Abstract
We present a history of the concepts and developments that have led us to focus on the resting state as an object of study. We then discuss resting state research performed in our laboratory since 2005 with an emphasis on papers of particular interest.
Introduction
It has been appreciated for at least two millennia that the brains of humans exhibit ongoing activity regardless of the presence or absence of any observable behaviors. As noted by Seneca in ~60 A.D., “The fact that the body is lying down is no reason for supposing that the mind is at peace. Rest is… far from restful” (Seneca, 1969). However, scientific investigation of the brain’s “resting state” presents conceptual as well as methodological challenges whereas studying the brain’s responses to controlled stimuli, that is, the experimental paradigm that has dominated systems neuroscience throughout the latter half of the 20th century, is comparatively straightforward. An expanded view of the tension between task-based and resting-state modes of investigation has been outlined in a recent review (Raichle, 2009). Below we present a brief account of scientific milestones that have shaped our view of the resting state. We then briefly review the history of resting state fMRI studies performed our laboratory.
“Resting state” defined
Given the apparently contradictory characterization of “rest” (see above) it is prudent to begin with a definition. In the context of experimentation, “rest” is an operational definition referring to a constant condition without imposed stimuli or other behaviorally salient events. The eyes may be closed or open, with or without visual fixation. The operational definition of “rest” may be generalized to encompass engagement in a controlled task as long as all imposed temporal structure is randomly phased with respect to the fMRI acquisition e.g., (Fransson, 2006). The objective of resting state experiments is to capture the statistical properties of endogenously generated (synonyms: spontaneous; intrinsic) neural activity. In contrast, the objective of event-related studies is to measure evoked or induced responses.
During quiet wakefulness, humans experience stimulus independent thoughts (Antrobus, 1968; Christoff et al., 2009; Mason et al., 2007), the cognitive content of which is not easily related to objectively measurable fMRI responses (for recent examinations of this issue see (Gruberger et al., 2011; Hasenkamp et al., 2011; Stawarczyk et al., 2011)). Hence, the resting state is uncontrolled according to the usual conventions that apply in cognitive neuroimaging. This circumstance has provoked strongly worded objections to resting state studies altogether (Morcom and Fletcher, 2007). But resting state studies are motivated in large part by questions of neurobiology, not cognitive theory (see below).
Moreover, although resting state fMRI as a technique does not support conventional measurement of event related responses, resting state studies during the past few years have shown that the statistical properties of endogenous activity are modulated by the state of the eyes (Bianciardi et al., 2009; McAvoy et al., 2008), by concurrent performance of semantic (Fransson, 2006) and motor tasks (Sun et al., 2007) and by concurrent sensory stimulation (Hampson et al., 2004). Similar modulations have also been observed immediately following task performance (Albert et al., 2009; Stevens et al., 2010; Waites et al., 2005). These effects provide clues regarding the physiological significance of resting state activity.
Before proceeding, we note that unconstrained cognition alone does not account for the greatest part of intrinsic activity although it undoubtedly contributes a small increment. The principal reasons for this assertion may be stated as follows: (i) Imposed tasks evoke responses that are modest in magnitude in comparison to intrinsic activity (Raichle and Mintun, 2006). This is why averaging is required to extract meaningful responses from the ongoing background. There is no reason to suppose that unconstrained thoughts are more energy demanding than constrained ones. (ii) Resting state activity persists, albeit in modified form, during slow wave sleep (Samann et al., 2011) and even during surgical anesthesia (Vincent et al., 2007), states in which cognition generally is assumed to be absent or at least very attenuated. Hence, something other than unconstrained cognition must be posited to account for most intrinsic activity (see (Raichle, 2009, 2010) for additional discussion of this point).1
Some history
The first scientist to explicitly address the significance of patterned nervous activity may have been Thomas Henry Huxley, in his book on the crayfish (Huxley. T, 1884). Huxley wrote, “If the nervous system were a mere bundle of nerve fibers extending between sensory organs and muscles, every muscular contraction would require the stimulation of that special point of the surface on which the appropriate sensory nerve ended. The contraction of several muscles at the same time, that is, the combination of movements towards one end, would be possible only if the appropriate nerves would be stimulated in the proper order, and every movement would be the direct result of external changes. The organism would be like a piano, which may be made to give out the most complicated harmonies, but is dependent on the depression of a separate key for every note that is sounded. But it is obvious that the crayfish needs no such separate impulses for the performance of highly complicated actions. … To carry the analogy of the musical instrument further, striking a single key gives rise, not to a single note, but to a more or less elaborate tune; as if the hammer struck not a single string, but pressed down the stop of a musical box.” Huxley emphasized the extent to which the crayfish exhibits highly organized behaviors in response to the simplest stimuli. This principle applies as well to more advanced animals including humans: Almost everything we do is automatic (Hikosaka and Isoda, 2010; Saling and Phillips, 2007)2. But, the crayfish has an extremely limited capacity to modify its behavioral repertoire on the basis of experience, that is, to acquire automatized responses. Hence, the crayfish does not require abundant ongoing neural activity unrelated to emergent behavior.
In 1933, George Bishop (Bishop, 1933) observed cyclic changes of excitability in the visual cortex of the rabbit during stimulation of the optic nerve. Bishop commented, “In general, it is not necessary to infer that each individual impulse traveling up a fiber from the retina arrives as a unit impulse in the cortex, and registers there as such. Rather, we would look upon the cortex as being in constant activity, the physiological activity of the whole network of neurons bearing some direct relationship to the ‘present state’ of the animal’s complex behavior which is sometimes referred to as his ‘mental state’”. Thus, Bishop clearly understood that the brain’s response to stimuli is modulated by fluctuating endogenous activity. More recent, fMRI-based examples of this principle include the demonstration that percepts as well as actions are modulated by ongoing activity (for review see (Sadaghiani et al., 2010)). The same perspective underlies studying the cognitive significance of trial-to-trial variability in evoked responses (Arieli et al., 1996; Debener et al., 2007). These experiments are grounded in the view, articulated in the early part of the 20th century by the physiologist T. Graham Brown (Brown, 1914), that the brain’s operations are mainly intrinsic, involving acquisition and maintenance of information for interpreting, responding to and even predicting environmental demands. In 1996, Lawrence R. Pinneo forcefully argued that ongoing neural activity (“noise”) is essential to brain function (Pinneo, 1966). Pinneo related tonic neural activity to arousal and suggested that this activity is what enables the brain to efficiently respond to environmental events. These ideas antedate by at least 25 years similar notions that today are discussed under the heading of stochastic resonance (McDonnell and Abbott, 2009).
In 1929, Hans Berger reported the first human EEG recordings (Berger, 1929) (for an English translation see (Gloor, 1969)). Berger understood that the EEG was related to mental activity and posed the question, “Is it possible to demonstrate the influence of intellectual work upon the human electroencephalogram, insofar as it has been reported here?” He concluded: “Of course, one should not at first entertain too high hopes with regard to this, because mental work, as I explained elsewhere, adds only a small increment to the cortical work which is going on continuously and not only in the waking state.” Recent studies have shown that, indeed, it is possible to detect volitionally induced changes in the statistical properties of the EEG and, using this technology, provide paralyzed humans with some control over their environment via robotic devices (Birbaumer and Cohen, 2007). However, the computational significance of the EEG as a whole remains largely unknown.
The advent of electronic computers following the second world war enabled the development of averaged evoked response potential (ERP) recording, introduced in 1954 by G. D. Dawson (Dawson, 1954). Heretofore, the EEG had remained much as developed by Berger, that is, polygraphic tracings on paper. Response averaging enabled researchers to extract reproducible waveforms from the ongoing EEG and relate these responses to controlled stimuli. This basic paradigm was carried forward as new techniques for acquiring physiological data became available, e.g., single unit recording, optical imaging and ultimately, fMRI. Until recently, the preponderance of neuroscience research has been conducted by averaging away anything not phase synchronous with events of interest.
Metabolic investigations of the resting state also date to the years following the second world war. In 1948, Seymour Kety and Carl Schmidt introduced the first quantitative measurements of human, whole-brain blood flow and metabolism (Kety and Schmidt, 1948). Kety, Sokoloff and their colleagues noted that, while the human brain is only 2% of the body weight, it accounts for 20% of the body’s energy consumption, ten times the amount expected on a per weight basis. These measurements were, of course, made in the resting state. In 1955, the same group had normal subjects perform a difficult mental arithmetic task while whole-brain blood flow and oxygen consumption were measured. When these measurements were compared with the resting state in the same subjects, no change in either whole-brain blood flow or oxygen consumption (a measure of energy expenditure) was observed (Sokoloff et al., 1955). These observations were extended to regional measurements with PET in the 1980s ((Fox et al., 1988); for reviews see (Raichle, 2010; Raichle and Mintun, 2006)) in which it was shown that locally induced changes in blood flow were accompanied by proportionate increase in glucose consumption but not oxygen utilization. These results made understandable the earlier observation of the absence of an energy cost for mental ‘work’ (Sokoloff et al., 1955). The local changes were too small to have been detected by methods designed to measure the energy consumption of the brain as a whole. As so often happens in science, these results were anticipated in a remarkably prescient analysis by the late Otto Creutzfeldt (Creutzfeldt, 1974).
An important additional perspective on the energy cost of the resting state emerged with the analysis of glutamate cycling by David Attwell and Simon Laughlin (Attwell and Laughlin, 2001) and others (e.g., see (Ames, 2000; Lennie, 2003)). These studies showed that transmitter cycling, a hallmark of brain functional activity, accounts for the overwhelming majority of the brain’s enormous metabolic cost. Studies employing MR spectroscopy and stable isotopes of glucose (Sibson et al., 1997; Sibson et al., 1998) came to the same conclusion. Thoughtfully considered, these data present a challenge to those wishing to study brain function when it is realized that most of the brain’s activity is intrinsic.
Thus, the resting state is not truly a resting state at all. But, having acknowledged this, it has not until recently been entirely clear how to undertake a study of intrinsic activity. It is for this reason that the vast majority of studies of the brain of humans and laboratory animals consisted of provoking a change in brain activity and monitoring the features of that change with microelectrodes, optical techniques and imaging devices such as MRI and PET. In fact, the idea that one would include a resting state in studies of the human brain was considered unacceptable by cognitive neuroscientists because it completely lacked the features of an adequately designed ‘control state’. Despite this concern some did, indeed, include a resting state in their imaging studies and we were, unashamedly, among them! The results of doing so were surprising and most interesting. These appeared as activity decreases from a resting state during the performance of goal-directed tasks.
The first formal characterization of task-induced activity decreases from a resting state derived from a large meta-analysis of published PET data from our group (Shulman et al., 1997). This study generated iconic images of a constellation of brain regions now generally referred to as the default mode network or DMN, after our later paper on a default mode of brain function (Raichle et al., 2001). The unique identity of the DMN was amply confirmed in later meta-analyses by Jeffery Binder and colleagues (Binder et al., 1999) and by Bernard Mazoyer and colleagues (Mazoyer et al., 2001). It is currently widely accepted that a specific set of brain areas decreases activity during performance of a remarkably wide range of tasks as compared to a control condition such as visual fixation (see also article by Jeff Binder in this volume).
The observation of task-induced activity decreases exhibiting a stereotypical topography was surprising because the involved areas had not previously been recognized as a functional system in the same sense as, say, the motor or visual systems. Compelling evidence of a DMN equivalent has since been demonstrated in the monkey (Mantini et al., 2011) and suggestive evidence has been found in the cat (Popa et al., 2009) and mouse (White et al., 2011). However, at the time, many argued, informally, that these activity decreases were simply activations induced by a poorly constrained resting state. To determine whether or not task-induced activity decreases were simply ‘activations’ in the absence of an imposed task we employed quantitative PET measurements of regional brain blood flow and oxygen consumption to define a physiologic baseline. The details of this work have been recounted on several occasions (Gusnard and Raichle, 2001; Raichle et al., 2001; Raichle and Mintun, 2006; Raichle and Snyder, 2007) and will not be repeated here. The PET results showed that activity within the DMN did not represent conventional activations in the resting state but, rather, a new view of the organization of the brain’s intrinsic activity. The operations represented in the DMN were attributed to ‘a default mode of brain function’ because they are most active when subjects are not engaged in goal-directed task performance (Raichle et al., 2001). It is important to note that the DMN is not unique in exhibiting both high levels of baseline metabolism and organized functional activity in the resting state. These are properties of all cortical functional systems and their subcortical connections.
Precursors of resting state fMRI
Spontaneous fluctuations in regional oxygen availability have been actively investigated since the 1950s (for an excellent review see (Hudetz et al., 1992)). In retrospect, it is clear that these fluctuations represent the same physiology as spontaneous fluctuations of the BOLD signal (Ogawa et al., 1990; Pauling and Coryell, 1936)) but the connection to fMRI was not established until much later (see also articles by Keith Thulborn, Peter Fox and Seiji Ogawa in this volume). In fact, these early physiological observations preceded all imaging by almost two decades! Oxygen availability was measured with oxygen sensitive electrodes placed on the cortex of experimental animals and patients undergoing evaluation for epilepsy surgery. Routinely observed in these studies were slow (<0.05 Hz) variations in oxygen availability along with stimulus/task induced increases (Cooper and Crow, 1975). Much speculation surrounded the origin and significance of these oxygen waves (for an interesting discussion see (Purvis, 1978)) concerning how they might relate to ongoing metabolism and whether might reflect opening and closing of capillaries. The metabolism hypothesis received support when it was demonstrated that oxygen waves reflected regional fluctuations in the brain’s redox state that, remarkably, were synchronous in homologous regions of both hemispheres (Vern et al., 1997, 1998). The issue of capillary opening and closing fell by the wayside, in part, because of the demonstration of homologous synchrony. Finally, these spontaneous waves of blood flow and oxygen availability were related to patterns of electrical activity in experimental animals (Golanov et al., 1994), a subject of immense current interest (e.g., (He et al., 2007b; Lu et al., 2007)). Bharat Biswal and his colleague, Antal Hudetz, were well aware of this legacy (Biswal and Hudetz, 1996) when they performed their seminal experiment on resting state fMRI correlations (Biswal et al., 1995) (see also article by Bharat Biswal in this volume).
Resting state fMRI
It had been known since the advent of fMRI that the BOLD signal exhibits slow (nominally, < 0.1 Hz) spontaneous fluctuations although this phenomenon was initially regarded as noise (Purdon and Weisskoff, 1998). However, that these fluctuations are of neural origin was not established until Bharat Biswal and colleagues demonstrated that resting state BOLD signals are temporally correlated within the somatomotor system (Biswal et al., 1995). The neuroscience community, with few exceptions, was remarkably slow to take note of this important result. For an excellent account of this history see (Lowe, 2010). Much emphasis was placed on non-neuronal, that is, artifactual sources of correlated fMRI signals, e.g., head motion (Friston et al., 1996), cardiac pulsations (Glover and Lee, 1995) and variable arterial pCO2 (Wise et al., 2004). To be sure, the importance of recognizing the existence of these artifacts and of developing techniques to minimize their impact (e.g., (Deckers et al., 2006; Jo et al., 2010; Jones et al., 2008)) cannot be overstated. However, during the first few years following the demonstration that resting-state BOLD correlations recapitulate the topography of task-evoked responses, skepticism regarding the biological significance of this effect outweighed acceptance. Most resting state fMRI studies used very short TRs to reduce the impact of physiological pulsations (e.g., (Lowe et al., 1998)), an unavoidable consequence of which was limited coverage. Another characteristic of this early period was the tendency to attribute coherent physiological signals to the vasculature rather than the brain (Mitra et al., 1997).
The significance of resting state BOLD signal correlations was brought forcefully to our attention when Michael Greicius and colleagues generated an image of the DMN using a seed region of interest in the posterior cingulate cortex (Greicius et al., 2003). Similar patterns of resting state coherence have now been documented in most cortical systems in the human brain (Fox and Raichle, 2007; Thomas Yeo et al., 2011) as well as their subcortical connections (Barnes et al., 2010; Buckner et al., 2011; Di Martino et al., 2008) (for reviews see (Cole et al., 2010; Fox and Raichle, 2007; Lowe, 2010; Uddin et al., 2011; Wig et al., 2011)).
Survey of resting state studies at the Washington University School of Medicine Neuroimaging Laboratories (NIL)
Seed-based correlation mapping is but one of several techniques used at the NIL to investigate the resting state. A comprehensive listing of all NIL papers on resting state activity published since 2005 is provided in Supplementary Information. Each paper is assigned to one or more related headings: Neuroscience; Analysis methodology; Mapping; Pathophysiology; Development and aging; State-dependent functional connectivity; Inter-individual differences; Review. These headings are briefly discussed below with an emphasis on selected contributions from the NIL.
Neuroscience
The “neuroscience” heading refers to investigations bearing on the fundamental nature of resting state activity. It is generally assumed, for good reasons, that intrinsic fluctuations of the BOLD signal reflect electrophysiological activity. Logothetis and colleagues have amply demonstrated this principle in the context of task performance (Goense and Logothetis, 2008; Logothetis et al., 2001) (see also article by Nikos Logothetis in this volume). Extension of this result to the resting state is attributable to (Shmuel and Leopold, 2008). Our contribution to this question includes a study showing a correspondence, in individuals, between the topography of BOLD resting state networks and the correlation structure of electrocorticographically (ECoG) recorded resting state slow cortical potentials (He et al., 2008). In related work, we and colleagues at the University of Chieti demonstrated a correspondence between fMRI RSNs and magnetoencephalographically (MEG) recorded intrinsic activity (de Pasquale et al., 2010). Similar results have since been obtained by others (Brookes et al., 2011) (see also article by Helmut Laufs in this volume). But, accepting that spontaneous BOLD signal fluctuations do reflect electrophysiology, this leaves unsolved the fundamental problem of defining what intrinsic activity contributes to brain function.
One set of results that informs this question derives from our demonstration that resting state networks, including the DMN, are present in anesthetized monkeys (Vincent et al., 2007). The implications of this finding extend far beyond establishing monkey-human homologies: (i) The persistence of RSNs in the anesthetized state represents one of the key observations that render untenable the notion that unconstrained cognition accounts for most resting state activity (see also above). Several laboratories have reported similar demonstrations in sedated humans (e.g., (Mhuircheartaigh et al., 2010; Peltier et al., 2005)). Anesthesia as an experimental intervention is closely related to the topic of state-dependent resting state activity, which is covered under a separate heading (see below) (ii) The existence of a DMN in subhuman primates raises interesting questions concerning the cognitive role of this functional system, which, in humans, is thought to include moral reasoning, model building and prospection (Buckner et al., 2008; Schacter et al., 2007) (see also article by Randy Buckner in this volume). The problem is that these labels suggest intellectual capacities found only in humans whereas the DMN is certainly present in monkeys (Mantini et al., 2011) and may be present as well in the mouse (White et al., 2011). If, as appears increasingly likely, all mammals have a DMN, currently prevailing theories concerning the cognitive operations represented in the DMN will have to be revised. (iii) Our report on RSNs in anesthetized monkeys (Vincent et al., 2007) also includes one of the earliest elaborations on the point that the correspondence between functional and anatomical (axonal) connectivity is not one to one (for a recent paper on this topic see (Deco et al., 2011)). Other work bearing on this question includes our demonstration that inter-hemispheric functional connectivity is effectively obliterated in the acute period (days) after complete section of the corpus callosum (Johnston et al., 2008). This result seems to reinforce the notion that functional connectivity is closely tied to anatomic connectivity. But other laboratories have shown that interhemispheric functional connectivity may be present decades after total corpus callosotomy (Uddin et al., 2008), which reinforces the point that RSNs substantially reorganize after injury. We have seen suggestions of such reorganization in the context of pre-surgical RSN mapping in patients with brain tumors (Zhang et al., 2009). This reorganization itself represents another clue regarding the physiological functions of resting state activity.
Analysis methodology
Two complementary analysis strategies, seed based correlation mapping and spatial ICA (sICA; see also article by Christian Beckmann in this volume) (Beckmann et al., 2005), currently dominate the field of resting state fMRI. Seed based correlation mapping provides a natural means of investigating the functional connectivity of a priori targeted regions of interest but requires extensive preprocessing to minimize the influence of non-neuronal sources of variance. In contrast, ICA provides a direct means of separating artifact from neural signals but is less suited to investigating targeted regions of interest.3 Both strategies yield highly reproducible results (Damoiseaux et al., 2006; Shehzad et al., 2009). However, the topography of certain resting state networks (RSNs) derived by the two methods systematically differ. Specifically, certain RSNs typically obtained by sICA (‘C’ and ‘D’ in (Damoiseaux et al., 2006)) are lateralized whereas seed-based correlation mapping generally yields highly symmetric maps (Vincent et al., 2006). It is therefore noteworthy that the topography of the DMN appears more or less the same regardless of which method is used; this invariance speaks to the highly robust character of the DMN.
Soon after the NIL became committed to resting state fMRI, several of us worked on improving technical aspects of seed-based correlation mapping. Global signal regression (GSR), that is, using the timeseries averaged over the whole brain as a nuisance regressor during preprocessing, was implemented as a strategy for reducing the influence of spurious variance, e.g., artifact attributable to fluctuating pCO2 (Birn et al., 2006; Chang and Glover, 2009) (see also article by Rasmus Birn in this volume). GSR was observed to dramatically improve the spatial specificity of correlation maps (Fox et al., 2009; Macey et al., 2004). One of the most striking features of correlation maps obtained using GSR is anti-correlation between the DMN and a set of regions that we named the “task positive network” (TPN) (Fox et al., 2005). Today, we identify the TPN as encompassing both the dorsal attention network (DAN) (Corbetta and Shulman, 2002) and areas associated with cognitive control (Dosenbach et al., 2007; Seeley et al., 2007; Vincent et al., 2008). Negatively correlated DMN-TPN activity in the resting state recapitulates the opposed DMN-TPN dynamics induced by task performance. We are persuaded that this recapitulation reflects a fundamental principle of brain organization. Most recently, the DMN vs. TPN distinction was found to correspond to regional differences in metabolic parameters (Vaishnavi et al., 2010), which, in turn, closely correspond to regional differences in susceptibility to the effects of Alzheimer’s disease (Bero et al., 2011; Vlassenko et al., 2010).
GSR has turned out to be hugely contentious (Anderson et al., 2011; Murphy et al., 2009). This is partly our fault, since we did not, in 2005 (Fox et al., 2005) or even in 2009 (Fox et al., 2009), clearly articulate that the BOLD signal everywhere represents a superposition of spatio-temporal components, some of which are neural and some of which are artifact; this superposition is implicit in ICA. Correlation mapping following GSR is algebraically similar to partial correlation mapping of order one controlling for widely shared variance. GSR removes widely shared variance, thereby uncovering more specific relations. But, a consequence of GSR is that, in all subsequently computed correlation maps, the mean value is algebraically constrained to be approximately zero. (This result is exactly correct in application to beta maps (Fox et al., 2009)). Hence, GSR may be viewed as artificially generating negative correlations. However, absent GSR, all correlations would be positively biased by spatially non-specific variance, some unknown fraction of which undoubtedly is artifact. Recent evidence suggests that the global signal includes a substantial component of neural origin (Scholvinck et al., 2010). Therefore, the advantages of GSR (improved spatial specificity) may come at the cost of removing from the analysis global components of truly neural origin.
It has been our practice, since 2005, to prepare resting state data for correlation mapping using several procedures in addition to conventional fMRI preprocessing (e.g., head motion correction, see (Shulman et al., 2009) for a complete account). Spatial smoothing (6 mm FWHM in each direction) and temporal smoothing (low pass, retaining frequencies below 0.1 Hz) are applied early in the pipeline, that is, before nuisance regression. The logic underlying the 0.1 Hz figure is that all available evidence indicates that BOLD modulations of neuronal origin are essentially absent above this frequency (Hathout et al., 1999), whereas artifacts of various origin, including aliased cardio-pulmonary pulsations, are not. We originally (Fox et al., 2005) excluded from correlation analyses frequencies below 0.009 Hz simply because that was standard practice in GLM-based analyses of task-based fMRI. However, it appears that intrinsic neuronal activity, at least as reflected in the BOLD signal, is “1/f-like” (He et al., 2010), which means that no advantage accrues from ignoring the lowest measureable frequencies; we no longer do so. Following spatio-temporal filtering, artifact reduction is accomplished by regression of multiple waveforms, in addition to the global signal: six rigid body head motion parameters (derived by retrospective motion correction) and the timeseries extracted from one white matter region and one cerebrospinal fluid region (Fox et al., 2005). Several laboratories have developed variants of this strategy avoiding GSR and relying, instead, on nuisance regressors derived from multiple regions of high noise-to-signal ratio (Chai et al., 2012; Jo et al., 2010; Weissenbacher et al., 2009). It is likely that we will employ similar multiple nuisance regressor techniques in the future. Finally, volume censoring (“scrubbing”; (Power et al.; Smyser et al., 2010)) has been standard practice in our laboratory during the past year or so.
NIL contributions to seed-based correlation mapping technique include our demonstration that partial correlation (order 4 but omitting GSR) may be used to map functional connectivity between thalamic voxels and broad parcels of the cerebral cortex (Zhang et al., 2008). This technique maps thalamo-cortical functional connectivity with specificity comparable to that obtained by diffusion tensor tractography (Zhang et al., 2010). A more thorough investigation of the relations between anatomic and functional connectivity will be undertaken in the context of the Human Connectome Project (HCP; <http://humanconnectome.org>; see also article by David van Essen and Kamil Ugurbil in this volume), in which the NIL is a participant. One of the objectives of the HCP is to compute parcellations of the cerebral cortex, at both the individual and group levels, on the basis of resting-state fMRI. Novel methodologies for determining the boundaries between RSNs developed at the NIL (Cohen et al., 2008) will be applied to this end. The NIL also has advanced the use of graph theoretic techniques (for reviews see (Bullmore and Sporns, 2009) and article by Olaf Sporns in this volume) to delineate RSNs (e.g., (Barnes et al., 2010; Power et al.)) and to track the development of RSNs as a function of age (Power et al., 2010). Seed-based correlation (including GSR) is at the front end of all graph-theoretic analyses so far performed at the NIL. Most recently, we have shown that head motion, even that of relatively modest amplitude, gives rise to artifact that biases measured correlations positively at short range but negatively at longer range; and, that this artifact is not eliminated by regression of motion parameters (Power et al.). Frame censoring (“scrubbing”) can be used to reduce the impact of this artifact in the context of correlation mapping.
Mapping
The immediate objective of mapping experiments is to delineate the topography of RSNs. The underlying objective is to study the topographic correspondences between task-evoked responses and RSNs (e.g., (Dosenbach et al., 2007; Fox et al., 2006; Fox et al., 2005; Lewis et al., 2009; Nelson et al., 2010; Sestieri et al., 2011; Shannon et al., 2011; Vincent et al., 2008; Vincent et al., 2006). Such correspondences offer a means of assigning functionality to observed RSNs, and, conversely, of reshaping theoretical models of cognition (see article by Russ Poldrack in this volume). Thus, for example, social cognition and episodic recall are both represented in the same RSN (the DMN). This association suggests a biological link between two seemingly disparate functions. We hasten to add that we do not here claim to understand this link, only that its existence is implied by evidence derived from resting state mapping studies. On a more practical note, we are exploring the utility of resting state RSN mapping to delineate eloquent cortex prior to neurosurgical procedures (Zhang et al., 2009). Several groups, using either sICA (Doucet et al., 2011) or seed-based correlation mapping (including GSR; (Thomas Yeo et al., 2011)) have advanced RSN mapping to the point that accounts for nearly the entire cerebral cortex. The NIL’s contribution to this effort uses both correlation mapping and graph-theoretic techniques (Power et al.).
Pathophysiology
Many neurological and psychiatric entities give rise to resting state functional connectivity changes (Fox and Greicius, 2010; Zhang and Raichle, 2010). Original NIL contributions to this field have concentrated on neglect and motor disability consequent to stroke (Carter et al., 2010; Carter et al., 2011; He et al., 2007b), Alzheimer’s disease (Sheline et al., 2010a; Sheline et al., 2010c), depression (Sheline et al., 2010b), traumatic brain injury (MacDonald et al., 2008), prematurity at birth (Smyser et al., 2010) and Tourette’s syndrome (Church et al., 2009).
Pizoli and colleagues (Pizoli et al., 2011) very recently reported dramatic improvement in functional connectivity architecture following anterior 2/3 corpus callosotomy in a child with epileptic encephalopathy (Lennox-Gastaut syndrome). The most notable feature of this case was the observation of pre-treatment resting state abnormalities attributable to nearly complete suppression of spontaneous BOLD fluctuations, which abnormality remitted after treatment in concert with a dramatic improvement in the patient’s clinical status and EEG. This paper represents, as far as we are aware, the first report of direct evidence linking spontaneous resting state activity with the development and maintenance of normal brain function.
Development and aging
NIL investigators have examined the development of RSNs in newborn (premature and term) infants (Smyser et al., 2010) and children (Fair et al., 2010; Fair et al., 2008; Fair et al., 2009; Fair et al., 2007). One theme that emerges from these studies in aggregate is that long-range functional connectivity between posterior and anterior nodes of the DMN retrogresses in old age (Andrews-Hanna et al., 2007) suggesting reversal of the sequence of development during early childhood.
State-dependent functional connectivity
NIL investigators have studied the effects of eye closure in quietly resting, awake humans (McAvoy et al., 2008), early sleep in normal humans (Larson-Prior et al., 2009) and variable depth anesthesia in monkeys (Vincent et al., 2007). Maurizio Corbetta and colleagues at the University of Chieti, Italy, have shown that intensive perceptual training (several hours per day for several days) alters functional connectivity within task-relevant parts of the brain (Lewis et al., 2009). This result is consistent with a Hebbian view of experience-dependent synaptic enhancement.
Inter-individual differences
Several laboratories have demonstrated a correspondence between individual cognitive performance measures and resting state functional connectivity (e.g., (Song et al., 2008)). At the NIL, Shannon and colleagues studied a cohort of 107 incarcerated juvenile offenders and observed that impulsivity scores correlated with functional connectivity bilaterally in dorsal premotor cortex (PMdr) (Shannon et al., 2011). In greater detail, resting state BOLD fluctuations in PMdr correlated with the DMN in impulsive individuals; conversely, fluctuations in PMdr correlated with functional systems associated with cognitive control in non-impulsive individuals. Moreover, in a separate cohort of normally developing teen-agers and young adults, the impulsivity-associated pattern was found to be a correlate of youth. Thus, impulsivity in juvenile offenders may be a consequence of delayed brain maturation rather than an immutable trait. This study is notable also for introducing a novel analysis technique, the Iterative Data-driven Evolutionary Algorithm (IDEA), for automatically isolating regions of interest (e.g., PMdr) whose functional connectivity maximally correlates with an independent variable (e.g., impulsivity).
Reviews
Several NIL investigators have authored reviews on the topic of BOLD functional connectivity in health and disease (Corbetta, 2010; Fox and Greicius, 2010; Fox and Raichle, 2007; He et al., 2007a; Raichle, 2011; Raichle et al., 2001; Raichle and Mintun, 2006; Raichle and Snyder, 2007; Smyser et al., 2011; Zhang and Raichle, 2010).
Supplementary Material
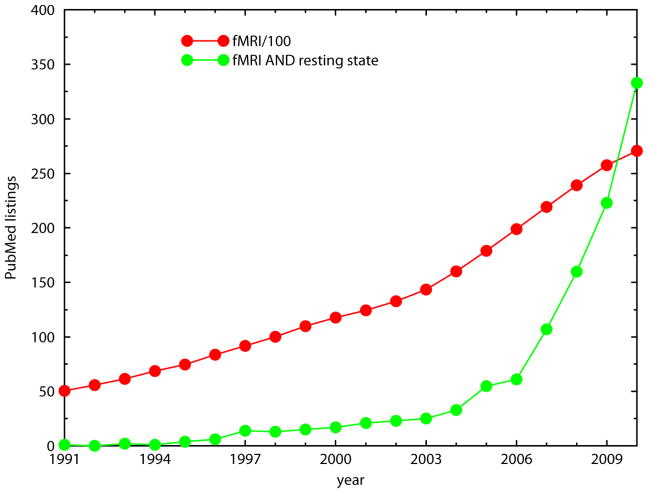
Figure 1.
Graph of papers/year retrieved by a PubMed search using either “fMRI” or “fMRI AND resting state”. N.B.: The “fMRI” scale is compressed by a factor of 100. Resting state fMRI papers are appearing at an exponentially increasing rate. The current doubling time is a little less than 2 years.
Footnotes
It is not self evident that unconstrained cognition does not, in large part, account for ongoing neural activity. In 2006, we submitted a manuscript on the default mode network (DMN) and the dorsal attention network (DAN; Corbetta, M., Shulman, G.L., 2002. Control of goal-directed and stimulus-driven attention in the brain. Nature reviews. Neuroscience 3, 201–215.), showing that the topography of resting state correlation maps closely matches responses obtained by conventional task-based fMRI. Although this work was ultimately published (Vincent, J.L., Snyder, A.Z., Fox, M.D., Shannon, B.J., Andrews, J.R., Raichle, M.E., Buckner, R.L., 2006. Coherent spontaneous activity identifies a hippocampal-parietal memory network. Journal of neurophysiology 96, 3517–3531.), a reviewer of the submitted manuscript wrote, “The absence of behavioral events does not necessarily mean that there is an absence of cognitive events. Perhaps fluctuations in voluntary attention to sensory stimuli (which are always present even in the absence of experimental presentation) and fluctuations in voluntary episodic recollection are driving the resting state correlations.”
Cognitive errors arising out of automatic behaviors often are comical (Reason, J., Mycielska, K., 1982. Absent Minded? Prentice-Hall, Englewood Cliffs, NJ.) but also are the cause of serious man-made disasters (Reason, J., 1990. Human Error. Cambridge University Press.)
Mugs and T-shirts are available to proponents of ICA and seed-based correlation mapping at <http://www.cafepress.com/neurobureau/7193595>. The ICA logo reads, “ICA is for people who don’t know what they want.” The alternative logo is “Seed-based Correlation Analysis. Because you have the balls to hypothesize.” “Balls” refers to the practice of centering spherical regions interest on a priori defined coordinates.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Current biology: CB. 2009;19:1023–1027. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A., 3rd CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000;34:42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Druzgal TJ, Lopez-Larson M, Jeong EK, Desai K, Yurgelun-Todd D. Network anticorrelations, global regression, and phase-shifted soft tissue correction. Human brain mapping. 2011;32:919–934. doi: 10.1002/hbm.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antrobus JS. Informnation theory and stimulus indenpendent thought. British Journal of Psychology. 1968;59:423–430. [Google Scholar]
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Barnes KA, Cohen AL, Power JD, Nelson SM, Dosenbach YB, Miezin FM, Petersen SE, Schlaggar BL. Identifying Basal Ganglia divisions in individuals using resting-state functional connectivity MRI. Frontiers in systems neuroscience. 2010;4:18. doi: 10.3389/fnsys.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. Uber des Elektrenkephalogramm des Menschen. Archiv fur Psychiatrie und Nervenkrankheiten. 1929;87:527–580. [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianciardi M, Fukunaga M, van Gelderen P, Horovitz SG, de Zwart JA, Duyn JH. Modulation of spontaneous fMRI activity in human visual cortex by behavioral state. Neuroimage. 2009;45:160–168. doi: 10.1016/j.neuroimage.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Cohen LG. Brain-computer interfaces: communication and restoration of movement in paralysis. The Journal of physiology. 2007;579:621–636. doi: 10.1113/jphysiol.2006.125633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Bishop G. Cyclic changes in excitability of the optic pathway of the rabbit. American Journal of Physiology. 1933;103:213–224. [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Hudetz AG. Synchronous oscillations in cerebrocortical capillary red blood cell velocity after nitric oxide synthase inhibition. Microvascular research. 1996;52:1–12. doi: 10.1006/mvre.1996.0039. [DOI] [PubMed] [Google Scholar]
- Brookes MJ, Woolrich M, Luckhoo H, Price D, Hale JR, Stephenson MC, Barnes GR, Smith SM, Morris PG. Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16783–16788. doi: 10.1073/pnas.1112685108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TG. On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. Journal of Physiology. 1914;48:18–46. doi: 10.1113/jphysiol.1914.sp001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The Organization of the Human Cerebellum Estimated By Intrinsic Functional Connectivity. Journal of neurophysiology. 2011 doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature reviews. Neuroscience. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DL, Shulman GL, Corbetta M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Annals of neurology. 2010;67:365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AR, Patel KR, Astafiev SV, Snyder AZ, Rengachary J, Strube MJ, Pope A, Shimony JS, Lang CE, Shulman GL, Corbetta M. Upstream Dysfunction of Somatomotor Functional Connectivity After Corticospinal Damage in Stroke. Neurorehabilitation and neural repair. 2011 doi: 10.1177/1545968311411054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH. Relationship between respiration, end-tidal CO2, and BOLD signals in resting-state fMRI. Neuroimage. 2009;47:1381–1393. doi: 10.1016/j.neuroimage.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JA, Fair DA, Dosenbach NU, Cohen AL, Miezin FM, Petersen SE, Schlaggar BL. Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain: a journal of neurology. 2009;132:225–238. doi: 10.1093/brain/awn223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AL, Fair DA, Dosenbach NU, Miezin FM, Dierker D, Van Essen DC, Schlaggar BL, Petersen SE. Defining functional areas in individual human brains using resting functional connectivity MRI. Neuroimage. 2008;41:45–57. doi: 10.1016/j.neuroimage.2008.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Frontiers in systems neuroscience. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R, Crow HJ. Changes in cerebral oxygenation during motor and mental tasks. In: Ingvar D, Lassen N, editors. Brain work: the coupling of function, metabolism and blood flow in the brain. Minksgaard, Copenhagen: 1975. pp. 389–392. [Google Scholar]
- Corbetta M. Functional connectivity and neurological recovery. Developmental psychobiology. 2010 doi: 10.1002/dev.20507. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature reviews. Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt OD. Neurophysiological correlates of different functional states of the brain. In: Ingvar DH, Lassen NA, editors. Brain Work: The coupling of function, metabolism, and blood flow in the brain; Proceedings of the alfred benzon symposium VIII; Munksgaard, Copenhagen: 1974. pp. 21–47. [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson GD. A summation technique for the detection of small evoked potentials. Electroencephalography and Clinical Neurophysiology. 1954;6:65–84. doi: 10.1016/0013-4694(54)90007-3. [DOI] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, Lewis C, Mantini D, Marzetti L, Belardinelli P, Ciancetta L, Pizzella V, Romani GL, Corbetta M. Temporal dynamics of spontaneous MEG activity in brain networks. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6040–6045. doi: 10.1073/pnas.0913863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Engel AK. Towards single-trial analysis in cognitive brain research. Trends in cognitive sciences. 2007;11:502–503. doi: 10.1016/j.tics.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Deckers RH, van Gelderen P, Ries M, Barret O, Duyn JH, Ikonomidou VN, Fukunaga M, Glover GH, de Zwart JA. An adaptive filter for suppression of cardiac and respiratory noise in MRI time series data. Neuroimage. 2006;33:1072–1081. doi: 10.1016/j.neuroimage.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nature reviews. Neuroscience. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cerebral cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet G, Naveau M, Petit L, Delcroix N, Zago L, Crivello F, Jobard G, Tzourio-Mazoyer N, Mazoyer B, Mellet E, Joliot M. Brain activity at rest: a multiscale hierarchical functional organization. Journal of neurophysiology. 2011;105:2753–2763. doi: 10.1152/jn.00895.2010. [DOI] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Mills KL, Dias TG, Blythe MS, Zhang D, Snyder AZ, Raichle ME, Stevens AA, Nigg JT, Nagel BJ. Maturing thalamocortical functional connectivity across development. Frontiers in systems neuroscience. 2010;4:10. doi: 10.3389/fnsys.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain’s default network. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS computational biology. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Frontiers in systems neuroscience. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gloor P. Hans Berger on the Electroencephalogram of Man. The Fourteen Original Reports on the Human Electroencephalogram. Electroencephalography and Clinical Neurophysiology Supplement. 1969;28:1–350. [PubMed] [Google Scholar]
- Glover GH, Lee AT. Motion artifacts in fMRI: comparison of 2DFT with PR and spiral scan methods. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1995;33:624–635. doi: 10.1002/mrm.1910330507. [DOI] [PubMed] [Google Scholar]
- Goense JB, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Current biology: CB. 2008;18:631–640. doi: 10.1016/j.cub.2008.03.054. [DOI] [PubMed] [Google Scholar]
- Golanov EV, Yamamoto S, Reis DJ. Spontaneous waves of cerebral blood flow associated with a pattern of electrocortical activity. Am J Physiol. 1994;266:R204–214. doi: 10.1152/ajpregu.1994.266.1.R204. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruberger M, Ben-Simon E, Levkovitz Y, Zangen A, Hendler T. Towards a neuroscience of mind-wandering. Frontiers in human neuroscience. 2011;5:56. doi: 10.3389/fnhum.2011.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hampson M, Olson IR, Leung HC, Skudlarski P, Gore JC. Changes in functional connectivity of human MT/V5 with visual motion input. Neuroreport. 2004;15:1315–1319. doi: 10.1097/01.wnr.0000129997.95055.15. [DOI] [PubMed] [Google Scholar]
- Hasenkamp W, Wilson-Mendenhall CD, Duncan E, Barsalou LW. Mind wandering and attention during focused meditation: A fine-grained temporal analysis of fluctuating cognitive states. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Hathout GM, Gopi RK, Bandettini P, Gambhir SS. The lag of cerebral hemodynamics with rapidly alternating periodic stimulation: modeling for functional MRI. Magnetic resonance imaging. 1999;17:9–20. doi: 10.1016/s0730-725x(98)00150-7. [DOI] [PubMed] [Google Scholar]
- He BJ, Shulman GL, Snyder AZ, Corbetta M. The role of impaired neuronal communication in neurological disorders. Current opinion in neurology. 2007a;20:655–660. doi: 10.1097/WCO.0b013e3282f1c720. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007b;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16039–16044. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Zempel JM, Snyder AZ, Raichle ME. The temporal structures and functional significance of scale-free brain activity. Neuron. 2010;66:353–369. doi: 10.1016/j.neuron.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Isoda M. Switching from automatic to controlled behavior: cortico-basal ganglia mechanisms. Trends in cognitive sciences. 2010;14:154–161. doi: 10.1016/j.tics.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz AG, Roman RJ, Harder DR. Spontaneous flow oscillations in the cerebral cortex during acute changes in mean arterial pressure. J Cereb Blood Flow Metab. 1992;12:491–499. doi: 10.1038/jcbfm.1992.67. [DOI] [PubMed] [Google Scholar]
- Huxley TH. An Introduction to the Study of Zoology Illustrated by the Crayfish. D. Appleton & Company; New York: 1884. [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage. 2010;52:571–582. doi: 10.1016/j.neuroimage.2010.04.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JM, Vaishnavi SN, Smyth MD, Zhang D, He BJ, Zempel JM, Shimony JS, Snyder AZ, Raichle ME. Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:6453–6458. doi: 10.1523/JNEUROSCI.0573-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TB, Bandettini PA, Birn RM. Integration of motion correction and physiological noise regression in fMRI. Neuroimage. 2008;42:582–590. doi: 10.1016/j.neuroimage.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety S, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. Journal of Clinical Investigation. 1948;27:107–119. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci U S A. 2009;106:4489–4494. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennie P. The cost of cortical computation. Curr Biol. 2003;13:493–497. doi: 10.1016/s0960-9822(03)00135-0. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lowe MJ. A historical perspective on the evolution of resting-state functional connectivity with MRI. Magma. 2010;23:279–288. doi: 10.1007/s10334-010-0230-y. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Lu H, Zuo Y, Gu H, Waltz JA, Zhan W, Scholl CA, Rea W, Yang Y, Stein EA. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18265–18269. doi: 10.1073/pnas.0705791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CL, Schwarze N, Vaishnavi SN, Epstein AA, Snyder AZ, Raichle ME, Shimony JS, Brody DL. Verbal memory deficit following traumatic brain injury: assessment using advanced MRI methods. Neurology. 2008;71:1199–1201. doi: 10.1212/01.wnl.0000327521.69520.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Macey KE, Kumar R, Harper RM. A method for removal of global effects from fMRI time series. Neuroimage. 2004;22:360–366. doi: 10.1016/j.neuroimage.2003.12.042. [DOI] [PubMed] [Google Scholar]
- Mantini D, Gerits A, Nelissen K, Durand JB, Joly O, Simone L, Sawamura H, Wardak C, Orban GA, Buckner RL, Vanduffel W. Default mode of brain function in monkeys. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:12954–12962. doi: 10.1523/JNEUROSCI.2318-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McAvoy M, Larson-Prior L, Nolan TS, Vaishnavi SN, Raichle ME, d’Avossa G. Resting states affect spontaneous BOLD oscillations in sensory and paralimbic cortex. Journal of neurophysiology. 2008;100:922–931. doi: 10.1152/jn.90426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MD, Abbott D. What is stochastic resonance? Definitions, misconceptions, debates, and its relevance to biology. PLoS computational biology. 2009;5:e1000348. doi: 10.1371/journal.pcbi.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhuircheartaigh RN, Rosenorn-Lanng D, Wise R, Jbabdi S, Rogers R, Tracey I. Cortical and subcortical connectivity changes during decreasing levels of consciousness in humans: a functional magnetic resonance imaging study using propofol. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:9095–9102. doi: 10.1523/JNEUROSCI.5516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra PP, Ogawa S, Hu X, Ugurbil K. The nature of spatiotemporal changes in cerebral hemodynamics as manifested in functional magnetic resonance imaging. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1997;37:511–518. doi: 10.1002/mrm.1910370407. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Fletcher PC. Does the brain have a baseline? Why we should be resisting a rest. Neuroimage. 2007;37:1073–1082. doi: 10.1016/j.neuroimage.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Cohen AL, Power JD, Wig GS, Miezin FM, Wheeler ME, Velanova K, Donaldson DI, Phillips JS, Schlaggar BL, Petersen SE. A parcellation scheme for human left lateral parietal cortex. Neuron. 2010;67:156–170. doi: 10.1016/j.neuron.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling L, Coryell CD. The Magnetic Properties and Structure of Hemoglobin, Oxyhemoglobin and Carbonmonoxyhemoglobin. Proc Natl Acad Sci U S A. 1936;22:210–216. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier SJ, Kerssens C, Hamann SB, Sebel PS, Byas-Smith M, Hu X. Functional connectivity changes with concentration of sevoflurane anesthesia. Neuroreport. 2005;16:285–288. doi: 10.1097/00001756-200502280-00017. [DOI] [PubMed] [Google Scholar]
- Pinneo LR. On noise in the nervous system. Psychological review. 1966;73:242–247. doi: 10.1037/h0023240. [DOI] [PubMed] [Google Scholar]
- Pizoli CE, Shah MN, Snyder AZ, Shimony JS, Limbrick DD, Raichle ME, Schlaggar BL, Smyth MD. Resting-state activity in development and maintenance of normal brain function. Proc Natl Acad Sci U S A. 2011;108:11638–11643. doi: 10.1073/pnas.1109144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa D, Popescu AT, Pare D. Contrasting activity profile of two distributed cortical networks as a function of attentional demands. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:1191–1201. doi: 10.1523/JNEUROSCI.4867-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject head motion. Neuroimage. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Vogel AC, Church JA, Barnes KA, Wig GS, Laumann TO, Meizin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdon PL, Weisskoff RM. Effect of temporal autocorrelation due to physiological noise and stimulus paradigm on voxel-level false-positive rates in fMRI. Human brain mapping. 1998;6:239–249. doi: 10.1002/(SICI)1097-0193(1998)6:4<239::AID-HBM4>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis MJ, editor. Cerebral Vascular Smooth Muscle and its Control. Elsevier; London: 1978. [Google Scholar]
- Raichle ME. A paradigm shift in functional brain imaging. J Neurosci. 2009;29:12729–12734. doi: 10.1523/JNEUROSCI.4366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. Two views of brain function. Trends Cogn Sci. 2010;14:180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Raichle ME. The restless brain. Brain Connectivity. 2011;1:3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1089. [DOI] [PubMed] [Google Scholar]
- Reason J. Human Error. Cambridge University Press; 1990. [Google Scholar]
- Reason J, Mycielska K. Absent Minded? Prentice-Hall; Englewood Cliffs, NJ: 1982. [Google Scholar]
- Sadaghiani S, Hesselmann G, Friston KJ, Kleinschmidt A. The relation of ongoing brain activity, evoked neural responses, and cognition. Frontiers in systems neuroscience. 2010;4:20. doi: 10.3389/fnsys.2010.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saling LL, Phillips JG. Automatic behaviour: efficient not mindless. Brain research bulletin. 2007;73:1–20. doi: 10.1016/j.brainresbull.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Samann PG, Wehrle R, Hoehn D, Spoormaker VI, Peters H, Tully C, Holsboer F, Czisch M. Development of the brain’s default mode network from wakefulness to slow wave sleep. Cerebral cortex. 2011;21:2082–2093. doi: 10.1093/cercor/bhq295. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nature reviews. Neuroscience. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneca LCA. Letters from a Stoic: Epistulae Morales ad Lucilium. Penguin Books; New York: 1969. [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:4407–4420. doi: 10.1523/JNEUROSCI.3335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon BJ, Raichle ME, Snyder AZ, Fair DA, Mills KL, Zhang D, Bache K, Calhoun VD, Nigg JT, Nagel BJ, Stevens AA, Kiehl KA. Premotor functional connectivity predicts impulsivity in juvenile offenders. Proc Natl Acad Sci U S A. 2011;108:11241–11245. doi: 10.1073/pnas.1108241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, Milham MP. The resting brain: unconstrained yet reliable. Cerebral cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D’Angelo G, Liu C, Dixit S, Benzinger T, Fagan A, Goate A, Mintun MA. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010a;30:17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America. 2010b;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biological psychiatry. 2010c;67:584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Human brain mapping. 2008;29:751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, Franke D, Pope DL, Snyder AZ, McAvoy MP, Corbetta M. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:4392–4407. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Behar KL, Rothman DL, Shulman RG. In vivo 13C NMR measurements of cerebral glutamine synthesis as evidence for glutamate-glutamine cycling. Proc Natl Acad Sci U S A. 1997;94:2699–2704. doi: 10.1073/pnas.94.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. Longitudinal analysis of neural network development in preterm infants. Cerebral cortex. 2010;20:2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Snyder AZ, Neil JJ. Functional connectivity MRI in infants: exploration of the functional organization of the developing brain. Neuroimage. 2011;56:1437–1452. doi: 10.1016/j.neuroimage.2011.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L, Mangold R, Wechsler RL, Kenney C, Kety SS. The effect of mental arithmetic on cerebral circulation and metabolism. J Clin Invest. 1955;34:1101–1108. doi: 10.1172/JCI103159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Zhou Y, Li J, Liu Y, Tian L, Yu C, Jiang T. Brain spontaneous functional connectivity and intelligence. Neuroimage. 2008;41:1168–1176. doi: 10.1016/j.neuroimage.2008.02.036. [DOI] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Maquet P, D’Argembeau A. Neural correlates of ongoing conscious experience: both task-unrelatedness and stimulus-independence are related to default network activity. PLoS One. 2011;6:e16997. doi: 10.1371/journal.pone.0016997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WD, Buckner RL, Schacter DL. Correlated low-frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category-preferential visual regions. Cerebral cortex. 2010;20:1997–2006. doi: 10.1093/cercor/bhp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FT, Miller LM, Rao AA, D’Esposito M. Functional connectivity of cortical networks involved in bimanual motor sequence learning. Cerebral cortex. 2007;17:1227–1234. doi: 10.1093/cercor/bhl033. [DOI] [PubMed] [Google Scholar]
- Thomas Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Menon V, Young CB, Ryali S, Chen T, Khouzam A, Minshew NJ, Hardan AY. Multivariate searchlight classification of structural magnetic resonance imaging in children and adolescents with autism. Biological psychiatry. 2011;70:833–841. doi: 10.1016/j.biopsych.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Mooshagian E, Zaidel E, Scheres A, Margulies DS, Kelly AM, Shehzad Z, Adelstein JS, Castellanos FX, Biswal BB, Milham MP. Residual functional connectivity in the split-brain revealed with resting-state functional MRI. Neuroreport. 2008;19:703–709. doi: 10.1097/WNR.0b013e3282fb8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A. 2010;107:17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vern BA, Leheta BJ, Juel VC, LaGuardia J, Graupe P, Schuette WH. Interhemispheric synchrony of slow oscillations of cortical blood volume and cytochrome aa3 redox state in unanesthetized rabbits. Brain Res. 1997;775:233–239. doi: 10.1016/s0006-8993(97)01028-7. [DOI] [PubMed] [Google Scholar]
- Vern BA, Leheta BJ, Juel VC, LaGuardia J, Graupe P, Schuette WH. Interhemispheric synchrony Oxygen Transport to Tissue. Plenum Press; New York: 1998. Slow oscillations of cytochrome oxidase redox state and blood volume in unanesthetized cat and rabbit cortex; pp. 561–570. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of neurophysiology. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. Journal of neurophysiology. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME, Mintun MA. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta) deposition. Proc Natl Acad Sci U S A. 2010;107:17763–17767. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites AB, Stanislavsky A, Abbott DF, Jackson GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Human brain mapping. 2005;24:59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- White BR, Bauer AQ, Snyder AZ, Schlaggar BL, Lee JM, Culver JP. Imaging of functional connectivity in the mouse brain. PLoS One. 2011;6:e16322. doi: 10.1371/journal.pone.0016322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS, Schlaggar BL, Petersen SE. Concepts and principles in the analysis of brain networks. Annals of the New York Academy of Sciences. 2011;1224:126–146. doi: 10.1111/j.1749-6632.2010.05947.x. [DOI] [PubMed] [Google Scholar]
- Wise RG, Ide K, Poulin MJ, Tracey I. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage. 2004;21:1652–1664. doi: 10.1016/j.neuroimage.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Zhang D, Johnston JM, Fox MD, Leuthardt EC, Grubb RL, Chicoine MR, Smyth MD, Snyder AZ, Raichle ME, Shimony JS. Preoperative sensorimotor mapping in brain tumor patients using spontaneous fluctuations in neuronal activity imaged with functional magnetic resonance imaging: initial experience. Neurosurgery. 2009;65:226–236. doi: 10.1227/01.NEU.0000350868.95634.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. Journal of neurophysiology. 2008;100:1740–1748. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 2010;20:1187–1194. doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.