Abstract
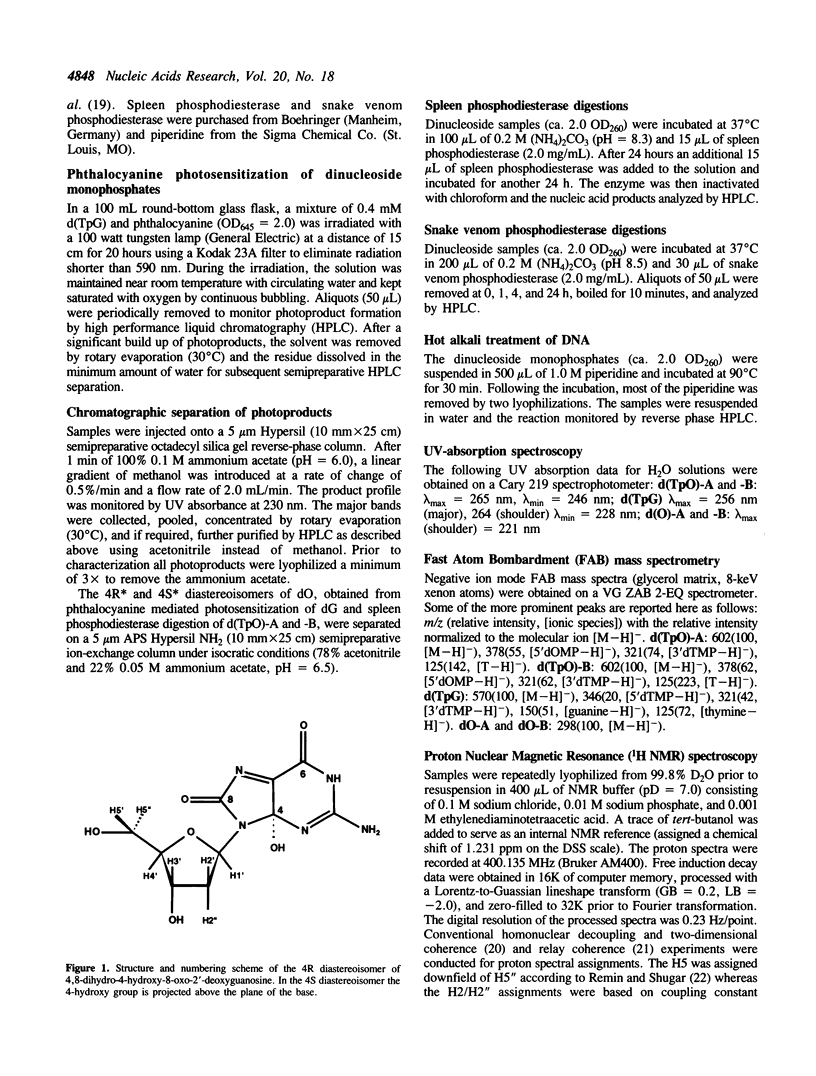
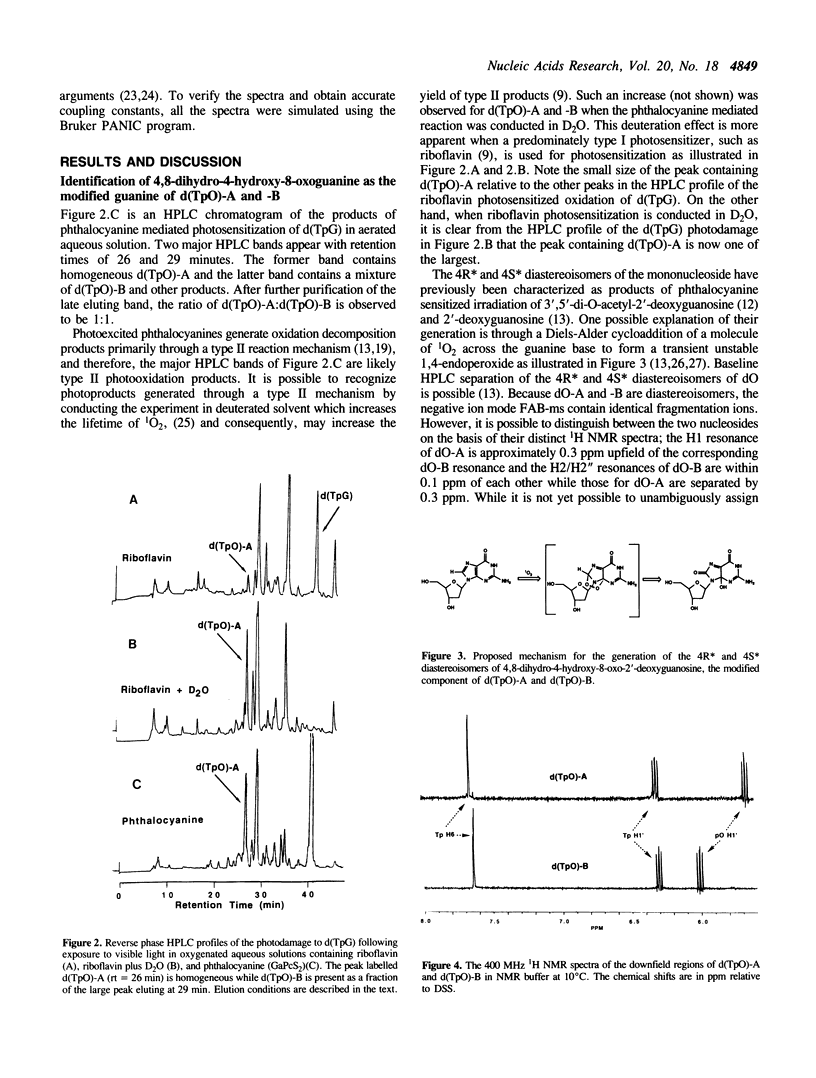
Phthalocyanine mediated photosensitization of 2'-deoxyguanosine (dG) in oxygen saturated aqueous solution has previously been shown to result in the addition of molecular oxygen to the guanine base generating the 4R* and 4S* diastereoisomers of 4,8-dihydro-4-hydroxy-8-oxo-2'-deoxyguanosine (dO) (the asterisk denotes unambiguous assignment of the 4R and 4S diastereoisomers). The data presented here show that the same guanine modified bases are generated in a 1:1 ratio when thymidylyl-(3',5')-2'-deoxyguanosine (d(TpG)) is similarly photo-oxidized. These modified dinucleoside monophosphates, labelled d(TpO)-A and -B, have been isolated by high performance liquid chromatography and characterized by proton NMR spectrometry, fast atom bombardment mass spectrometry, and enzymatic digestions. Photosensitization in D2O instead of H2O leads to an increase in the rate of d(TpO) formation that is consistent with a type II (singlet oxygen) reaction mechanism. Three interesting properties of these modified dinucleoside monophosphates are: i) the rate of their digestion with spleen phosphodiesterase is greatly reduced relative to d(TpG), ii) they are not digested by snake venom phosphodiesterase, and iii) they are stable to 1.0 M piperidine at 90 degrees C for 30 min. The latter observation indicates that 4,8-dihydro-4-hydroxy-8-oxoguanine is not a base lesion responsible for the strand breaks observed following hot piperidine treatment of DNA exposed to type II photosensitizers or chemically generated singlet oxygen.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blazek E. R., Peak J. G., Peak M. J. Singlet oxygen induces frank strand breaks as well as alkali- and piperidine-labile sites in supercoiled plasmid DNA. Photochem Photobiol. 1989 May;49(5):607–613. doi: 10.1111/j.1751-1097.1989.tb08431.x. [DOI] [PubMed] [Google Scholar]
- Cadet J., Berger M., Decarroz C., Wagner J. R., van Lier J. E., Ginot Y. M., Vigny P. Photosensitized reactions of nucleic acids. Biochimie. 1986 Jun;68(6):813–834. doi: 10.1016/s0300-9084(86)80097-9. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Davies D. B., Danyluk S. S. Nuclear magnetic resonance studies of 5'-ribo- and deoxyribonucleotide structures in solution. Biochemistry. 1974 Oct 8;13(21):4417–4434. doi: 10.1021/bi00718a027. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J. Photosensitizers: therapy and detection of malignant tumors. Photochem Photobiol. 1987 Jun;45(6):879–889. doi: 10.1111/j.1751-1097.1987.tb07898.x. [DOI] [PubMed] [Google Scholar]
- Floyd R. A. Oxidative damage to behavior during aging. Science. 1991 Dec 13;254(5038):1597–1597. doi: 10.1126/science.1684251. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., West M. S., Eneff K. L., Schneider J. E. Methylene blue plus light mediates 8-hydroxyguanine formation in DNA. Arch Biochem Biophys. 1989 Aug 15;273(1):106–111. doi: 10.1016/0003-9861(89)90167-7. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Brown D. M. Base-specific reactions useful for DNA sequencing: methylene blue--sensitized photooxidation of guanine and osmium tetraoxide modification of thymine. Nucleic Acids Res. 1978 Feb;5(2):615–622. doi: 10.1093/nar/5.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner W. D., Rodriguez L. O., Hecht S. M., Haseltine W. A. gamma Ray induced deoxyribonucleic acid strand breaks. 3' Glycolate termini. J Biol Chem. 1983 Jan 25;258(2):711–713. [PubMed] [Google Scholar]
- Langlois R., Ali H., Brasseur N., Wagner J. R., van Lier J. E. Biological activities of phythalocyanines--IV. Type II sensitized photooxidation of L-tryptophan and cholesterol by sulfonated metallo phthalocyanines. Photochem Photobiol. 1986 Aug 2;44(2):117–123. doi: 10.1111/j.1751-1097.1986.tb03574.x. [DOI] [PubMed] [Google Scholar]
- Liuzzi M., Weinfeld M., Paterson M. C. Enzymatic analysis of isomeric trithymidylates containing ultraviolet light-induced cyclobutane pyrimidine dimers. I. Nuclease P1-mediated hydrolysis of the intradimer phosphodiester linkage. J Biol Chem. 1989 Apr 15;264(11):6355–6363. [PubMed] [Google Scholar]
- Marx J. L. Oxygen free radicals linked to many diseases. Science. 1987 Jan 30;235(4788):529–531. doi: 10.1126/science.3810154. [DOI] [PubMed] [Google Scholar]
- Müller E., Boiteux S., Cunningham R. P., Epe B. Enzymatic recognition of DNA modifications induced by singlet oxygen and photosensitizers. Nucleic Acids Res. 1990 Oct 25;18(20):5969–5973. doi: 10.1093/nar/18.20.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiarowski W., Uznanski B. Substrate specificity and stereospecificity of calf spleen phosphodiesterase towards deoxyribonucleosidyl 3'-(4-nitrophenyl phosphates) and phosphorothioates. Eur J Biochem. 1985 Nov 15;153(1):145–153. doi: 10.1111/j.1432-1033.1985.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Ogilvie K. K., Hruska F. H. Affect of spleen and snake venom phosphodiesterases on nucleotides containing nucleosides in the syn conformation. Biochem Biophys Res Commun. 1976 Jan 26;68(2):375–378. doi: 10.1016/0006-291x(76)91155-4. [DOI] [PubMed] [Google Scholar]
- OhUigin C., McConnell D. J., Kelly J. M., van der Putten W. J. Methylene blue photosensitised strand cleavage of DNA: effects of dye binding and oxygen. Nucleic Acids Res. 1987 Sep 25;15(18):7411–7427. doi: 10.1093/nar/15.18.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J. Biological consequences associated with DNA oxidation mediated by singlet oxygen. J Photochem Photobiol B. 1991 Dec;11(3-4):241–260. doi: 10.1016/1011-1344(91)80030-l. [DOI] [PubMed] [Google Scholar]
- Piette J., Moore P. D. DNA synthesis on phi X174 template damaged by proflavine and light treatment. Photochem Photobiol. 1982 May;35(5):705–708. doi: 10.1111/j.1751-1097.1982.tb02633.x. [DOI] [PubMed] [Google Scholar]
- Remin M., Shugar D. Conformation of the exocyclic 5'-CH 2 OH in nucleosides and nucleotides in aqueous solution from specific assignments of the H 5' and H 5'' signals in the NMR spectra. Biochem Biophys Res Commun. 1972 Aug 7;48(3):636–642. doi: 10.1016/0006-291x(72)90395-6. [DOI] [PubMed] [Google Scholar]
- Rodgers M. A. Time resolved studies of 1.27 micron luminescence from singlet oxygen generated in homogeneous and microheterogeneous fluids. Photochem Photobiol. 1983 Jan;37(1):99–103. doi: 10.1111/j.1751-1097.1983.tb04440.x. [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Price S., Maidt L., Gutteridge J. M., Floyd R. A. Methylene blue plus light mediates 8-hydroxy 2'-deoxyguanosine formation in DNA preferentially over strand breakage. Nucleic Acids Res. 1990 Feb 11;18(3):631–635. doi: 10.1093/nar/18.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinfeld M., Liuzzi M., Paterson M. C. Selective hydrolysis by exo- and endonucleases of phosphodiester bonds adjacent to an apurinic site. Nucleic Acids Res. 1989 May 25;17(10):3735–3745. doi: 10.1093/nar/17.10.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinfeld M., Soderlind K. J. 32P-postlabeling detection of radiation-induced DNA damage: identification and estimation of thymine glycols and phosphoglycolate termini. Biochemistry. 1991 Jan 29;30(4):1091–1097. doi: 10.1021/bi00218a031. [DOI] [PubMed] [Google Scholar]


