Abstract
Tyrosine hydroxylase (TH) is the rate limiting enzyme in norepinephrine synthesis, and its expression and activity are regulated by many factors in sympathetic neurons. Cytokines that act through gp130, such as CNTF (ciliary neurotrophic factor) decrease norepinephrine production in sympathetic neurons by suppressing TH mRNA and stimulating degradation of TH protein, leading to the loss of enzyme. Their effect on the activity of TH is unclear, but recent in vivo observations suggest that cytokines may stimulate TH activity. We investigated this issue by quantifying TH protein levels and activity in cultured sympathetic neurons. We also examined the state of TH phosphorylation on Serine 31 and 40, sites known to affect TH activity and degradation. We found that CNTF, acting through gp130, stimulated the rate of L-DOPA (L-3,4-dihydroxyphenylalanine) production while at the same time decreasing TH enzyme levels, thereby increasing the specific activity of the enzyme. We also found that phosphorylation of TH on Ser31 was increased, and phosphorylation on Ser40 was decreased, after four days of CNTF exposure. Our data are consistent with previous findings that Ser31 phosphorylation stimulates TH activity, whereas Ser40 phosphorylation can target TH for proteasomal degradation.
Introduction
Tyrosine hydroxylase (TH), the rate limiting enzyme in norepinephrine synthesis, catalyzes the hydroxylation of L-tyrosine to form L-3,4-dihydroxyphenylalanine (L-DOPA). Many factors are known to regulate both the activity and expression of this enzyme including nerve activity, catecholamines, neurotrophins, and inflammatory cytokines (Lewis-Tuffin et al. 2004; Landis 1996; Daubner et al. 2011; Dunkley et al. 2004). For example, acute stimulation of sympathetic nerves or depolarization of PC12 cells increases TH activity via phosphorylation (Zigmond et al. 1989; Salvatore et al. 2001), while chronic nerve stimulation or cell depolarization increases TH mRNA and protein levels (Zigmond 1980; Zigmond 1988; Lewis-Tuffin et al. 2004).
In contrast to the stimulatory effects of nerve activity, inflammatory cytokines that act through gp130, such as LIF (leukemia inhibitory factor) and CNTF (ciliary neurotrophic factor), decrease TH content and catecholamine production in sympathetic neurons (Saadat et al. 1989; Yamamori et al. 1989). These cytokines decrease TH mRNA (Lewis et al. 1994; Nawa et al. 1991) and stimulate the proteasomal degradation of TH protein (Shi and Habecker 2012), leading to the loss of enzyme. Their effect on TH activity is unclear, but recent in vivo observations suggest that cytokines may stimulate TH activity. TH enzyme levels in the peri-infarct region of the left ventricle, corrected for neuronal loss, are reduced by ~70–80% following myocardial infarction (Li et al. 2004; Parrish et al. 2008; Parrish et al. 2009a). The loss of peri-infarct TH is triggered by cytokines, and is absent in mice whose sympathetic neurons lack the gp130 receptor (Parrish et al. 2009a). However, peri-infarct NE levels are similar in wild type and gp130 knockout mice (Parrish et al. 2009a), despite the depletion of TH in wild type neurons. These data suggest the possibility that inflammatory cytokines influence TH enzyme activity in addition to their effect on enzyme levels.
We examined this issue by treating cultured sympathetic neurons with recombinant CNTF, and measuring TH protein levels by western blot analysis and TH activity using an HPLC method to measure L-DOPA production. We also examined the state of TH phosphorylation on Ser31 and Ser40, sites that are thought to affect TH activity (Daubner et al. 2011; Dunkley et al. 2004) and TH turnover (Nakashima et al. 2011).
Methods
Animals
Pregnant adult Sprague Dawley rats were obtained from Charles River. Wildtype C57BL/6J mice were obtained from Jackson Laboratories. The gp130DBH-Cre/lox mice, whose sympathetic neurons lack gp130, were generated as previously described (Stanke et al. 2006). All animals were housed individually with a 12 hr:12 hr light dark cycle with ad libitum access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee, and comply with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996).
Primary Cell culture
Superior cervical ganglia (SCG) from newborn rats or mice (P0-P1) were dissociated and grown in cell culture as previously described (Dziennis and Habecker 2003; Shi and Habecker 2012) using C2 medium supplemented with 50 ng/mL nerve growth factor (NGF, BD Bioscience), and 3% fetal bovine serum (ATCC)(Pellegrino et al. 2011). Neurons were grown under sterile conditions in a humidified 5% CO2 incubator at 37°C. Sympathetic neurons were grown in 24-well plates pre-coated with 100 μg/mL poly-L-lysine (Sigma) and 10 μg/mL collagen (BD bioscience). Non-neuronal cells were eliminated by treating the cultures with the anti-mitotic agent, cytosine arabinoside (Ara C, 1 μM, Sigma) for 2 days.
Recombinant CNTF (100 ng/mL, Pepro Tech) was diluted in culture medium before addition to the culture plates. Neurons were maintained in 100 ng/mL CNTF for 4 days because previous studies showed that TH content was reliably decreased by this treatment paradigm (Shi and Habecker 2012), mimicking the cytokine-induced loss of enzyme in vivo (Parrish et al. 2009b).
In vitro TH enzyme activity assay
Cultures of sympathetic neurons (20,000 cells/sample for rat cultures or 12,000 cells/sample for mouse) were homogenized in 150 μL of 5 mM Tris-acetate buffer (pH 6.0) containing 0.1% Triton X-100, protease inhibitor cocktail (Roche) and sodium orthovanadate (1 mM). TH enzyme activity was determined by measuring the initial rate of L-DOPA production from L-tyrosine under standardized conditions (Acheson et al. 1984). A 60 μL aliquot of the homogenate was combined with an equal volume of reaction buffer and assayed for TH activity under the following conditions: 120 mM Tris-acetate buffer (pH 6.0), 3 mM 6-methyl-5, 6, 7, 8 tetrahydropterin HCl (BH4), 10 mM 2-mercaptoethanol, catalase (200,000 U/mL), 100 μM L-tyrosine, and the internal standard, 1.0 μM 3,4-dihydroxybenzylamine (DHBA). The reaction mixtures were incubated at 37°C. Aliquots (20 μL) of the reaction mixture were removed at regular intervals (every 3 min. for rat cultures and every 4 min. for mouse cultures) and added to 1 mL of a chilled solution containing 0.5 M Tris-acetate buffer (pH 8.0), 0.1 M EDTA, and 15 mg of alumina to stop the reaction. The L-DOPA and DHBA were purified by an alumina extraction procedure described previously (Li et al. 2004), and the production of L-DOPA was measured by HPLC using electrochemical detection as previously described (Li et al. 2004).
Immunoblot Analysis
Cells were washed with ice-cold PBS and lysed at 4°C in homogenization buffer as described above. Lysates were fractionated on SDS-polyacrylamide gels and transferred to nitrocellulose membranes. Blots were incubated in 5% low fat milk in Tris-buffered saline (pH 7.4) containing 0.1% tween-20 at room temperature. Membranes were subsequently incubated at 4°C overnight with rabbit antiTH (1:5000, Millipore), rabbit anti-pTH Ser31 (1:1000, Cell Signaling), or rabbit anti-pTH Ser40 (1:1000, Cell Signaling). The immunoblots were incubated with horseradish peroxidase HRP - conjugated secondary antibody and immune complexes were visualized by chemiluminescence (Super Signal Dura, Pierce). Band intensity was recorded by a −40°C CCD camera and analyzed using LabWorks software (UVP, Upland, CA), quantifying only sub-saturating exposures. To quantify TH phosphorylation levels, the band density obtained for phospho-specific TH was normalized to the total TH protein in that sample. Total and mean band density gave similar results.
Statistical analyses
Data are presented as mean values± SEM. Significant differences were assessed by one-way analysis of variance (ANOVA) using GraphPad Prism 5 (GraphPad Software, Inc.). Tukey’s post hoc test was used to compare to all conditions. P values <0.05 were considered significant.
Results
In order to determine if cytokines alter neurotransmitter production in sympathetic neurons, we treated sympathetic neuron cultures with 100 ng/mL CNTF for 4 days, and then measured TH activity in cell homogenates. Cytokines significantly decreased TH protein levels after 4 days (Fig. 1A & B). TH activity was quantified by measuring the initial rate of L-DOPA formation under standardized conditions (Acheson et al. 1984) by a sensitive HPLC method using electrochemical detection (Li et al. 2004). Under these conditions, L-DOPA production in control cells was linear with respect to protein concentration and time (Fig. 1C). We normalized the rate of L-DOPA formation in each condition to the total amount of TH protein present, as determined by western blotting. The rate of L-DOPA production, corrected for TH protein levels, was significantly increased (Fig. 1D). In order to rule out the possibility L-DOPA was further converted to dopamine by aromatic amino acid decarboxylase under our assay conditions, we examined the reaction mixture for the presence of dopamine by HPLC but were unable to detect it. Moreover, the present or absence of carbidopa, an aromatic amino acid decarboxylase inhibitor, in the reaction mixture did not alter the rate of L-DOPA formation (data not shown).
Figure 1.
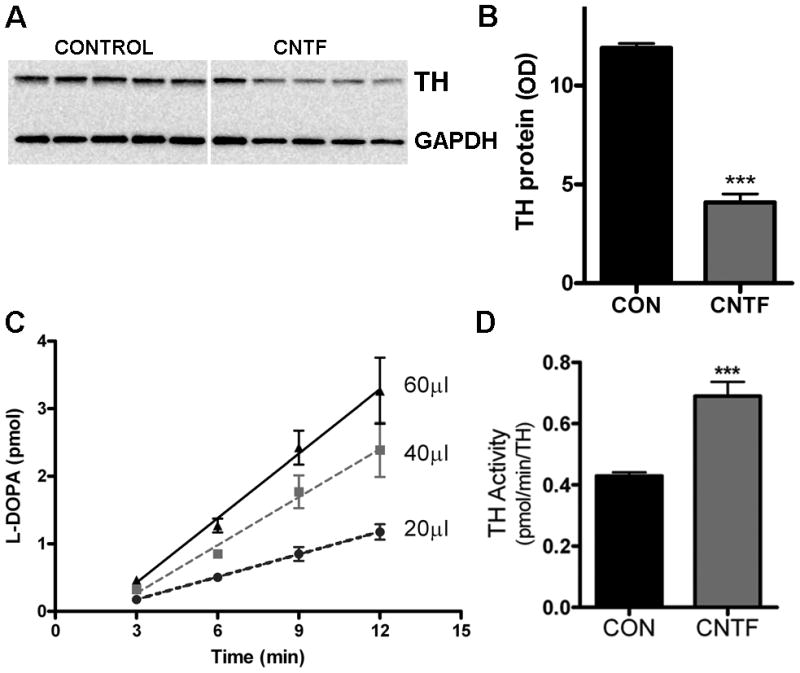
CNTF increases TH activity while decreasing TH levels. A&B) TH content was assayed by western blot. A representative blot identifying TH and GAPDH in 5 control and 5 CNTF-treated samples is shown in (A), with quantification of TH by optical density units (OD) shown in (B). Data are mean±sem, n=5 samples, ***p<0.001, and are representative of results from 3 independent experiments. C) TH activity was quantified by measuring the initial rate L-DOPA synthesis. L-DOPA production in control cells was linear with respect to protein concentration and time. Slopes for 20, 40, and 60 μL samples were 0.1±0.01, 0.2±0.03, and 0.3±0.04 pmol L-DOPA/min, respectively. D) TH specific activity in neurons treated with control media (CON) or 100 ng/ml CNTF for 4 days (mean±sem, n=5 samples, ***p<0.001). Data are representative of 3 independent experiments. Activity was calculated as the rate of L-DOPA production normalized to TH content in the cell (pmol L-DOPA/min/OD unit of TH).
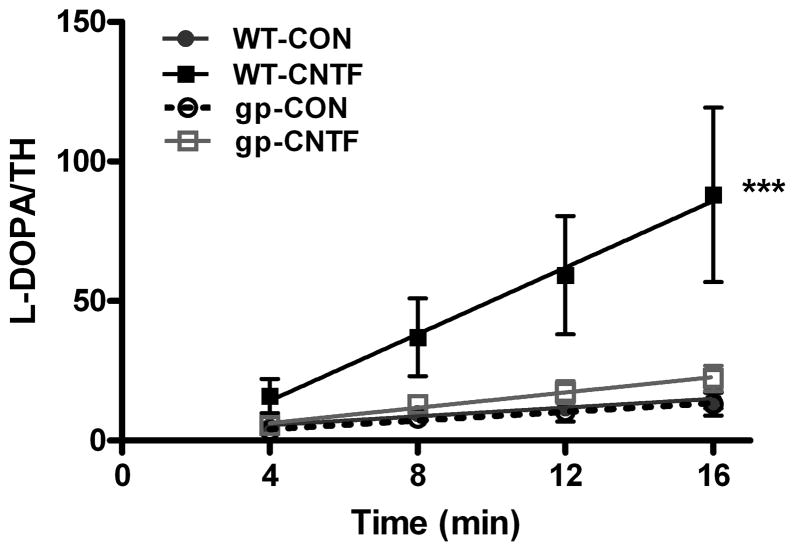
Cytokine suppression of TH content requires activation of the gp130 receptor. Therefore, we asked whether the CNTF-stimulated increase in TH activity was gp130-dependent. Cultures of neurons from wild type mice and from mice whose sympathetic neurons lack gp130 were treated with CNTF for 4 days, and TH activity was assayed. CNTF increased the specific activity of TH in wild type neurons as previously shown in rat sympathetic neurons, but had no effect on TH activity in neurons lacking gp130 signaling (Fig. 2). This confirms that CNTF influences TH activity through the gp130 receptor, and that similar changes occur in neurons derived from rat and mouse.
Figure 2.
CNTF stimulates TH activity via gp130. Wild type (WT) sympathetic neurons and neurons lacking gp130 (gp) were maintained for four days in control media (CON) or in media supplemented with 100 ng/ml CNTF. TH activity was quantified by measuring the rate of L-DOPA formation (pmol) normalized to TH content (OD units). Data are mean±sem, n=3 samples, ***p<0.005, and are representative of 2 independent experiments.
Changes in the phosphorylation of TH can regulate TH activity and catecholamine production. To determine if the CNTF-induced changes in TH activity correlated with changes in TH phosphorylation, we used western blots to assess the phosphorylation of two key sites, Ser31 and Ser40, thought to be involved in the regulation of TH activity (Fig. 3A). In untreated control neurons, the ratio of phospho-Ser31 (pSer31) to total TH averaged 0.8±0.1 (mean±sem, n=3 experiments) while the ratio of pSer40 to total TH averaged 2.0±0.5 (mean±sem, average of 3 experiments). One day of CNTF treatment increased the portion of TH that was phosphorylated on Ser31 (165±11% of control; mean±sem, n=3 experiments) and decreased the fraction of TH phosphorylated on Ser40 (57±14 % of control; mean±sem, n=3 experiments). These changes were maintained through four days of CNTF treatment, with more TH phosphorylated on Ser31 and less phosphorylated on Ser40 compared to control cells (Fig. 3). Data from a representative experiment are graphed in Figure 3(B, C) as the ratio of phospho TH:total TH rather than percent of control.
Figure 3.
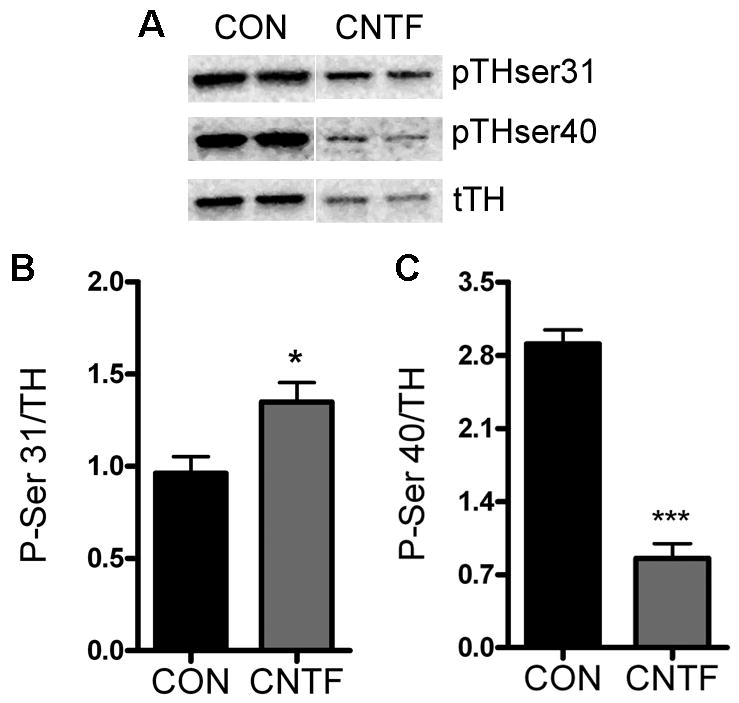
TH phosphorylation. Neurons were treated for four days with control media (CON) or media supplemented with 100 ng/ml CNTF and blotted for total and phosphorylated TH. A) Sample western blot showing total TH (tTH), TH phosphorylated on Ser31 (pTHser31), and TH phosphorylated on Ser40 (pTHser40) in the same samples. B) Quantification of Ser31 phosphorylated TH normalized to total TH. Data are mean±sem, n=4–5 samples, *p<0.05. C) Quantification of Ser40 phosphorylated TH normalized to total TH. Data are mean±sem, n=5 samples, ***p<0.0001. These data are representative of results from 3 independent experiments.
Discussion
Following myocardial infarction, cytokines acting through the gp130 receptor reduce the levels of TH enzyme in sympathetic neurons in the peri-infarct region of the left ventricle. Myocardial infarction studies in mice whose sympathetic neurons lack the gp130 receptor suggested that cytokines might cause a compensatory increase in the activity of TH that at least partially counteracts the loss of enzyme (Parrish et al. 2009b). We confirmed that prediction in the present study. The specific activity of TH in sympathetic neurons treated with CNTF is ~80% greater than that in untreated neurons. Furthermore, in neurons lacking gp130 signaling, the specific activity of TH and level of TH protein in CNTF treated cells was the same as that in untreated neurons. These results suggest that CNTF, acting through the gp130 receptor complex, influences not only the level TH protein but also the activity of the enzyme.
In order to compare the activity of TH enzyme in cultured sympathetic neurons, we developed an HPLC assay to measure the initial rate of L-DOPA production under standardized conditions in which the substrates, L-tyrosine and BH4, were present in saturating concentrations (Acheson et al. 1984). We have shown that under our assay conditions L-DOPA production is linear for at least 16 minutes and increased with increasing volume of homogenate. Our assays attempted to control factors that could potentially affect our comparisons of TH activity. For example, we ruled out further metabolism of L-DOPA by showing that carbidopa, a decarboxylase inhibitor, had no effect on the rate of L-DOPA production and that dopamine was undetectable even at reaction times as long as 60 minutes.
Our observation that CNTF stimulates TH enzyme activity conflicts with earlier reports that TH activity was either unchanged or decreased in neurons treated with inflammatory cytokines (Iacovitti et al. 1981; Swerts et al. 1983; Wolinsky and Patterson 1983). Several factors could account for the discrepancy between our results and those of the earlier studies with respect to the effect of cytokines on TH activity. First, previous studies used conditioned media and tissue extracts which are mixtures of cytokines and other factors that may influence TH activity (Iacovitti et al. 1981;Swerts et al. 1983; Wolinsky and Patterson 1983), whereas we used recombinant CNTF. Second, we treated the neurons for 4 days while other studies treated cultured neurons for several weeks and maintained the cultures in different media and growth factor conditions. Finally, we quantified L-DOPA production using HPLC with electrochemical detection, a more sensitive assay of TH activity than measuring the accumulation of labeled catecholamines. For these reasons, we have confidence that CNTF and related cytokines increase the activity of TH in addition to decreasing TH levels.
The mechanism of how CNTF influences TH activity is not entirely clear and needs additional investigation. One possibility is that CNTF affects TH activity by a post-translational modification, such as altering the phosphorylation state of the enzyme. TH is phosphorylated at several serine residues: Ser8, Ser19, Ser31, and Ser40 (Daubner et al. 2011; Dunkley et al. 2004). Phosphorylation of Ser31 and Ser40 are clearly associated with stimulation of TH activity (Bobrovskaya et al. 1998), with less evidence for Ser8 or 19 playing a direct role in stimulating TH catalytic activity (Daubner et al. 2011; Dunkley et al. 2004). We investigated the effect of CNTF treatment on pSer31 and pSer40, and found that chronic treatment with CNTF increased the fraction of TH phosphorylated on Ser31 while decreasing the fraction of TH phosphorylated on Ser40. The increased phosphorylation at Ser31 may contribute to the stimulation of TH activity observed in our study. This is consistent with studies of depolarization-induced stimulation of TH, where phosphorylation of Ser31 stimulated TH activity while increases in Ser40 phosphorylation had no effect on catecholamine production (Salvatore et al. 2001). The major kinases that phosphorylate TH on Ser31 are ERK1/2 (Dunkley et al. 2004). CNTF stimulates ERK1/2 activation in sympathetic neurons (Dziennis and Habecker 2003; Shi and Habecker 2012), suggesting that CNTF stimulation of ERK 1/2 activity is responsible for the increase in Ser31 phosphorylation. Ser31 (and Ser40) phosphorylation can increase TH activity by altering the binding affinity of TH for its cofactor, BH4. However, BH4 is present in saturating concentrations under our assay conditions, so the specific mechanism by which CNTF increases TH activity in our study remains unknown.
Phosphorylation of TH affects enzyme stability in addition to altering catalytic activity (Moy and Tsai 2004; Mockus et al. 1997; Nakashima et al. 2011). The observation that phosphorylation at Ser40 targets TH for degradation by the ubiquitin-proteasome system is of particular interest (Nakashima et al. 2011). CNTF increases proteasomal degradation of TH in sympathetic neurons (Shi and Habecker 2012), contributing to the loss of enzyme observed in our experiments. Thus, the decrease in Ser40 phosphorylated TH our studies may result from degradation of that pool of enzyme rather than changes in kinase or phosphatase activity. Our results, taken together with previous studies of TH phosphorylation, suggest that CNTF-stimulated Ser31 phosphorylation contributes to the increase in TH activity, whereas Ser40 phosphorylation may target TH for proteasomal degradation. These contradictory effects of inflammatory cytokines on TH have interesting implications for neurodegenerative diseases, where cytokines may promote the loss of TH protein while at the same time increasing the catalytic activity of remaining enzyme.
Acknowledgments
This work was supported by NIH R01 HL068231 and HL093056.
The abbreviations used are
- TH
tyrosine hydroxylase
- L-DOPA
L-3,4-dihydroxyphenylalanine
- CNTF
ciliary neurotrophic factor
- DHBA
3,4-dihydroxybenzylamine
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- NGF
nerve growth factor
- PBS
phosphate-buffered saline
- BH4
(6R)-5,6,7,8-tetrahydropterin
Footnotes
The authors have no financial conflict of interest.
Reference List
- Acheson AL, Naujoks K, Thoenen H. Nerve growth factor-mediated enzyme induction in primary cultures of bovine adrenal chromaffin cells: specificity and level of regulation. J Neurosci. 1984;4:1771–1780. doi: 10.1523/JNEUROSCI.04-07-01771.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrovskaya L, Cheah TB, Bunn SJ, Dunkley PR. Tyrosine hydroxylase in bovine adrenal chromaffin cells: angiotensin II- stimulated activity and phosphorylation of Ser19, Ser31, and Ser40. J Neurochem. 1998;70:2565–2573. doi: 10.1046/j.1471-4159.1998.70062565.x. [DOI] [PubMed] [Google Scholar]
- Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys. 2011;508:1–12. doi: 10.1016/j.abb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley PR, Bobrovskaya L, Graham ME, Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem. 2004;91:1025–1043. doi: 10.1111/j.1471-4159.2004.02797.x. [DOI] [PubMed] [Google Scholar]
- Dziennis S, Habecker BA. Cytokine Suppression of Dopamine-beta -hydroxylase by Extracellular Signal-regulated Kinase-dependent and -independent Pathways. J Biol Chem. 2003;278:15897–15904. doi: 10.1074/jbc.M212480200. [DOI] [PubMed] [Google Scholar]
- Iacovitti L, Joh TH, Park DH, Bunge RP. Dual expression of neurotransmitter synthesis in cultured autonomic neurons. J Neurosci. 1981;1:685–690. doi: 10.1523/JNEUROSCI.01-07-00685.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis SC. The development of cholinergic sympathetic neurons: a role for neuropoietic cytokines? Perspect Dev Neurobiol. 1996;4:53–63. doi: 10.1080/0907676x.1996.9961274. [DOI] [PubMed] [Google Scholar]
- Lewis SE, Rao MS, Symes AJ, Dauer WT, Fink JS, Landis SC, Hyman SE. Coordinate regulation of choline acetyltransferase, tyrosine hydroxylase, and neuropeptide mRNAs by ciliary neurotrophic factor and leukemia inhibitory factor in cultured sympathetic neurons. J Neurochem. 1994;63:429–438. doi: 10.1046/j.1471-4159.1994.63020429.x. [DOI] [PubMed] [Google Scholar]
- Lewis-Tuffin LJ, Quinn PG, Chikaraishi DM. Tyrosine hydroxylase transcription depends primarily on cAMP response element activity, regardless of the type of inducing stimulus. Mol Cell Neurosci. 2004;25:536–547. doi: 10.1016/j.mcn.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Li W, Knowlton D, Van Winkle DM, Habecker BA. Infarction alters both the distribution and noradrenergic properties of cardiac sympathetic neurons. Am J Physiol Heart Circ Physiol. 2004;286:H2229–H2236. doi: 10.1152/ajpheart.00768.2003. [DOI] [PubMed] [Google Scholar]
- Mockus SM, Kumer SC, Vrana KE. A chimeric tyrosine/tryptophan hydroxylase - The tyrosine hydroxylase regulatory domain serves to stabilize enzyme activity. J Mol Neurosci. 1997;9:35–48. doi: 10.1007/BF02789393. [DOI] [PubMed] [Google Scholar]
- Moy LY, Tsai LH. Cyclin-dependent Kinase 5 Phosphorylates Serine 31 of Tyrosine Hydroxylase and Regulates Its Stability. J Biol Chem. 2004;279:54487–54493. doi: 10.1074/jbc.M406636200. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Mori K, Kaneko YS, Hayashi N, Nagatsu T, Ota A. Phosphorylation of the N-terminal portion of tyrosine hydroxylase triggers proteasomal digestion of the enzyme. Biochem Biophys Res Commun. 2011;407:343–347. doi: 10.1016/j.bbrc.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Nawa H, Nakanishi S, Patterson PH. Recombinant cholinergic differentiation factor (leukemia inhibitory factor) regulates sympathetic neuron phenotype by alterations in the size and amounts of neuropeptide mRNAs. J Neurochem. 1991;56:2147–2150. doi: 10.1111/j.1471-4159.1991.tb03479.x. [DOI] [PubMed] [Google Scholar]
- Parrish DC, Alston EN, Rohrer H, Hermes SM, Aicher SA, Nkadi P, Woodward WR, Stubbusch J, Gardner RT, Habecker BA. The absence of gp130 in dopamine {beta} hydroxylase-expressing neurons leads to autonomic imbalance and increased reperfusion arrhythmias. Am J Physiol Heart Circ Physiol. 2009a;297:H960–H967. doi: 10.1152/ajpheart.00409.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish DC, Alston EN, Rohrer H, Nkadi P, Woodward WR, Schutz G, Habecker BA. Infarction-induced cytokines cause local depletion of tyrosine hydroxylase in cardiac sympathetic nerves. Exp Physiol. 2009b;95:304–314. doi: 10.1113/expphysiol.2009.049965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish DC, Gritman K, Van Winkle DM, Woodward WR, Bader M, Habecker BA. Postinfarct sympathetic hyperactivity differentially stimulates expression of tyrosine hydroxylase and norepinephrine transporter. Am J Physiol Heart Circ Physiol. 2008;294:H99–H106. doi: 10.1152/ajpheart.00533.2007. [DOI] [PubMed] [Google Scholar]
- Pellegrino MJ, Parrish DC, Zigmond RE, Habecker BA. Cytokines inhibit norepinephrine transporter expression by decreasing Hand2. Mol Cell Neurosci. 2011;46:671–680. doi: 10.1016/j.mcn.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat S, Sendtner M, Rohrer H. Ciliary neurotrophic factor induces cholinergic differentiation of rat sympathetic neurons in culture. J Cell Biol. 1989;108:1807–1816. doi: 10.1083/jcb.108.5.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Waymire JC, Haycock JW. Depolarization-stimulated catecholamine biosynthesis: involvement of protein kinases and tyrosine hydroxylase phosphorylation sites in situ. J Neurochem. 2001;79:349–360. doi: 10.1046/j.1471-4159.2001.00593.x. [DOI] [PubMed] [Google Scholar]
- Shi X, Habecker BA. gp130 cytokines stimulate proteasomal degradation of tyrosine hydroxylase via extracellular signal regulated kinases 1 and 2. J Neurochem. 2012;120:239–247. doi: 10.1111/j.1471-4159.2011.07539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke M, Duong CV, Pape M, Geissen M, Burbach G, Deller T, Gascan H, Otto C, Parlato R, Schutz G, Rohrer H. Target-dependent specification of the neurotransmitter phenotype: cholinergic differentiation of sympathetic neurons is mediated in vivo by gp 130 signaling. Development. 2006;133:141–150. doi: 10.1242/dev.02189. [DOI] [PubMed] [Google Scholar]
- Swerts JP, Le Van TA, Vigny A, Weber MJ. Regulation of enzymes responsible for neurotransmitter synthesis and degradation in cultured rat sympathetic neurons. I. Effects of muscle- conditioned medium. Dev Biol. 1983;100:1–11. doi: 10.1016/0012-1606(83)90195-1. [DOI] [PubMed] [Google Scholar]
- Wolinsky E, Patterson PH. Tyrosine hydroxylase activity decreases with induction of cholinergic properties in cultured sympathetic neurons. J Neurosci. 1983;3:1495–1500. doi: 10.1523/JNEUROSCI.03-07-01495.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T, Fukada K, Aebersold R, Korsching S, Fann MJ, Patterson PH. The cholinergic neuronal differentiation factor from heart cells is identical to leukemia inhibitory factor. Science. 1989;246:1412–1416. doi: 10.1126/science.2512641. [DOI] [PubMed] [Google Scholar]
- Zigmond RE. The long-term regulation of ganglionic tyrosine hydroxylase by preganglionic nerve activity. Fed Proc. 1980;39:3003–3008. [PubMed] [Google Scholar]
- Zigmond RE. A comparison of the long-term and short-term regulations of tyrosine hydroxylase activity. J Physiol (Paris ) 1988;83:267–271. [PubMed] [Google Scholar]
- Zigmond RE, Schwarzschild MA, Rittenhouse AR. Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation. Annu Rev Neurosci. 1989;12:415–461. doi: 10.1146/annurev.ne.12.030189.002215. [DOI] [PubMed] [Google Scholar]