanical injury that induces vascular inflammation. Vascular inflammation in this setting involves complex interactions between endothelial cells, smooth muscle cells, platelets, and inflammatory cells including neutrophils, monocytes, and lymphocytes. Signaling molecules produced by cells at the site of vascular injury stimulate mobilization of bone marrow derived EPCs and SMPCs which are recruited to the sites of vascular inflammation. The cellular and molecular processes that control vascular injury responses direct repair and vascular healing however, dysregulation of these responses can result in adverse arterial remodeling, neointimal proliferation and restenosis. DES effectively reduce neointimal proliferation but also slow the reendothelialization and the healing process. DES also appear to influence the mobilization, homing and differentiation of reparative stem cells. Despite the potential for delayed vascular healing with DES, analysis of clinical trials have demonstrated equivalent safety to BMS in the setting of extended dual antiplatelet use. In the future, improved DES technologies may be able to abolish restenosis and further improve safety by inhibiting maladaptive neointimal proliferation while promoting reendothelialization and repair.
Acknowledgments
Funding Sources
This work was supported in part by grants from the National Heart, Lung, and Blood Institute to D.S. (HL85816, HL57506 MERIT Award, HL73852), and to K.C. (1K08HL086672), a Future Leaders in Cardiovascular Medicine Fellowship Grant to K.C., an award from the Michael Lerner Foundation to K.C., a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, by a grant from Kimura Foundation to T.I and K.N., and by a research grant from the Japan Foundation of Cardiovascular Research to T.I and K.N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no significant conflicts of interest. All authors contributed to the writing and revision of the manuscript.
References
- 1.Goueffic Y, Potter-Perigo S, Chan CK, Johnson PY, Braun K, Evanko SP, Wight TN. Sirolimus blocks the accumulation of hyaluronan (ha) by arterial smooth muscle cells and reduces monocyte adhesion to the ecm. Atherosclerosis. 2007;195:23–30. doi: 10.1016/j.atherosclerosis.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hilker M, Buerke M, Guckenbiehl M, Schwertz H, Buhler J, Moersig W, Hake U, Oelert H. Rapamycin reduces neointima formation during vascular injury. Vasa. 2003;32:10–13. doi: 10.1024/0301-1526.32.1.10. [DOI] [PubMed] [Google Scholar]
- 3.Park J, Ha H, Ahn HJ, Kang SW, Kim YS, Seo JY, Kim MS. Sirolimus inhibits platelet-derived growth factor-induced collagen synthesis in rat vascular smooth muscle cells. Transplant Proc. 2005;37:3459–3462. doi: 10.1016/j.transproceed.2005.09.066. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia V, Bhatia R, Dhindsa M. Drug-eluting stents: New era and new concerns. Postgrad Med J. 2004;80:13–18. doi: 10.1136/pmj.2003.009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa MA, Simon DI. Molecular basis of restenosis and drug-eluting stents. Circulation. 2005;111:2257–2273. doi: 10.1161/01.CIR.0000163587.36485.A7. [DOI] [PubMed] [Google Scholar]
- 6.Lemos PA, van Mieghem CA, Arampatzis CA, Hoye A, Ong AT, McFadden E, Sianos G, van der Giessen WJ, de Feyter PJ, van Domburg RT, Serruys PW. Post-sirolimus-eluting stent restenosis treated with repeat percutaneous intervention: Late angiographic and clinical outcomes. Circulation. 2004;109:2500–2502. doi: 10.1161/01.CIR.0000130173.63105.4E. [DOI] [PubMed] [Google Scholar]
- 7.Garg P, Mauri L. The conundrum of late and very late stent thrombosis following drugeluting stent implantation. Curr Opin Cardiol. 2007;22:565–571. doi: 10.1097/HCO.0b013e3282f02100. [DOI] [PubMed] [Google Scholar]
- 8.McFadden EP, Stabile E, Regar E, Cheneau E, Ong AT, Kinnaird T, Suddath WO, Weissman NJ, Torguson R, Kent KM, Pichard AD, Satler LF, Waksman R, Serruys PW. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364:1519–1521. doi: 10.1016/S0140-6736(04)17275-9. [DOI] [PubMed] [Google Scholar]
- 9.Webster MW, Ormiston JA. Drug-eluting stents and late stent thrombosis. Lancet. 2007;370:914–915. doi: 10.1016/S0140-6736(07)61424-X. [DOI] [PubMed] [Google Scholar]
- 10.Jaffe R, Strauss BH. Late and very late thrombosis of drug-eluting stents: Evolving concepts and perspectives. J Am Coll Cardiol. 2007;50:119–127. doi: 10.1016/j.jacc.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 11.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: Delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 12.Nakazawa G, Finn AV, Joner M, Ladich E, Kutys R, Mont EK, Gold HK, Burke AP, Kolodgie FD, Virmani R. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: An autopsy study. Circulation. 2008;118:1138–1145. doi: 10.1161/CIRCULATIONAHA.107.762047. [DOI] [PubMed] [Google Scholar]
- 13.Kotani J, Awata M, Nanto S, Uematsu M, Oshima F, Minamiguchi H, Mintz GS, Nagata S. Incomplete neointimal coverage of sirolimus-eluting stents: Angioscopic findings. J Am Coll Cardiol. 2006;47:2108–2111. doi: 10.1016/j.jacc.2005.11.092. [DOI] [PubMed] [Google Scholar]
- 14.Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis: Strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–2441. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- 15.Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, Virmani R. Vascular responses to drug eluting stents: Importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27:1500–1510. doi: 10.1161/ATVBAHA.107.144220. [DOI] [PubMed] [Google Scholar]
- 16.Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr Opin Hematol. 2007;14:55–61. doi: 10.1097/00062752-200701000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Finn AV, Kolodgie FD, Harnek J, Guerrero LJ, Acampado E, Tefera K, Skorija K, Weber DK, Gold HK, Virmani R. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation. 2005;112:270–278. doi: 10.1161/CIRCULATIONAHA.104.508937. [DOI] [PubMed] [Google Scholar]
- 18.Nebeker JR, Virmani R, Bennett CL, Hoffman JM, Samore MH, Alvarez J, Davidson CJ, McKoy JM, Raisch DW, Whisenant BK, Yarnold PR, Belknap SM, West DP, Gage JE, Morse RE, Gligoric G, Davidson L, Feldman MD. Hypersensitivity cases associated with drug-eluting coronary stents: A review of available cases from the research on adverse drug events and reports (radar) project. J Am Coll Cardiol. 2006;47:175–181. doi: 10.1016/j.jacc.2005.07.071. [DOI] [PubMed] [Google Scholar]
- 19.Virmani R, Guagliumi G, Farb A, Musumeci G, Grieco N, Motta T, Mihalcsik L, Tespili M, Valsecchi O, Kolodgie FD. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: Should we be cautious? Circulation. 2004;109:701–705. doi: 10.1161/01.CIR.0000116202.41966.D4. [DOI] [PubMed] [Google Scholar]
- 20.Pallero MA, Talbert Roden M, Chen YF, Anderson PG, Lemons J, Brott BC, Murphy-Ullrich JE. Stainless steel ions stimulate increased thrombospondin-1-dependent tgf-beta activation by vascular smooth muscle cells: Implications for in-stent restenosis. J Vasc Res. 2010;47:309–322. doi: 10.1159/000265565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byrne RA, Joner M, Kastrati A. Polymer coatings and delayed arterial healing following drug-eluting stent implantation. Minerva Cardioangiol. 2009;57:567–584. [PubMed] [Google Scholar]
- 22.Wilson GJ, Nakazawa G, Schwartz RS, Huibregtse B, Poff B, Herbst TJ, Baim DS, Virmani R. Comparison of inflammatory response after implantation of sirolimus- and paclitaxel-eluting stents in porcine coronary arteries. Circulation. 2009;120:141–149. 141–142. doi: 10.1161/CIRCULATIONAHA.107.730010. [DOI] [PubMed] [Google Scholar]
- 23.Fuke S, Maekawa K, Kawamoto K, Saito H, Sato T, Hioka T, Ohe T. Impaired endothelial vasomotor function after sirolimus-eluting stent implantation. Circ J. 2007;71:220–225. doi: 10.1253/circj.71.220. [DOI] [PubMed] [Google Scholar]
- 24.Shin DI, Kim PJ, Seung KB, Kim DB, Kim MJ, Chang K, Lim SM, Jeon DS, Chung WS, Baek SH, Lee MY. Drug-eluting stent implantation could be associated with long-term coronary endothelial dysfunction. Int Heart J. 2007;48:553–567. doi: 10.1536/ihj.48.553. [DOI] [PubMed] [Google Scholar]
- 25.Maekawa K, Kawamoto K, Fuke S, Yoshioka R, Saito H, Sato T, Hioka T. Images in cardiovascular medicine. Severe endothelial dysfunction after sirolimus-eluting stent implantation. Circulation. 2006;113:e850–e851. doi: 10.1161/CIRCULATIONAHA.105.597948. [DOI] [PubMed] [Google Scholar]
- 26.Togni M, Windecker S, Cocchia R, Wenaweser P, Cook S, Billinger M, Meier B, Hess OM. Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J Am Coll Cardiol. 2005;46:231–236. doi: 10.1016/j.jacc.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 27.Hofma SH, van der Giessen WJ, van Dalen BM, Lemos PA, McFadden EP, Sianos G, Ligthart JM, van Essen D, de Feyter PJ, Serruys PW. Indication of long-term endothelial dysfunction after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:166–170. doi: 10.1093/eurheartj/ehi571. [DOI] [PubMed] [Google Scholar]
- 28.Tesfamariam B. Drug release kinetics from stent device-based delivery systems. J Cardiovasc Pharmacol. 2008;51:118–125. doi: 10.1097/FJC.0b013e318158540f. [DOI] [PubMed] [Google Scholar]
- 29.Kamath KR, Barry JJ, Miller KM. The taxus drug-eluting stent: A new paradigm in controlled drug delivery. Adv Drug Deliv Rev. 2006;58:412–436. doi: 10.1016/j.addr.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Waugh J, Wagstaff AJ. The paclitaxel (taxus)-eluting stent: A review of its use in the management of de novo coronary artery lesions. Am J Cardiovasc Drugs. 2004;4:257–268. doi: 10.2165/00129784-200404040-00006. [DOI] [PubMed] [Google Scholar]
- 31.McKeage K, Murdoch D, Goa KL. The sirolimus-eluting stent: A review of its use in the treatment of coronary artery disease. Am J Cardiovasc Drugs. 2003;3:211–230. doi: 10.2165/00129784-200303030-00007. [DOI] [PubMed] [Google Scholar]
- 32.Raman VK, Edelman ER. Coated stents: Local pharmacology. Semin Interv Cardiol. 1998;3:133–137. [PubMed] [Google Scholar]
- 33.Shin DI, Seung KB, Kim PJ, Chang K, Choi JK, Jeon DS, Kim MJ, Lee MY, Chung WS. Long-term coronary endothelial function after zotarolimus-eluting stent implantation A 9 month comparison between zotarolimus-eluting and sirolimus-eluting stents. Int Heart J. 2008;49:639–652. doi: 10.1536/ihj.49.639. [DOI] [PubMed] [Google Scholar]
- 34.Kim JW, Suh SY, Choi CU, Na JO, Kim EJ, Rha SW, Park CG, Seo HS, Oh DJ. Six-month comparison of coronary endothelial dysfunction associated with sirolimus-eluting stent versus paclitaxel-eluting stent. JACC Cardiovasc Interv. 2008;1:65–71. doi: 10.1016/j.jcin.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Hamilos MI, Ostojic M, Beleslin B, Sagic D, Mangovski L, Stojkovic S, Nedeljkovic M, Orlic D, Milosavljevic B, Topic D, Karanovic N, Wijns W. Differential effects of drugeluting stents on local endothelium-dependent coronary vasomotion. J Am Coll Cardiol. 2008;51:2123–2129. doi: 10.1016/j.jacc.2007.12.059. [DOI] [PubMed] [Google Scholar]
- 36.Thanyasiri P, Kathir K, Celermajer DS, Adams MR. Endothelial dysfunction and restenosis following percutaneous coronary intervention. Int J Cardiol. 2007;119:362–367. doi: 10.1016/j.ijcard.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Bavry AA, Kumbhani DJ, Helton TJ, Bhatt DL. Risk of thrombosis with the use of sirolimus-eluting stents for percutaneous coronary intervention (from registry and clinical trial data) Am J Cardiol. 2005;95:1469–1472. doi: 10.1016/j.amjcard.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 38.Bavry AA, Kumbhani DJ, Helton TJ, Bhatt DL. What is the risk of stent thrombosis associated with the use of paclitaxel-eluting stents for percutaneous coronary intervention?: A meta-analysis. J Am Coll Cardiol. 2005;45:941–946. doi: 10.1016/j.jacc.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 39.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 40.Moreno R, Fernandez C, Hernandez R, Alfonso F, Angiolillo DJ, Sabate M, Escaned J, Banuelos C, Fernandez-Ortiz A, Macaya C. Drug-eluting stent thrombosis: Results from a pooled analysis including 10 randomized studies. J Am Coll Cardiol. 2005;45:954–959. doi: 10.1016/j.jacc.2004.11.065. [DOI] [PubMed] [Google Scholar]
- 41.Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schomig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS, Goy JJ, Park SJ, Sabate M, Suttorp MJ, Kelbaek H, Spaulding C, Menichelli M, Vermeersch P, Dirksen MT, Cervinka P, Petronio AS, Nordmann AJ, Diem P, Meier B, Zwahlen M, Reichenbach S, Trelle S, Windecker S, Juni P. Outcomes associated with drug-eluting and bare-metal stents: A collaborative network meta-analysis. Lancet. 2007;370:937–948. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 42.Ong AT, Hoye A, Aoki J, van Mieghem CA, Rodriguez Granillo GA, Sonnenschein K, Regar E, McFadden EP, Sianos G, van der Giessen WJ, de Jaegere PP, de Feyter P, van Domburg RT, Serruys PW. Thirty-day incidence and six-month clinical outcome of thrombotic stent occlusion after bare-metal, sirolimus, or paclitaxel stent implantation. J Am Coll Cardiol. 2005;45:947–953. doi: 10.1016/j.jacc.2004.09.079. [DOI] [PubMed] [Google Scholar]
- 43.Ong AT, Serruys PW, Aoki J, Hoye A, van Mieghem CA, Rodriguez-Granillo GA, Valgimigli M, Sonnenschein K, Regar E, van der Ent M, de Jaegere PP, McFadden EP, Sianos G, van der Giessen WJ, de Feyter PJ, van Domburg RT. The unrestricted use of paclitaxel- versus sirolimus-eluting stents for coronary artery disease in an unselected population: One-year results of the taxus-stent evaluated at rotterdam cardiology hospital (t-search) registry. J Am Coll Cardiol. 2005;45:1135–1141. doi: 10.1016/j.jacc.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 44.Urban P, Gershlick AH, Guagliumi G, Guyon P, Lotan C, Schofer J, Seth A, Sousa JE, Wijns W, Berge C, Deme M, Stoll HP. Safety of coronary sirolimus-eluting stents in daily clinical practice: One-year follow-up of the e-cypher registry. Circulation. 2006;113:1434–1441. doi: 10.1161/CIRCULATIONAHA.104.532242. [DOI] [PubMed] [Google Scholar]
- 45.Williams DO, Abbott JD, Kip KE. Outcomes of 6906 patients undergoing percutaneous coronary intervention in the era of drug-eluting stents: Report of the descover registry. Circulation. 2006;114:2154–2162. doi: 10.1161/CIRCULATIONAHA.106.667915. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka H, Sukhova GK, Swanson SJ, Clinton SK, Ganz P, Cybulsky MI, Libby P. Sustained activation of vascular cells and leukocytes in the rabbit aorta after balloon injury. Circulation. 1993;88:1788–1803. doi: 10.1161/01.cir.88.4.1788. [DOI] [PubMed] [Google Scholar]
- 47.Welt FG, Rogers C. Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol. 2002;22:1769–1776. doi: 10.1161/01.atv.0000037100.44766.5b. [DOI] [PubMed] [Google Scholar]
- 48.Welt FG, Edelman ER, Simon DI, Rogers C. Neutrophil, not macrophage, infiltration precedes neointimal thickening in balloon-injured arteries. Arterioscler Thromb Vasc Biol. 2000;20:2553–2558. doi: 10.1161/01.atv.20.12.2553. [DOI] [PubMed] [Google Scholar]
- 49.Evangelista V, Manarini S, Rontondo S, Martelli N, Polischuk R, McGregor JL, de Gaetano G, Cerletti C. Platelet/polymorphonuclear leukocyte interaction in dynamic conditions: Evidence of adhesion cascade and cross talk between p-selectin and the b2 integrin cd11b/cd18. Blood. 1996;88:4183–4194. [PubMed] [Google Scholar]
- 50.Hamburger SA, McEver RP. Gmp-140 mediates adhesion of stimulated platelets to neutrophils. Blood. 1990;75:550–554. [PubMed] [Google Scholar]
- 51.Larsen E, Celi A, Gilbert GE, Furie BC, Erban JK, Bonfanti R, Wagner DD, Furie B. Padgem protein: A receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989;59:305–312. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- 52.McEver RP, Cummings RD. Role of psgl-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100:S97–S103. [PubMed] [Google Scholar]
- 53.Simon DI, Chen Z, Xu H, Li CQ, Dong J, McIntire LV, Ballantyne CM, Zhang L, Furman MI, Berndt MC, Lopez JA. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin mac-1 (cd11b/cd18) J Exp Med. 2000;192:193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diacovo TG, Roth SJ, Buccola JM, Bainton DF, Springer TA. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of p-selectin and the beta 2-integrin cd11b/cd18. Blood. 1996;88:146–157. [PubMed] [Google Scholar]
- 55.Rogers C, Edelman ER, Simon DI. A mab to the beta2-leukocyte integrin mac-1 (cd11b/cd18) reduces intimal thickening after angioplasty or stent implantation in rabbits. Proc Natl Acad Sci U S A. 1998;95:10134–10139. doi: 10.1073/pnas.95.17.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simon DI, Dhen Z, Seifert P, Edelman ER, Ballantyne CM, Rogers C. Decreased neointimal formation in mac-1(−/−) mice reveals a role for inflammation in vascular repair after angioplasty. J Clin Invest. 2000;105:293–300. doi: 10.1172/JCI7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Inoue T, Sakai Y, Hoshi K, Yaguchi I, Fujito T, Morooka S. Lower expression of neutrophil adhesion molecule indicates less vessel wall injury and might explain lower restenosis rate after cutting balloon angioplasty. Circulation. 1998;97:2511–2518. doi: 10.1161/01.cir.97.25.2511. [DOI] [PubMed] [Google Scholar]
- 58.Inoue T, Sakai Y, Morooka S, Hayashi T, Takayanagi K, Takabatake Y. Expression of polymorphonuclear leukocyte adhesion molecules and its clinical significance in patients treated with percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1996;28:1127–1133. doi: 10.1016/S0735-1097(96)00308-7. [DOI] [PubMed] [Google Scholar]
- 59.Inoue T, Sohma R, Miyazaki T, Iwasaki Y, Yaguchi I, Morooka S. Comparison of activation process of platelets and neutrophils after coronary stent implantation versus balloon angioplasty for stable angina pectoris [in process citation] Am J Cardiol. 2000;86:1057–1062. doi: 10.1016/s0002-9149(00)01159-0. [DOI] [PubMed] [Google Scholar]
- 60.Inoue T, Uchida T, Yaguchi I, Sakai Y, Takayanagi K, Morooka S. Stent-induced expression and activation of the leukocyte integrin mac-1 is associated with neointimal thickening and restenosis. Circulation. 2003;107:1757–1763. doi: 10.1161/01.CIR.0000060487.15126.56. [DOI] [PubMed] [Google Scholar]
- 61.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–966. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 62.Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, Onitsuka I, Matsui K, Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 64.Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV, Russell SJ, Litzow MR, Edwards WD. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci U S A. 2003;100:4754–4759. doi: 10.1073/pnas.0730743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strauss BH, MacLeod DC, de Feyter PJ, van Suylen RJ, Uitterlinden AG, de Leeuw WJ, van Trommelen GJ, Serruys PW. Analysis of vntr loci amplified by the polymerase chain reaction for investigating the origin of intimal smooth muscle cells in a coronary artery lesion developing after heart transplantation in man. Am Heart J. 1993;125:1176–1180. doi: 10.1016/0002-8703(93)90137-x. [DOI] [PubMed] [Google Scholar]
- 66.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 67.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 68.Schober A, Hoffmann R, Opree N, Knarren S, Iofina E, Hutschenreuter G, Hanrath P, Weber C. Peripheral cd34+ cells and the risk of in-stent restenosis in patients with coronary heart disease. Am J Cardiol. 2005;96:1116–1122. doi: 10.1016/j.amjcard.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 69.Inoue T, Sata M, Hikichi Y, Sohma R, Fukuda D, Uchida T, Shimizu M, Komoda H, Node K. Mobilization of cd34-positive bone marrow-derived cells after coronary stent implantation: Impact on restenosis. Circulation. 2007;115:553–561. doi: 10.1161/CIRCULATIONAHA.106.621714. [DOI] [PubMed] [Google Scholar]
- 70.Elemer GS, Edgington TS. Two independent sets of monoclonal antibodies define neoepitopes linked to soluble ligand binding and leukocyte adhesion functions of activated alpha m beta 2. Circ Res. 1994;75:165–171. doi: 10.1161/01.res.75.1.165. [DOI] [PubMed] [Google Scholar]
- 71.Fukuda D, Sata M, Tanaka K, Nagai R. Potent inhibitory effect of sirolimus on circulating vascular progenitor cells. Circulation. 2005;111:926–931. doi: 10.1161/01.CIR.0000155612.47040.17. [DOI] [PubMed] [Google Scholar]
- 72.Imanishi T, Kobayashi K, Kuki S, Takahashi C, Akasaka T. Sirolimus accelerates senescence of endothelial progenitor cells through telomerase inactivation. Atherosclerosis. 2006;189:288–296. doi: 10.1016/j.atherosclerosis.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 73.Chen X, Kelemen SE, Autieri MV. Expression of granulocyte colony-stimulating factor is induced in injured rat carotid arteries and mediates vascular smooth muscle cell migration. Am J Physiol Cell Physiol. 2005;28/:C81–C88. doi: 10.1152/ajpcell.00322.2004. [DOI] [PubMed] [Google Scholar]
- 74.Zohlnhofer D, Ott I, Mehilli J, Schomig K, Michalk F, Ibrahim T, Meisetschlager G, von Wedel J, Bollwein H, Seyfarth M, Dirschinger J, Schmitt C, Schwaiger M, Kastrati A, Schomig A. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: A randomized controlled trial. Jama. 2006;295:1003–1010. doi: 10.1001/jama.295.9.1003. [DOI] [PubMed] [Google Scholar]
- 75.Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, Kim YJ, Soo Lee D, Sohn DW, Han KS, Oh BH, Lee MM, Park YB. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: The magic cell randomised clinical trial. Lancet. 2004;363:751–756. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 76.Starckx S, Van den Steen PE, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase b and chemokines in leukocytosis and stem cell mobilization. Leuk Lymphoma. 2002;43:233–241. doi: 10.1080/10428190290005982. [DOI] [PubMed] [Google Scholar]
- 77.Wize J, Sopata I, Smerdel A, Maslinski S. Ligation of selectin l and integrin cd11b/cd18 (mac-1) induces release of gelatinase b (mmp-9) from human neutrophils. Inflamm Res. 1998;47:325–327. doi: 10.1007/s000110050336. [DOI] [PubMed] [Google Scholar]
- 78.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires mmp-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pelus LM, Bian H, King AG, Fukuda S. Neutrophil-derived mmp-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of g-csf and the chemokines grobeta/cxcl2 and grobetat/cxcl2delta4. Blood. 2004;103:110–119. doi: 10.1182/blood-2003-04-1115. [DOI] [PubMed] [Google Scholar]
- 80.Schober A, Knarren S, Lietz M, Lin EA, Weber C. Crucial role of stromal cell-derived factor-1alpha in neointima formation after vascular injury in apolipoprotein edeficient mice. Circulation. 2003;108:2491–2497. doi: 10.1161/01.CIR.0000099508.76665.9A. [DOI] [PubMed] [Google Scholar]
- 81.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 82.Walter DH, Cejna M, Diaz-Sandoval L, Willis S, Kirkwood L, Stratford PW, Tietz AB, Kirchmair R, Silver M, Curry C, Wecker A, Yoon YS, Heidenreich R, Hanley A, Kearney M, Tio FO, Kuenzler P, Isner JM, Losordo DW. Local gene transfer of phvegf-2 plasmid by gene-eluting stents: An alternative strategy for inhibition of restenosis. Circulation. 2004;110:36–45. doi: 10.1161/01.CIR.0000133324.38115.0A. [DOI] [PubMed] [Google Scholar]
- 83.Kutryk MJKMA. In vivo endothelial progenitor cell seeding for the accelerated endothelialization of endovascular divices. Am J Cardiol. 2003;92:94L. [Google Scholar]
- 84.Blindt R, Vogt F, Astafieva I, Fach C, Hristov M, Krott N, Seitz B, Kapurniotu A, Kwok C, Dewor M, Bosserhoff AK, Bernhagen J, Hanrath P, Hoffmann R, Weber C. A novel drug-eluting stent coated with an integrin-binding cyclic arg-gly-asp peptide inhibits neointimal hyperplasia by recruiting endothelial progenitor cells. J Am Coll Cardiol. 2006;47:1786–1795. doi: 10.1016/j.jacc.2005.11.081. [DOI] [PubMed] [Google Scholar]
- 85.Aoki J, Serruys PW, van Beusekom H, Ong AT, McFadden EP, Sianos G, van der Giessen WJ, Regar E, de Feyter PJ, Davis HR, Rowland S, Kutryk MJ. Endothelial progenitor cell capture by stents coated with antibody against cd34: The healing-fim (healthy endothelial accelerated lining inhibits neointimal growth-first in man) registry. J Am Coll Cardiol. 2005;45:1574–1579. doi: 10.1016/j.jacc.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 86.Miglionico M, Patti G, D'Ambrosio A, Di Sciascio G. Percutaneous coronary intervention utilizing a new endothelial progenitor cells antibody-coated stent: A prospective single-center registry in high-risk patients. Catheter Cardiovasc Interv. 2008;71:600–604. doi: 10.1002/ccd.21437. [DOI] [PubMed] [Google Scholar]
- 87.Assmus B, Urbich C, Aicher A, Hofmann WK, Haendeler J, Rossig L, Spyridopoulos I, Zeiher AM, Dimmeler S. Hmg-coa reductase inhibitors reduce senescence and increase proliferation of endothelial progenitor cells via regulation of cell cycle regulatory genes. Circ Res. 2003;92:1049–1055. doi: 10.1161/01.RES.0000070067.64040.7C. [DOI] [PubMed] [Google Scholar]
- 88.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 89.Duckers H, Onuma Y, Benit E, de Winter R, Wijns W, Grisold M, Verheye S, Silber S, Teiger E, Hill J, Serruys PW. Abstract 5998: Final results of the healing 2b trial to evaluate a bioengineered cd34 antibody coated stent (genoustmstent) designed to promote vascular healing by capture of circulating endothelial progenitor cells in cad patients. Circulation. 2008;118:S_1042–S_1043. [Google Scholar]
- 90.Gensch C, Clever YP, Werner C, Hanhoun M, Bohm M, Laufs U. The ppar-gamma agonist pioglitazone increases neoangiogenesis and prevents apoptosis of endothelial progenitor cells. Atherosclerosis. 2007;192:67–74. doi: 10.1016/j.atherosclerosis.2006.06.026. [DOI] [PubMed] [Google Scholar]