Abstract
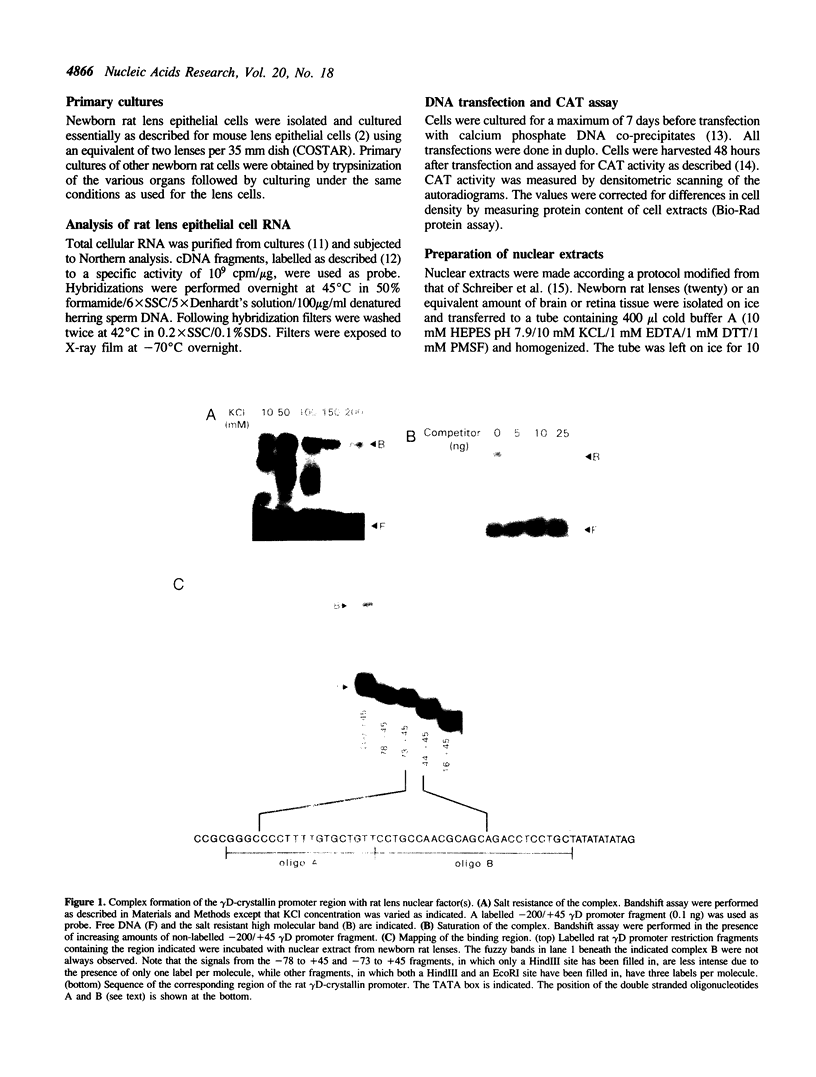
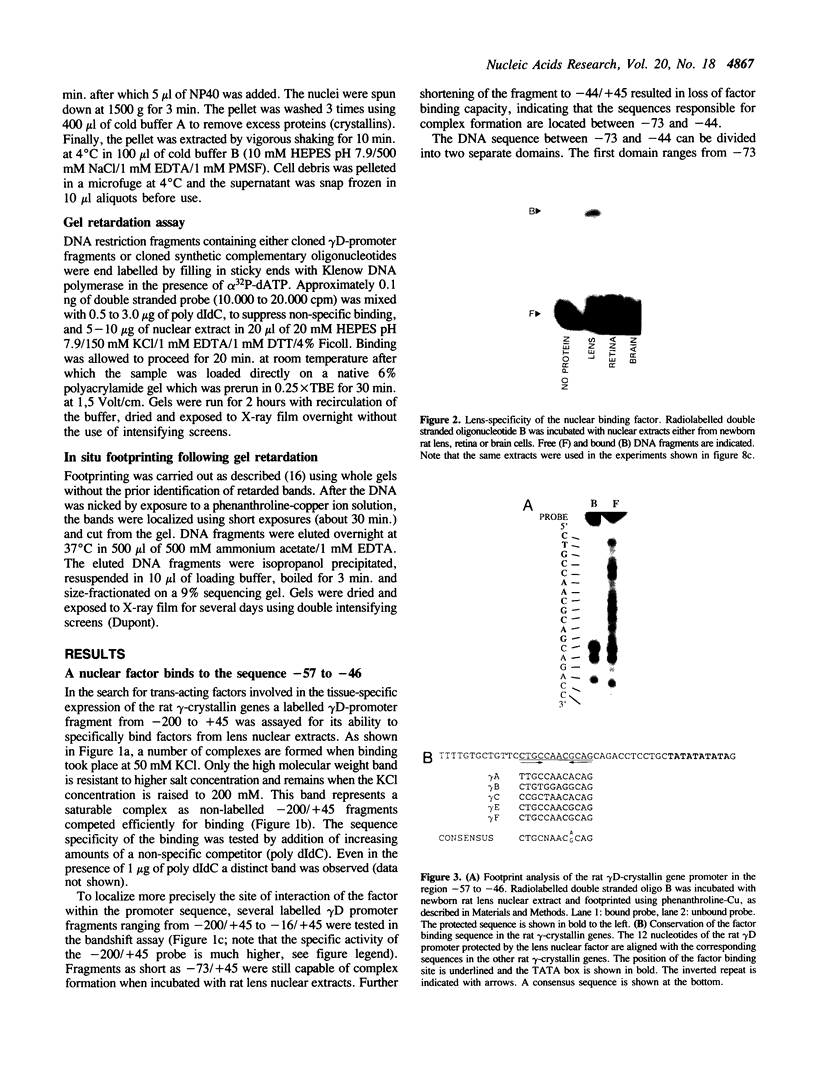
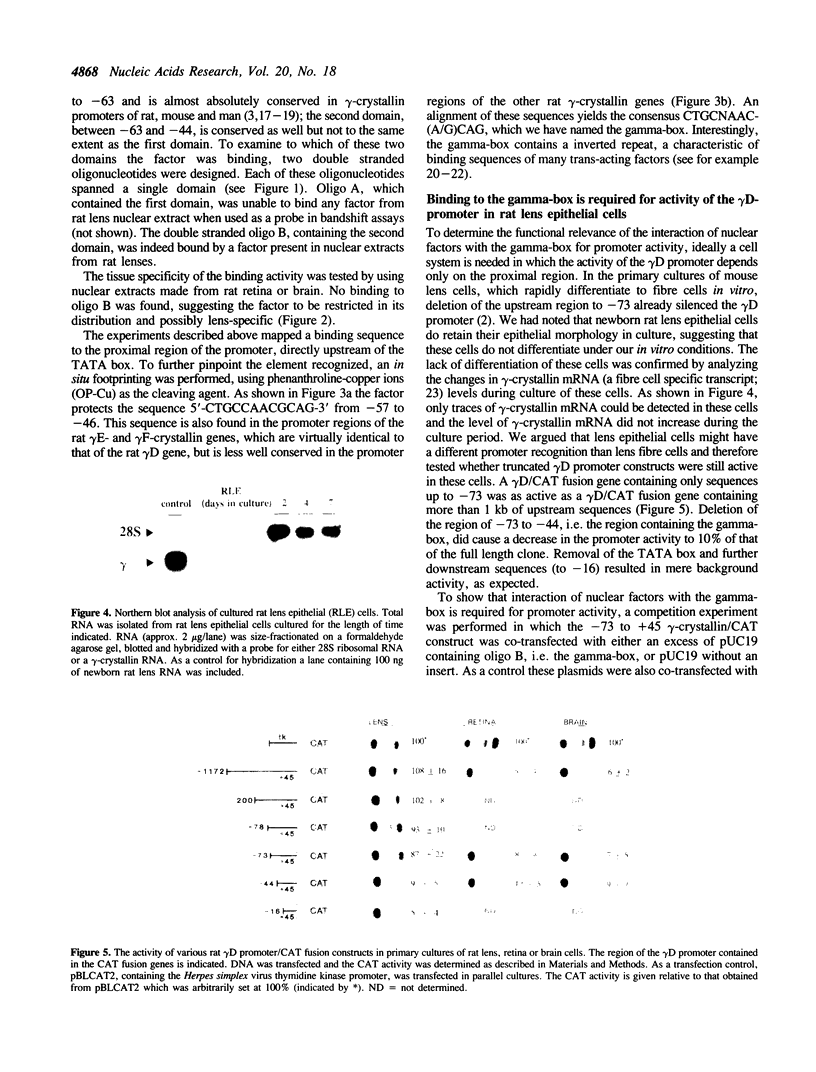
Rat lens nuclear extracts contain a factor that binds to position -57 to -46 of the rat gamma D-crystallin promoter region. This factor protects the sequence 5'-CTGCCAACGCAG-3' in a footprint analysis. Binding to this region is crucial for maximal promoter activity in rat lens cells, but this sequence was unable to act as an enhancer when cloned in front of a heterologous promoter. A region directly upstream from this activating sequence, between position -85 to -67, acts as a strong silencer of promoter activity in non-lens cells. This silencing effect is mediated by trans-acting factor(s). Our data provide evidence for two regulatory elements in rat gamma D-crystallin gene expression, an activating sequence active in lens cells and a silencing sequence active only in non-lens cells. The factor that binds to the activating sequence could be detected only in lens cells and may be a determinant of the lens-specific expression of the gamma-crystallin genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrisani O. M., Pot D. A., Zhu Z., Dixon J. E. Three sequence-specific DNA-protein complexes are formed with the same promoter element essential for expression of the rat somatostatin gene. Mol Cell Biol. 1988 May;8(5):1947–1956. doi: 10.1128/mcb.8.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakenhoff R. H., Ruuls R. C., Jacobs E. H., Schoenmakers J. G., Lubsen N. H. Transgenic Xenopus laevis tadpoles: a transient in vivo model system for the manipulation of lens function and lens development. Nucleic Acids Res. 1991 Mar 25;19(6):1279–1284. doi: 10.1093/nar/19.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough N. M. Rapid and quantitative preparation of cytoplasmic RNA from small numbers of cells. Anal Biochem. 1988 Aug 15;173(1):93–95. doi: 10.1016/0003-2697(88)90164-9. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Kuwabara M. D., Sigman D. S. Footprinting DNA-protein complexes in situ following gel retardation assays using 1,10-phenanthroline-copper ion: Escherichia coli RNA polymerase-lac promoter complexes. Biochemistry. 1987 Nov 17;26(23):7234–7238. doi: 10.1021/bi00397a006. [DOI] [PubMed] [Google Scholar]
- Liu Q. R., Tini M., Tsui L. C., Breitman M. L. Interaction of a lens cell transcription factor with the proximal domain of the mouse gamma F-crystallin promoter. Mol Cell Biol. 1991 Mar;11(3):1531–1537. doi: 10.1128/mcb.11.3.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok S., Breitman M. L., Chepelinsky A. B., Piatigorsky J., Gold R. J., Tsui L. C. Lens-specific promoter activity of a mouse gamma-crystallin gene. Mol Cell Biol. 1985 Sep;5(9):2221–2230. doi: 10.1128/mcb.5.9.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok S., Stevens W., Breitman M. L., Tsui L. C. Multiple regulatory elements of the murine gamma 2-crystallin promoter. Nucleic Acids Res. 1989 May 11;17(9):3563–3582. doi: 10.1093/nar/17.9.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok S., Tsui L. C., Shinohara T., Piatigorsky J., Gold R., Breitman M. Analysis of the mouse gamma-crystallin gene family: assignment of multiple cDNAs to discrete genomic sequences and characterization of a representative gene. Nucleic Acids Res. 1984 Jun 11;12(11):4517–4529. doi: 10.1093/nar/12.11.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meakin S. O., Breitman M. L., Tsui L. C. Structural and evolutionary relationships among five members of the human gamma-crystallin gene family. Mol Cell Biol. 1985 Jun;5(6):1408–1414. doi: 10.1128/mcb.5.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Donovan D. M., Hamada K., Sax C. M., Norman B., Flanagan J. R., Ozato K., Westphal H., Piatigorsky J. Regulation of the mouse alpha A-crystallin gene: isolation of a cDNA encoding a protein that binds to a cis sequence motif shared with the major histocompatibility complex class I gene and other genes. Mol Cell Biol. 1990 Jul;10(7):3700–3708. doi: 10.1128/mcb.10.7.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek R., McAvoy J. W., Lubsen N. H., Schoenmakers J. G. Rise and fall of crystallin gene messenger levels during fibroblast growth factor induced terminal differentiation of lens cells. Dev Biol. 1992 Jul;152(1):152–160. doi: 10.1016/0012-1606(92)90165-d. [DOI] [PubMed] [Google Scholar]
- Peek R., Niessen R. W., Schoenmakers J. G., Lubsen N. H. DNA methylation as a regulatory mechanism in rat gamma-crystallin gene expression. Nucleic Acids Res. 1991 Jan 11;19(1):77–83. doi: 10.1093/nar/19.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek R., van der Logt P., Lubsen N. H., Schoenmakers J. G. Tissue- and species-specific promoter elements of rat gamma-crystallin genes. Nucleic Acids Res. 1990 Mar 11;18(5):1189–1197. doi: 10.1093/nar/18.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Sommer B., Chepelinsky A. B., Piatigorsky J. Binding of nuclear proteins to promoter elements of the mouse alpha A-crystallin gene. J Biol Chem. 1988 Oct 25;263(30):15666–15672. [PubMed] [Google Scholar]
- Thompson M. A., Hawkins J. W., Piatigorsky J. Complete nucleotide sequence of the chicken alpha A-crystallin gene and its 5' flanking region. Gene. 1987;56(2-3):173–184. doi: 10.1016/0378-1119(87)90135-1. [DOI] [PubMed] [Google Scholar]
- Van Leen R. W., Breuer M. L., Lubsen N. H., Schoenmakers J. G. Developmental expression of crystallin genes: in situ hybridization reveals a differential localization of specific mRNAs. Dev Biol. 1987 Oct;123(2):338–345. doi: 10.1016/0012-1606(87)90392-7. [DOI] [PubMed] [Google Scholar]
- den Dunnen J. T., Moormann R. J., Lubsen N. H., Schoenmakers J. G. Concerted and divergent evolution within the rat gamma-crystallin gene family. J Mol Biol. 1986 May 5;189(1):37–46. doi: 10.1016/0022-2836(86)90379-7. [DOI] [PubMed] [Google Scholar]
- den Dunnen J. T., Moormann R. J., Lubsen N. H., Schoenmakers J. G. Intron insertions and deletions in the beta/gamma-crystallin gene family: the rat beta B1 gene. Proc Natl Acad Sci U S A. 1986 May;83(9):2855–2859. doi: 10.1073/pnas.83.9.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]