Abstract
This review provides an overview of metal-based anticancer drugs and drug candidates. In particular, we focus on metal complexes that can be activated in the reducing environment of cancer cells, thus serving as prodrugs. There are many reports of Pt and Ru complexes as redox-activatable drug candidates, but other d-block elements with variable oxidation states have a similar potential to serve as prodrugs in this manner. In this context are compounds based on Fe, Co, or Cu chemistry, which are also covered. A trend in the field of medicinal inorganic chemistry has been toward molecularly targeted, metal-based drugs obtained by functionalizing complexes with biologically active ligands. Another recent activity is the use of nanomaterials for drug delivery, exploiting passive targeting of tumors with nanosized constructs made from Au, Fe, carbon, or organic polymers. Although complexes of all of the above mentioned metals will be described, this review focuses primarily on Pt compounds, including constructs containing nanomaterials.
Keywords: Prodrugs, Pt anticancer drugs, Ru anticancer drugs, activation by reduction, medicinal inorganic chemistry, nanocarriers
1. Introduction
Most drugs are organic or biologically derived compounds, and the pharmaceutical industry focuses on organic chemistry with some exceptions [1]. Metal-based therapeutics comprise only a small percentage of available drugs. After the discovery and successful clinical applications of the Pt-based anticancer drug cisplatin, however, research on metal-based drugs became increasingly more important. There is a growing interest in metal-containing drugs, and medicinal inorganic chemistry covering applications of metals in therapeutics and diagnostics is a field of increasing prominence [2]. The major advantage of metal-based over organic-based drugs is the ability to vary coordination number, geometry, and redox states. Metals can also change the pharmacological properties of organic-based drugs by forming coordination complexes with them [1]. Cisplatin is one of the most widely used anticancer drugs, administered particularly for ovarian and testicular cancer. If testicular tumors are discovered early, an impressive cure rate of almost 100% is achieved. Significant side-effects due to systemic toxicity include nausea, bone-marrow suppression, and nephrotoxicity. Drug resistance, inherent or acquired, also poses a great problem [3]. In an effort to diminish side effects and resistance caused by cisplatin, other metal-based drugs are being investigated.
Metal-based pharmaceuticals can be arranged into seven categories depending on the function of the metal and ligand moieties according to Hambley et al. [4]: 1) the metal complex is active in its inert form, 2) the metal complex is active in its reactive form, 3) the metal serves as a radiation enhancer, 4) the compound contains a radioactive metal, 5) the metal or its biotransformation product is active, 6) a ligand is biologically active, and 7) only a fragment of the complex is active. The two latter categories apply to compounds presented in this review and represent two major strategies explored for the design of metal-containing drugs: targeting and prodrug concept. The targeting strategy uses selective transport of functionalized drug molecules recognized by receptors highly or only expressed on the surface of cancer cells. The prodrug strategy comprises the delivery of a cytotoxic compound that is only activated under conditions present in the targeted tissue or cells. Deactivation by cellular components before reaching the target would ideally be inhibited in the prodrug form. Triggers for prodrug activation can be pH, light or the redox environment. When compared to organic molecules, access to redox chemistry is a clear advantage of metal-containing compounds, since they generally have biologically accessible redox potentials. Accordingly, among the different strategies for releasing metal-containing fragments from prodrugs, the most successful is redox activation, in which the active species is formed in a reductive cellular environment [5].
Tumors are characterized by low oxygen concentration levels which give rise to a more reducing environment compared to normal tissue. The reductive microenvironment of hypoxic tumors results from insufficient formation of new blood vessels during growth [6]. Also, a large concentration of cellular reducing agents like glutathione is present in cancer cells, contributing to a reductive environment. Tumor hypoxia has been linked to unsuccessful treatments using chemotherapy and radiotherapy due to distance from blood vessels and resulting low oxygen levels [7]. Tumor cells that can survive in hypoxic conditions upregulate drug resistance genes [8]. Although hypoxia is considered a serious problem in cancer therapy, it can be exploited for therapeutic selectivity, since it differentiates cancerous and healthy tissue. One promising avenue is the development of bioreductive drugs that are selectively activated under such intracellular conditions. Literature known bioreductively activatable agents comprise quinones, nitroimidazoles, aromatic N-oxides, mustard nitrogens, and a few metal-containing compounds [8, 9]. Metal compounds are to be delivered to the target environment in an inert oxidized state (without prior reduction), and metabolized when they reach the reductive environment of the cancer cell rendering the inactive compound cytotoxic. Three Pt(IV) and two Ru(III) compounds have already been in clinical trials – to date the most promising bioreductive pharmaceuticals based on metals [6].
This review covers applications of metal-based drugs for anticancer applications using the redox-activated prodrug strategy. Electrochemical aspects of redox-activatable Pt and, particularly, Ru compounds have been reviewed in detail recently [6]. Thus, this review focuses on literature that has been published since then and electrochemical aspects will be touched only superficially.
2. Platinum
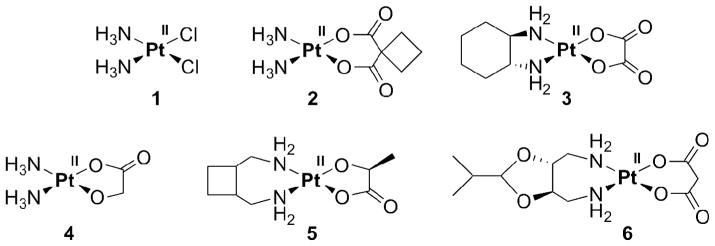
Cisplatin (1, Figure 1), one of the most successful anticancer drugs, and its analogs carboplatin and oxaliplatin (2, 3) are currently in routine clinical use worldwide. Pt anticancer agents only approved in Asia include nedaplatin, lobaplatin, and heptaplatin (4–6).
Figure 1.
Pt-based drugs in worldwide clinical use: cis- (1), carbo- (2), and oxaliplatin (3), and clinical use only in some Asian countries: neda- (4), loba- (5) and heptaplatin (6).
Once in the cell, where the chloride concentration drops from about 100 mM in extracellular fluids to 4 mM, cisplatin is aquated by substitution of its chlorido ligands with water ligands. Only 1% (or less) of the administered cisplatin ends up binding to the biological target DNA, which results in apoptotic cell death. The majority of cisplatin reacts with proteins and low molecular weight biomolecules, especially with those containing sulfur. This non-discriminate binding causes side-effects of cisplatin like nephrotoxicity [3]. Carboplatin (2) displays a more tolerable toxicological profile due to the higher stability of the chelating 1,1-cyclobutanedicarboxylato ligand when compared to the chlorido ligands in cisplatin. No nephrotoxicity is observed [10]. Oxaliplatin (3) is also active against cancer cell lines that are resistant to cisplatin and carboplatin [10].
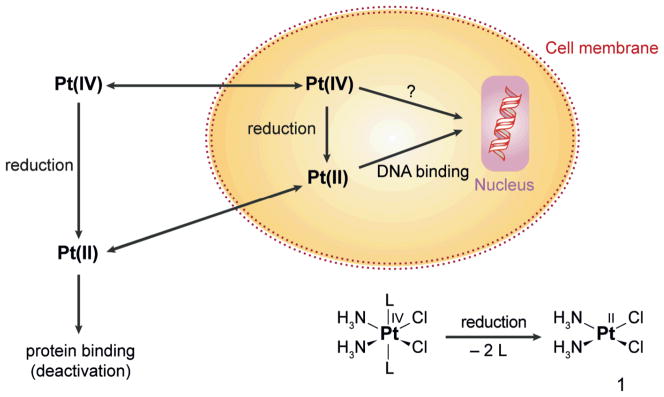
Clinical complications associated with the use of cisplatin, such as nephro- and neurotoxicity and the fact that only some tumors can be cured, might be overcome by using prodrugs with platinum in the more inert +IV oxidation state (Scheme 1) [2]. The administration of non-toxic Pt(IV) prodrugs that can be activated selectively by reduction at the tumor sites might reduce unwanted reactions with biomolecules and thus minimize undesired side-effects. Even oral administration is conceivable, since in contrast to the quite reactive Pt(II), Pt(IV) compounds are more stable in biological fluids. Degradation in the gastrointestinal tract is thus less likely, and the Pt(IV) prodrugs may reach the cellular target without prior transformation.
Scheme 1.
Activation by reduction: A Pt(IV) prodrug carrying axial ligands L, ammine ligands NH3, and chlorido ligands Cl− in the equatorial positions yields cisplatin (1) upon reduction accompanied by the loss of both axial ligands. Most probably, a Pt(II) species then binds to nuclear DNA.
Pt(IV) has a low-spin d6 electron configuration and exhibits octahedral geometry. This configuration is relatively inert to substitution; reactions with biological nucleophiles are thus disfavored compared to Pt(II) complexes, and the lifetime in biological fluids is expected to increase. It is not yet established, however, that Pt(IV) complexes survive long enough in vivo to be delivered to the tumor sites. The platinum compound could be also reduced extracellularly and enter the cell as Pt(II) (Scheme 1). As investigated by XANES (X-ray absorption near edge structure) analyses, the cellular distribution of Pt(IV) compounds 24 h after exposure is indeed similar to that of cisplatin. This result may indicate that the compound is readily reduced over that time period [11]. Microprobe SRIXE (synchrotron radiation-induced X-ray emission) is another excellent method for evaluating Pt compounds in whole and sectioned cells. This method has provided valuable insights into the distribution patterns and oxidation states of Pt drugs in cells [12].
Variation of the axial ligands in Pt(IV) complexes affords a valuable strategy for altering their lipophilicity and redox potentials, factors that may affect the ability to enter tumor cells before being reduced to the active Pt(II) compound and, subsequently, the cytotoxicity. For a series of different Pt(IV) complexes which, upon reduction, yield the same Pt(II) species there was an 800-fold range in cytotoxicity [13]. Although the pharmacology of Pt(IV) compounds seems to depend strongly on the nature of the axial ligands, activity can be also influenced by the potency of the Pt(II) complex formed upon reduction [14].
Lipophilic drugs are supposed to diffuse readily through cell membranes, thereby increasing their uptake. Lipophilicity, however, has to fall within an optimal window, otherwise the complex either becomes too insoluble in aqueous media or is trapped within the membrane. Platinum compounds with intermediate lipophilicity are therefore advantageous for cell uptake and anticancer activity [15].
More electronegative ligands tend to destabilize Pt(IV) compounds. Most easily reduced are those having axial chlorido ligands, and the most difficult are compounds with hydroxido ligands [10, 16]. The latter (L=OH) have reduction potentials that fall in the −900 mV range vs. Ag/AgCl in Pt(IV) complexes cis,trans,cis-[PtCl2L2(en)] and - [PtCl2L2(NH3)2], whereas analogous complexes with axial chlorido ligands (L=Cl) have reduction potentials of ~ −250 mV vs. Ag/AgCl. Related complexes carrying axial acetato ligands fall in between, with reduction potentials ~ −600 mV vs. Ag/AgCl [15, 17–19]. The reduction potential becomes more positive for bulkier carboxylate ligands (propionate, butyrate) [18]. For complexes of formula cis,trans,cis-|PtCl2L2(NH3)2], the net electron donor character correlates well with reduction rates and redox potentials in the axial ligand order L = OH− < alkylCOO− < Cl− < CF3COO− [17].
Potential reducing agents for Pt(IV) in the cell are glutathione [20–22], for which E0 = −240 mV at pH 7.0 [23], ascorbate (vitamin C), NAD(P)H, and cysteine-containing proteins [14, 24]. Metallothionein, a protein consisting of 61 amino acids with 20 cysteine residues, is a potential binding and redox partner for Pt(IV). Increased metallothionein levels are proposed as a mechanism by which cells become resistant to platinum drugs [25]. Serum albumin, a small protein with a 35% cysteine content, also represents a potential binding partner for cisplatin as well as Pt(IV) compounds [19]. Although Pt(IV) can be reduced by both extra- and intracellular reducing agents (Scheme 1), higher concentrations of these species are present within cells; e.g., for glutathione, the levels are 1–10 mM in cells vs. 2 μM in plasma [26]. Besides reducing Pt(IV) complexes efficiently, thiols at high concentrations can also coordinate to and deactivate the resulting Pt(II) species, thereby reducing their efficacy.
The DNA binding properties of Pt complexes of the type cis,trans,cis-[PtCl2L2(en)] correlate well with the reduction potentials; the more readily reduced complexes are better able to bind DNA [18]. Although reduction seems to be required for activity, and reactivity is enhanced in the presence of reducing agents [22, 27], Pt(IV) complexes can apparently also bind directly to DNA (Scheme 1) [28–30]. Reduction of Pt(IV) has even been claimed to occur by DNA itself, either by the nucleobases or the sugars of the sugar-phosphate backbone [14]. Upon arrival at the target site, the kinetic preference of a Pt(IV) complex for reduction by, versus binding to, DNA is then the deciding factor [14]. These arguments, while intriguing, would benefit by isolation and identification of the DNA fragments that are oxidized in the process.
Correlations of Pt(IV)/Pt(II) redox potentials with biological activity have been explored within the context of quantitative structure-activity relationships (QSAR). Several Pt(IV) complexes with various equatorial and axial ligands were synthesized to develop a predictive QSAR model. Theoretical descriptors were included in this model together with physicochemical data like lipophilicity, reduction peak potential, and the number of oxygen atoms in the molecular formula of the ligand [31]. In vitro cytotoxicity could be predicted based on this QSAR model, but the actual effects in vivo still have to be verified, because others have not been able to correlate reduction potential with cytotoxicity across a large series of different Pt(IV) complexes [32].
The effects of axial and carrier ligands on the reduction potential and cytotoxic properties of Pt(IV) complexes have also been investigated to define a relationship between reduction rate, redox potential, and in vitro cytotoxicity of Pt(IV) compounds. The reduction rates depended on the electronegativity and the steric hindrance of the axial and carrier ligands. Complexes carrying bulkier and more electron-withdrawing ligands were reduced more rapidly and showed higher reduction potentials. Reduction rates and the cytotoxicity of complexes with different carrier or axial ligands were correlated [17].
As can be concluded from the above examples and other factors, the selection of drug candidates based on in vitro activity can be misleading. The most active species is usually chosen for animal studies, whereas the most inert species in vitro might actually show the highest activity in vivo [4]. It is therefore important that the Pt(II) species derived from a Pt(IV) precursor have proven in vivo activity. The effects of simulated hypoxia and the use of models like spheroids, which mimic the tumor microenvironment, have been examined to predict the behavior of compounds in solid tumors. For example, a spheroid-based tumor model revealed that Pt(IV) complexes, although active, were unable to display selectivity for hypoxic cells [33].
In octahedral Pt(IV) complexes, the two additional ligands are not only valuable for modifying solubility in biological media and tuning the redox potential, but they can also provide targeting functionalities. Modifications at the axial sites offer the advantage of selectivity against cancerous tissue. This feature complements the potential for Pt(IV) complexes to be administered orally due to greater stability in the gastrointestinal tract as well as their potentially lower side effects due to their greater kinetic inertness compared to Pt(II) compounds. Targeting, directing a Pt(IV) complex to tumor cells and/or a subcellular target therein, can be accompanied by attachment of a membrane receptor substrate to an axial ligand or, alternatively, by release of therapeutically active axial ligands following reduction to Pt(II) in the cancer cell.
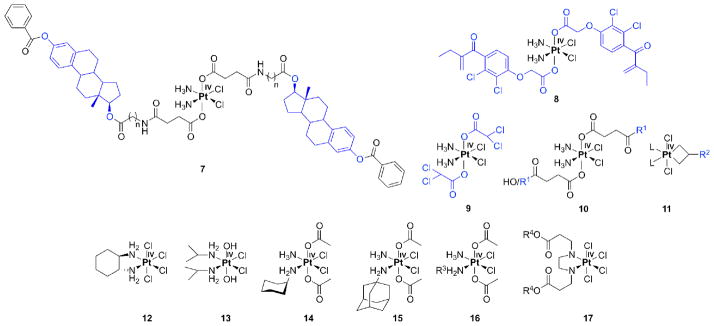
For targeting estrogen-receptor positive (ER(+)) cancers like breast and ovarian, estradiol-tethered Pt(IV) complexes have been employed (7, Figure 2, targeting groups are depicted in blue). Reduction of the Pt(IV) complex in the intracellular environment releases cisplatin and two equivalents of estradiol. The latter can upregulate expression of the protein HMGB1, which can prevent repair of cisplatin-damaged DNA by shielding the platinated DNA from the excision repair machinery [34]. Such upregulation of HMGB1 has the potential to sensitize cells to cisplatin. Cytotoxicity in ER(+) cells increased by up to 2-fold when compared to ER(−) cells [35].
Figure 2.
Pt(IV) compounds with bioactive ligands (in blue, 7–11): estrogen-tethered Pt(IV) complex (7, n=1–5), ethacraplatin (8), mitaplatin (9), peptide tethered Pt(IV) (10, R1 = RGD, NGR, c(CRGDC), c(RGDfK)), platinacyclobutane (11, L = pyridine for R2 = thymidine, proline, and L = 2,2′-bipyridine for R2 = glucose, cholesterol). Tetra- (12), ipro- (13), satraplatin (14), LA-12 (15), cis,cis,trans-[PtCl2(NH3)(NH2R3)(OOCCH3)2] (16, R3 = H, isopropyl, cyclohexyl), [PtCl4(R42eddp)] (17, R4 = ethyl, n-propyl).
For overcoming drug resistance, ethacrynic acid was coupled in another Pt(IV) construct termed ethacraplatin (8). The cytosolic enzyme glutathione-S-transferase (GST) was inhibited by the two equivalents ethacrynic acid released per Pt(IV) upon reduction. In three out of four cell lines the cytotoxicity was increased. After short incubation times (24 h) ethacraplatin was up to 2.5 times more effective than cisplatin as measured by IC50 values, whereas after 72 h both compounds displayed comparable cytotoxicity [36].
In pursuit of a similar prodrug strategy, the Pt(IV) compound mitaplatin, cis,cis,trans-[PtCl2(NH3)2(OOCCHCl2)2] (9), was prepared. Mitaplatin combines the orphan drug dichloroacetate (DCA) with cisplatin. DCA alters the mitochondrial membrane potential gradient in cancer but not normal cells. As a consequence, cytochrome c is released and apoptosis inducing factor is translocated to the nucleus [37]. Mitaplatin can thus efficiently target both nuclear DNA with released cisplatin and mitochondria with released DCA in cancer cells. The cytotoxicity of mitaplatin equals or exceeds that of most other Pt(IV) compounds and is comparable to that of cisplatin in a variety of cancer cell lines [38].
Small peptides recognizable by cancer tissue-specific receptors, the integrins, have also been used as targeting moieties. In order to target cells expressing integrins on their surface, Pt(IV) was coupled to several small peptides (3–5 amino acids) containing RGD or NGR by either one or two amide linkages through either one or two succinato groups (10, R1 = RGD, NGR, c(CRGDC), c(RGDfK)) [39]. Cytotoxicity was tested in several endothelial and human cancer cells. RGD-tethered Pt(IV) complexes were more cytotoxic than non-targeting Pt(IV) compounds and RGD tri- and pentapeptide moieties alone. NGR conjugates were less inhibitory than RGD counterparts, but were still more active than nonspecific Pt(IV)-peptide analogues.
Platinacyclobutane complexes (11), in which biologically relevant molecules like thymidine, cholesterol, glucose, and proline (R2), are linked to cyclobutane moiety in an equatorial position, have also been synthesized. The biocomponents were coupled to cyclopropylmethanol and then allowed to react with Zeise’s dimer ([Pt(C2H4)Cl2]2) resulting in platinacycles. It is anticipated that the presence of the biomolecules would lead to increased water solubility and cancer targeting, but thus far there has been no evaluation of these compounds either in vitro or in vivo [40, 41].
Pt(IV) compounds lacking biologically active ligands have also been quite successful in vitro. Four such octahedrally coordinated Pt(IV) complexes have even entered clinical trials, namely, tetra- (12), ipro- (13) and satraplatin (14, formerly JM-216, diacetatoam-minedichlorido(cyclohexylamine)platinum(IV)) as well as LA-12 (15). LA-12, however, failed in phase I. Tetraplatin could not be investigated further after phase I studies due to its high neurotoxicity. Iproplatin showed only limited success in phase II due to its low reactivity. Satraplatin, the first orally available Pt-based drug candidate, had to be abandoned recently in phase III [42]. Thus none of the platinum(IV) compounds has yet found its way to the clinics because of lower efficacy than cisplatin, variability in drug uptake, or the production of severe side-effects [6]. Satraplatin, although failing to provide an increase in overall survival above statistical significance when compared to cisplatin, was better tolerated and showed no signs of inducing nephrotoxicity [43].
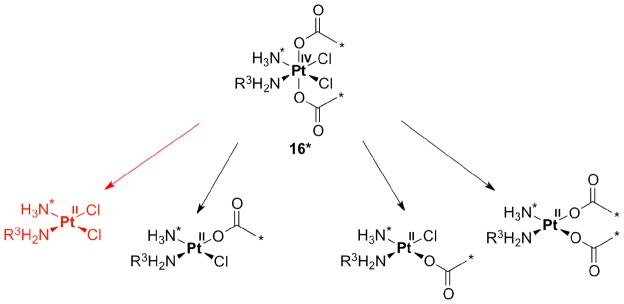
The initial success of the acetato complexes satraplatin (14) and LA-12 (15) motivated researchers to investigate reduction pathways for Pt(IV) complexes having acetato ligands in the axial position. Reduction of 13C- and 15N-labelled cis,cis,trans-[PtCl2(15NH3)(NH2R3)(OOC13CH3)2] (16*, R3 = H, isopropyl or cyclohexyl) was effected by different reducing agents like ascorbate, cytochrome c, NADH, or glutathione. Four reduction products were identified by monitoring the reactions using two-dimensional NMR spectroscopy and ESI-MS. In addition to the anticipated cisplatin analogue generated by loss of the axial acetato ligands (Scheme 2, red), additional products formed by elimination of one acetate and equatorial chloride (middle structures, in Scheme 2) or two chlorido ligands (structure at the right, Scheme 2).
Scheme 2.
Possible products following reduction of isoptopically labeled 16*.
These results challenge the commonly held supposition that, upon reduction, Pt(IV) compounds derived from cisplatin and related Pt(II) complexes, only release their axial ligands; this assumption might have to be reassessed for Pt(IV) derivatives carrying axial acetate groups [44].
The reduction of satraplatin (14) in extracts from three different cancer cell lines was monitored by 2D NMR spectroscopy. The cellular reduction rates followed the order A2780cisR > A2780 > HT-29, where HT-29 are colon cancer cells and A2780cisR is a cisplatin resistant cell line obtained from A2780 ovarian cancer cells by exposure to cisplatin. Reduction of satraplatin was not performed by low molecular weight (MW) anti-oxidants such as ascorbate and glutathione, but primarily by cellular components having MW > 3000 [44]; the metalloproteins cytochrome c and hemoglobin in the presence of NADH were competent to reduce satraplatin [45]. The most rapid reduction occurred in A2780cisR cell extracts, perhaps due to its intrinsically elevated levels of glutathione [46].
Finally, a novel Pt(IV) complex [PtCl4(R42eddp)], where R4 = ethyl or n-propyl (17), containing esters of ethylenediamine-N,N′-di-3-propionic acid (eddp) as bidentate ligands, was described. Although the ligands themselves were not cytotoxic, the complexes had IC50 values at least ten times higher when compared to cisplatin. The cytotoxic activity was correlated with Pt uptake. The compound having R4 = n-propyl reacted with plasmid DNA as judged by gel electrophoresis studies [47].
3. Ruthenium
The biological activity of Ru compounds was first recognized in the 1950s [48], and reports of their anticancer activity appeared in the 1960s [49]. Several Ru-based compounds show significant efficacy against various types of tumors in vivo [50] while having lower toxicity than cisplatin in vitro [51, 52].
Of particular interest are Ru(II) arene compounds and bioreducible Ru(III) complexes with heterocyclic N-donor ligands. The only non-platinum transition metal compounds currently in clinical trials are two Ru coordination compounds of the latter class, [ImH][trans-RuCl4(DMSO)Im] (NAMI-A, 18, Figure 3) and [InH][trans-RuCl4In2] (KP1019, 19) [53, 54]. The first Ru-based anticancer drug candidate in clinical trials was NAMI-A, followed by KP1019 in 2003. Both have successfully completed phase I. This achievement focused much attention on the medicinal properties of Ru compounds, reviews of which are available [55–60]. KP1019 is active against colon cancer [5]; NAMI-A has only low activity against primary tumors. NAMI-A is anti-angiogenic; its anti-invasive properties render it active against metastatic cancer.
Figure 3.
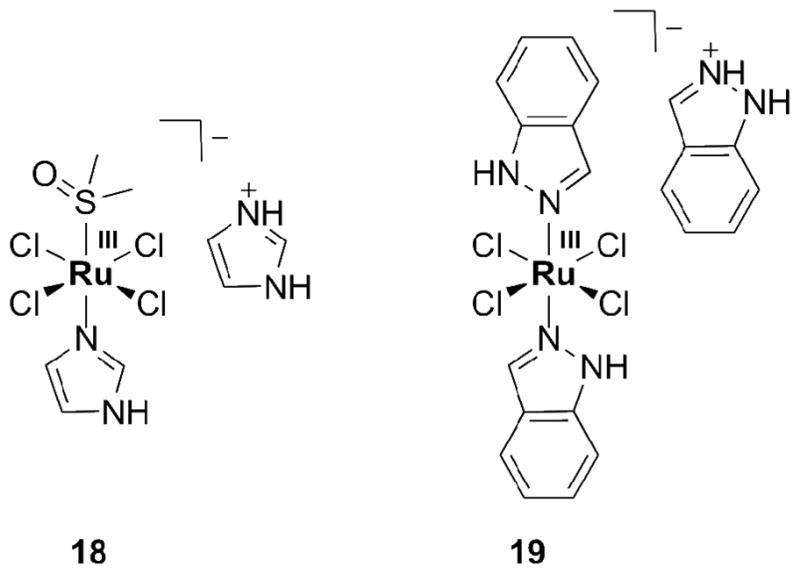
Ru(III) compounds NAMI-A (18) and KP1019 (19).
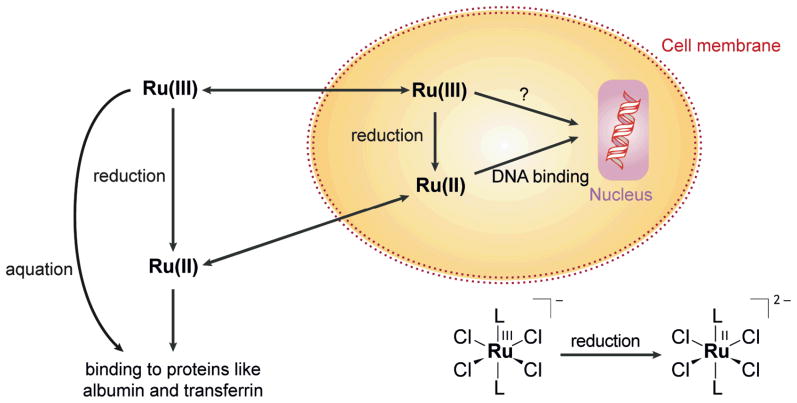
The mechanism of action of these Ru(III) compounds remains unknown and their in vivo chemistry is ambiguous [52]. Although Ru(III), like Pt(IV), can be reduced by ascorbate or glutathione under physiological conditions, the resulting Ru(II) complexes maintain their octahedral ligand set (Scheme 3) [54]. The biological target of Ru compounds has not been discovered. DNA adducts can be formed by both NAMI-A and KP1019. The resulting Ru-DNA adducts alter the duplex conformation [61]. KP1019 can unwind and bend DNA [62]. As for Pt(II) compounds, sulfur-containing biomolecules might bind to reduced Ru(II) species before they reach nuclear DNA.
Scheme 3.
Reduction pathways of Ru(III) compounds inside and outside the cell.
NAMI-A undergoes aquation reactions within minutes [54], whereas KP1019 is more stable, and better taken up by cells. About half of the intracellular Ru delivered in the form of KP1019 appears in the nucleus, which is quite high by comparison to other metal complexes; only 10% of cisplatin accumulates within the nucleus [63]. Ru compounds not only target DNA but also proteins. KP1019 binds to transferrin [64] (Scheme 3), an Fe(III) transport protein, and is released from the protein as Ru(II) after reduction by ascorbate or glutathione [6]. Interference with the Fe metabolism may also be an explanation for the anticancer activity of Ru(III) complexes [54].
The antitumor activity of Ru(III) compounds is postulated to depend on its reduction to Ru(II), since an increase in NAMI-A anti-metastatic activity occurs in the presence of biological reductants [55]. The more readily reduced Ru(III) complexes are more cytotoxic [65].
Transformations of Ru(III) complexes in mammalian cells have been monitored by XANES and EXAFS (X-ray absorption fine structure). It was concluded from these measurements that Ru(III) mainly binds to N-donors; however, gel filtration studies showed that Ru(III) was completely protein-bound [54]. A comparative study of a series of Ru(III) compounds using X-ray absorption spectroscopy (XAS) at the Cl K-edge and Ru L-edge revealed that the indazole ligand (In) in KP1019, a weak donor toward Ru, facilitates facile reduction [52]. As for Pt, the use of microprobe X-ray fluorescence might provide information about a potential Pt(IV)-like ‘activation by reduction’ [6] and clarify the role of transferrin in Ru(III) transport [53].
Ru(II)/Ru(III) redox chemistry has been studied thoroughly as an aspect of the mechanism of action of Ru(III) prodrugs, recently reviewed [6]. The redox potentials of KP1019 and NAMI-A are 30 mV and 25 mV, respectively, vs. NHE. The redox potentials are tunable by modification of the azole ligand (In and Im, respectively) [6].
These redox potential values reveal that NAMI-A and KP1019 can be reduced by glutathione and ascorbate under physiological conditions [66, 67]. As described in the introduction, in addition to providing reducing agents, cancer cells also harbor a hypoxic environment that promotes reduction and subsequent reactivity [52]. Increased levels of Ru-DNA adducts occurred when the O2 partial pressure was low, indicating a greater amount of educed, Ru(II), species [68].
Ru(III) phosphane complexes in which the metal is coordinated by two P,O,O-tridentate tris(o-anisyl) phosphane ligands are highly cytotoxic even in cisplatin-resistant cell lines [69]. Another construct involves NAMI A-type ligands conjugated through pyridyl or bipyridyl rings to yield Ru porphyrin conjugates (meso-4′-tetrapyridylporphyrin or meso-(p-bipyridyl-phenyl)porphyrin). The number of Ru fragments attached to the porphyrins ranged from 1 to 4. Conjugation of porphyrins to the Ru center was an attempt to obtain additive antitumor effects originating from the phototoxic and tumor-localizing properties of the porphyrin together with the cytotoxic properties of Ru(III) [70]. Thus far, however, the compounds have not been tested in vitro or in vivo.
An interesting conjugate was generated by coupling tamoxifen, a chemotherapeutic agent for patients with hormone-dependent breast cancer, with the organometallic Ru compound ruthenocene. This tamoxifen analog acts as an antiestrogen by competitive binding to the estrogen receptor in ER(+) breast cancer cells but not in ER(−) cells [71, 72]. This result is in contrast to that for the Fe analog ferrocifen (20), which is active in both cell lines (vide infra). Since the structural differences between the Fe and Ru derivative are marginal, the different redox properties of the metal ions may be responsible for the different patterns of activity [71].
Although Os belongs to the same group in the periodic table as Fe and Ru, and thus should be also able to interact with biomolecules like proteins and nucleic acids, there are only a few studies on the biological activity of Os complexes. In comparison to analogous Ru compounds, Os complexes are more substitution inert and less prone to hydrolysis and interactions with nucleobases. Nevertheless, they also have potential as anticancer agents. By examining homologous Os compounds, one might even obtain useful information about the mechanism of action of Ru drugs [73–75].
Os(III)-NAMI-A-type compounds (LH)[trans-Os(III)Cl4(DMSO)(L)] with L = 1H-indazole (In), 1H-pyrazole, 1H-benzimidazole, 1H-imidazole (Im), 1H-1,2,4-triazole, and DMSO, are kinetically stable in aqueous solution, but exhibit similar or even higher cytotoxicity when compared with the analogous Ru compounds. For example, the indazole complex (L = In) had an IC50 value ca. ten times lower than that of the analogous Ru complex against HT-29 cells. Whereas hydrolysis is a prerequisite for the antimetastatic activity of NAMI-A in vivo, hydrolyzed species were not required for the in vitro antiproliferative activity of analogous Os(III) complexes [76, 77].
4. Iron, Cobalt, Copper
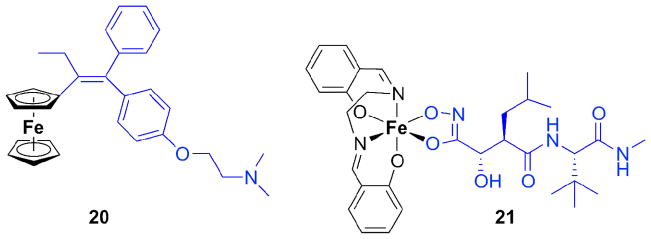
Ferrocifen (20, Figure 4), like the corresponding Ru compound described above, is an organometallic derivative of the breast cancer drug tamoxifen. The ferrocene analog of hydroxytamoxifen, however, acts against both ER(+) and ER(−) human breast cancer cells, in contrast to the properties of hydroxytamoxifen alone [62, 78]. This behavior is surprising since the latter cells lack an obvious molecular target for tamoxifen. It can be concluded that the antiproliferative effect stems from the antiestrogenic effect of the tamoxifen moiety plus the cytotoxicity of the redox-active ferrocenyl group. Ferrocene interconverts inside the cell between oxidation states II and III, represented by ferrocene and ferrocenium ions. The cytotoxicity is not caused by direct linkage to DNA but by formation of reactive oxygen species, which can damage DNA.
Figure 4.
Ferrocifen (20), a ferrocene derivative of tamoxifen, shown in blue. The third phenyl ring in tamoxifen is replaced by ferrocene. At the right is an Fe(III) complex (21) of the MMP inhibitor marimastat, depicted in blue.
Not only ferrocene derivatives containing Fe(II) but also ferrocenium derivatives containing Fe(III) are cytotoxic, generating radicals and thus inducing DNA damage [79]. Pyrazole conjugated to ferrocene serves as a ligand (L) for Co, Ni, and Fe (M), forming ML2 and ML3 complexes, respectively. The ligand L and the three ML2 metal complexes induced cytotoxicity in MCF-7 breast cancer cells, with IC50 values ranging from 46 to 73 μM; CoL2 exhibited the lowest IC50 value. As the redox potential increased, the toxicity of the metal complexes decreased [80].
Marimastat was once considered to be a promising anticancer drug, acting by inhibiting MMPs (matrix metalloproteinases) overexpressed in cancer cells. Clinical development of marimastat was discontinued, however, due to the lack of therapeutic benefits in patients. The hydroxamic acid portion of the compound (blue in 21, Fig. 4) may have reacted avidly with metal ions in biological fluids, especially Fe(III) [81]. The potential for high metal affinity was intentionally exploited subsequently to generate a metal-carrying prodrug containing marimastat and other ligands. A tetradentate ligand set was provided by salen (N,N-bis(salicylidene)-ethane-1,2-diimine) (cf. black part in 21). The octahedral Fe(III) marimastat salen complex (21) inhibited MMP activity in vitro approx-imately 30-times less efficiently than marimastat alone. Reduced inhibitory activity by Fe complexation of the hydroxamate functionality in marimastat and electrochemical data indicate a potential for developing a prodrug that might be activated bioreductively [82].
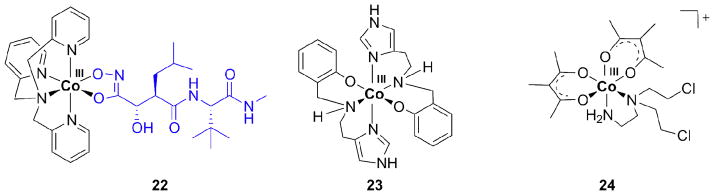
Co(III) has also been complexed by the hydroxamate moiety in marimastat, although the mechanism of action of such Co complexes is expected to differ from that of analogous Fe(III) compounds, which are prone to generate reactive oxygen species. Instead, Co(III) can be reduced to Co(II) in biological environments, leading to the release of marimastat. Co(III) thus provides an inert framework for the transport of the MMP inhibitor, protecting the hydroxamate moiety prior to reaching the tumor. In this case, Co is not the active species itself but only a protecting group for an otherwise cytotoxic ligand. Similarly, Au(I) has been claimed to protect phosphane ligands from oxidation in Au complexes like auranofin used for the treatment of rheumatoid arthritis [83].
As in the Fe(III) complex, a tetradentate ligand, here tpa for tris(methylpyridyl)-amine, was combined with the hydroxamate to form the octahedral complex (22, Figure 5) [81, 84]. Increased cytotoxicity was observed for the prodrug in comparison to the inhibitor alone. In an in vivo study free marimastat and its Co complex inhibited tumor growth in the mammary fat pad in mice; but only the Co complex showed a statistically relevant difference compared to the control group. The complex was 2–3 times more effective in reducing the tumor growth than the MMP inhibitor alone. Both marimastat and its Co complex, however, potentiated metastasis when compared to controls [81].
Figure 5.
Examples of Co(III) complexes with marimastat (22), N2O donor ligands (23), and acetylacetonato ligands (24).
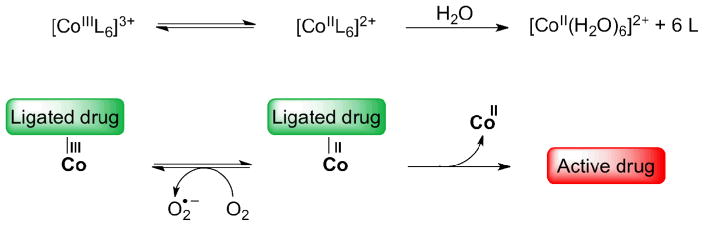
Co(III) complexes have reduction potentials in a range appropriate for being reduced under cellular conditions. In oxic cells, however, this is inhibited by the presence of oxygen, which can compete with the complex for cellular reductants (redox cycling, Scheme 4) [85, 86]. Thus, in vivo, back-oxidation is prevented by the hypoxic nature of the tumor tissue [8]. Co(II) complexes generated from Co(III) prodrugs under hypoxic conditions can undergo substitution reactions to release their neutral ligands, ideally cytotoxic drugs, and to form hexaaqua species (Scheme 4). Ligands that have been studied thus far include nitrogen mustards [87–90], amines [91], quinolines, macrocyclics [92], and tripodal ligands such as the one in the complex with marimastat (22) [4, 81, 93].
Scheme 4.
Prodrug mechanism based on Co(III) complexes [1]. Redox cycling in the presence of oxygen and release of the active drug by substitution with aqua ligands under hypoxic conditions.
The redox behavior of Co(III) complexes with tridentate N2O donor ligands (as in 23) was investigated and reduction was found to occur at −1000 to −800 mV vs. NHE. The reduced, Co(II) forms of the compounds, inhibited cell growth of S. cerevisae more efficiently than cisplatin (IC50 0.5 vs. 0.6 mM). The oxidized Co(III) forms were less cytotoxic than the reduced forms, indicating that the Co(III) species serve as prodrugs. A compound analogous to 23 in which the imidazoles were replaced by pyridines behaved similarly [94].
Nitrogen mustards are highly toxic due to their DNA cross-linking activity. In vivo they act unselectively, but can be deactivated by coordination to Co(III) and be released when reduction to Co(II) occurs in hypoxic tumor tissue, thereby reducing systemic toxicity [91, 95].
Some nitrogen mustard ligands showed hypoxic selectivity in tumor cells [95]. The Co complex [Co(Meacac)2(DCE)]+ (24, acac = acetylacetonate, DCE = N,N-bis(2-chloroethyl)ethylenediamine) had 20-times greater activity against hypoxic than oxic cancer cells [96]. In a series of Co acac complexes, the redox potential was of importance for hypoxic selectivity. The optimal value was −305 mV vs. NHE [96]. For 24, hypoxic selectivity did not rely on redox cycling, i. e. reoxidation by O2 in oxic tissue, because reoxidation was too slow relative to ligand release [8, 85].
Co(III) cyclen complexes having azachloromethylbenzindoline in the coordination sphere can be reduced under hypoxic conditions to release this agent, a potent DNA minor groove alkylator [92].
The chemistry of copper is attractive for the development of hypoxia selective drugs, because Cu has two oxidation states and the reduction potential in accessible within the cellular potential range [97].
As for Co(III), Cu(II) complexes can be reduced to form Cu(I) complexes of low stability to release an active ligand. Furthermore, radioactive isotopes of Cu can be used, providing the combination of radiation therapy with bioreduction. For this purpose, the tirapazamine ligand has been suggested as a hypoxic cytotoxin for use with radioactive Cu, combining the antitumor activity of the copper complex with its radioactivity, the Cu isotopes being 64Cu or 67Cu [98]. Radiolabelled 64Cu complexes carried by bis(thiocarbazone) and bis(salicylaldimine) ligands showed hypoxic selectivity against Chinese hamster ovary cancer cells [99].
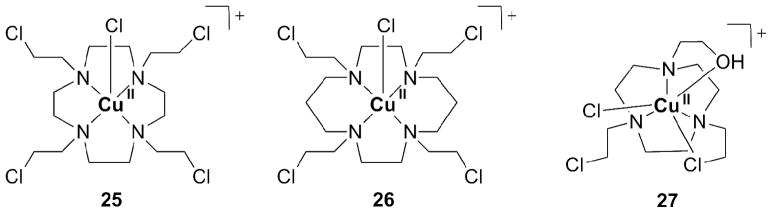
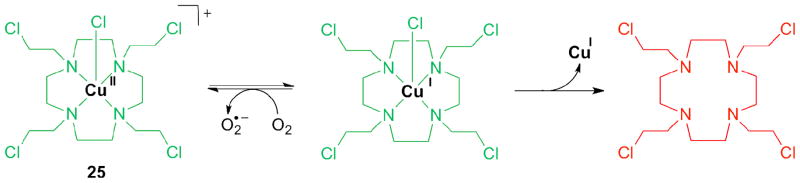
Macrocyclic ligand systems used for Cu(II) include cyclen, cyclam and tacn. Complexes with nitrogen mustards based on these macrocyclics are cytotoxic against leukemia cells. The complexes were also cytotoxic in lung cancer cells selectively under hypoxic conditions. The stability of the reduced Cu(I) species was investigated by cyclic vol-tammetry. Its redox chemistry is reversible, indicating that the reduced complex is stable. A Cu(II) complex with a mustard derivative of cyclen (1,4,7-tetraazacyclodecane, 25, Figure 6) exhibited good aqueous stability and, in vitro a 24-fold increased cytotoxicity under hypoxic conditions vs. oxic conditions. In aqueous solution, the redox behavior of the complex and its stability correlated well with hypoxia selectivity. The hypoxia selectivity is thought to originate from redox cycling in oxic tissue, similar to the behavior of Co(III) prodrugs described above (Scheme 5). Complexes 26 and 27, derived from the macrocyclic ligands cyclam and tacn, respectively, release the ligand independent of O2 partial pressure. This result indicates that bioreduction is less relevant for the latter systems, excluding their applications as prodrugs [89].
Figure 6.
Examples of Cu(II) complexes for releasing nitrogen mustards based on cyclen (25), cyclam (26), and tacn (27).
Scheme 5.
Mechanism of prodrugs based on a Cu(II) nitrogen mustard cyclen complex (25).
Copper diacetyl-bis(N4-methylthiosemicarbazone) is a promising compound for imaging hypoxic tissue based on PET using Cu radionuclides (60Cu, 61Cu, 62Cu, and 64Cu). A lower, i.e. more negative, reduction potential corresponded with increased selectivity of bioreduction and release of the ligand. Insight into the chemical and electronic properties underlying these previously observed structure-activity relationships was obtained recently by density functional theory (DFT) calculations [100].
Cu(II) complexes with ligands based on 3-aminoquinoxaline-2-carbonitrile-N1,N4-dioxide were developed to serve as selective hypoxic cytotoxins [101]. The complexes were evaluated under hypoxic and aerobic conditions in V79 cells. The complexes were equally low cytotoxic with the ligands under aerobic conditions, but more cytotoxic under hypoxic conditions [102].
Quinoxaline-N1,N4-dioxides themselves are bioreductively activatable drugs. However, they exhibit low aqueous solubility and a short half life [103]. Their vanadyl complexes VOL2, with L being a quinoxaline-N1,N4-dioxide ligand, however, showed improved solubility and greater cytotoxicity than the free quinoxaline ligands, with high hypoxia selectivity [104].
Pyrophosphate-bridged binuclear complexes of Cu(II) display low nanomolar toxicity against adriamycin-resistant ovarian cancer cells. The complexes were tested in glutathione assays to simulate cellular conditions. Cu(I) was formed in this reductive environment and the system produced hydrogen peroxide from molecular oxygen, producing oxidative stress. The generation of oxidative stress might explain the high cytotoxicity of this class of coordination compounds [105].
5. Nanobased Drug Delivery Systems
Delivery systems can mediate transport of drug molecules to desired cell populations. Nanosized systems have been successfully applied in the last years, exploiting the EPR, or enhanced permeability and retention, effect as a means of passively targeting cancer tissue. A combination of metal prodrugs with nano-vehicles can be achieved either by surface tethering or encapsulation. Encapsulation protects the drug from degradation before reaching cancer cells as well as the non-cancerous cells from the effects of the drug. Depending on the encapsulating material, controlled release of the drug from the nanoparticles is possible. Peptides, antibodies, or aptamers can be attached to the particle surface for active targeting of cancer tissue. In this section, we describe accumulation and active targeting as strategies for selectively delivering Pt(IV) prodrugs to cancer cells with the help of nanoparticle materials. Although more stable in the +IV oxidation state, Pt(IV) compounds are not impervious to degradation, as we have already discussed (section 2). Their combination with nanomaterials offers a promising opportunity to transport and protect these complexes from premature reduction in the blood stream.
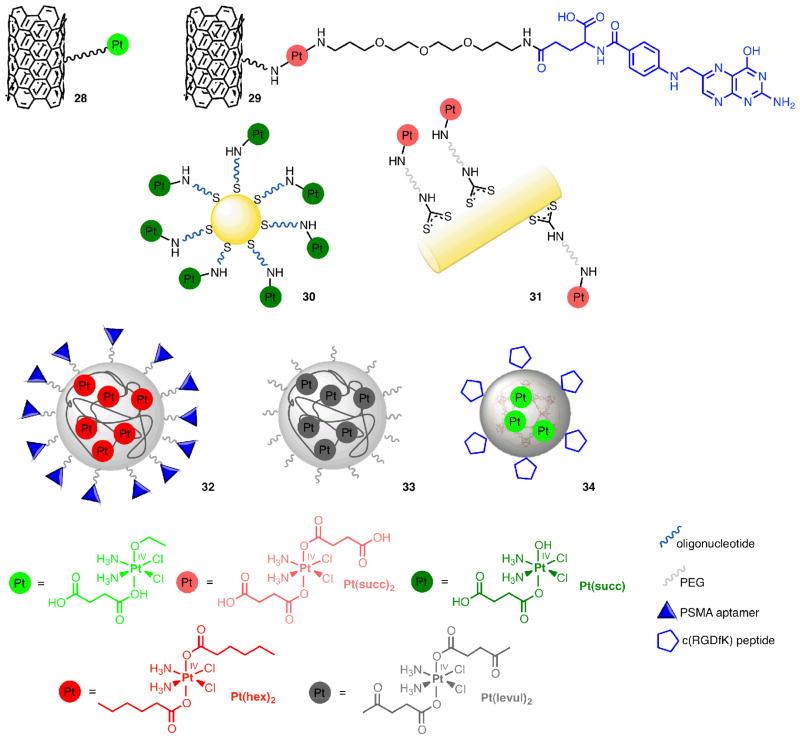
In one manifestation of this strategy, Pt(IV) was attached to single-walled carbon nano-tubes (SWNT) for shuttling into cells (28, Figure 7). Such soluble functionalized SWNTs nanotubes cross the cell membrane by clathrin-dependent endocytosis. A substantial increase in cytotoxicity was obtained when compared to cisplatin and the untethered Pt(IV) complex. In one experiment, the SWNT “longboat” carried 65 Pt “passengers” as determined by atomic absorption spectroscopy [106]. For targeting of folate receptors on cancer cells, the Pt(IV) prodrug was also coupled to folate (29) by using a bisuccinate precursor, Pt(succ)2, Figure 7. One carboxyl group allowed coupling to folate via an amino linker, while the other allowed tethering to the aminated nanotube surface. The toxicity in cell lines expressing the folate receptor increased by up to 9-fold by comparison to cisplatin [107].
Figure 7.
Metal-based prodrugs based on carbon nanotubes (28, 29), Au nanoparticles and -rods (30, 31), polymeric nanoparticles made of PLGA-PEG (32) and PLA-PEG (33), and iron-carboxylate nanoscale metal-organic frameworks with silica coating (34). Loaded or coupled Pt(IV) compounds are shown below. As above (Figures 2, 4 and 5), targeting units are shown in blue.
Gold nanoparticles have also been used as delivery systems for potentially therapeutic antisense oligonucleotides as well as Pt(IV) via covalent attachment (30). The nanoparticles were functionalized with thiolated 28mer oligonucleotides containing a terminal dodecyl amine for conjugation. The Pt(IV) complex Pt(succ) (Figure 7) was tethered to the amino-functionalized DNA-Au-NP surface by forming an amide linkage [108]. The constructs showed high levels of cellular uptake in different cell types and higher cytotoxicity when compared to cisplatin and the Pt(IV) precursor Pt(succ).
Gold nanorods, on the other hand, are even more promising vehicles than gold nanoparticles due to longer circulation times when compared to the spherical particles. PE-Gylated nanorods were loaded with disuccinato Pt(IV) (Pt(succ)2, 31, Figure 7). Cytotoxicity in HeLa, A549, and MCF7 cells was increased by up to 66-fold when compared to cisplatin. Cytotoxicity could be correlated with Pt uptake as measured by ICP-MS [109]. Better protection of the Pt(IV) prodrug can potentially be provided by encapsulation into particles as opposed to tethering on their surface. Encapsulation protects the prodrug but also can ameliorate unwanted side-effects of the drug. Cisplatin itself shows only low loading efficiency (< 1 wt%) within the hydrophobic interiors of a polymer [110], but an increase of internalization into nanoparticles could be achieved by using a hydrophobic Pt(IV) compound instead. Nanoparticles based on poly(D,L-lactic-co-glycolic acid)-block-poly(ethylene glycol) (PLGA-b-PEG) combine hydrophobic PLGA for encapsulation and hydrophilic PEG for aqueous solubility [111]. A Pt(IV) compound carrying hydrophobic hexyl chains, Pt(hex)2 (Figure 7), was released from PLGA-PEG nanoparticles in a controlled fashion over 60 h. The nanoparticles were taken up by cells by receptor-mediated endocytosis. Besides being designed for passive targeting (EPR), the nanoparticles were also expected to be guided to tumor tissue by active targeting. For targeting prostate-specific membrane antigen (PSMA) over-expressed on prostate cancer cells, an A10 2-fluoropyrimidine RNA aptamer was coupled to the surface of the nanoparticle (32) [111]. This system showed better cytotoxic activity in vitro than cisplatin and selectivity for the cells expressing the receptors targeted. In vivo studies with the PSMA-decorated Pt(IV) nanoparticle (32) demonstrated enhanced pharmacokinetics, biodistribution, and tolerability in rats and mice when compared to cisplatin. Efficacy in a PSMA-expressing LNCaP xenograft mouse model of prostate cancer was also higher. For obtaining the same degree of tumor volume reduction only 1/3 the dose of cisplatin was required [112].
Conjugation of dilevulinate Pt(IV) complexes Pt(levul)2 (Figure 7, levulinic acid = 4-oxopentanoic acid) with a hydrazinated PEG-PLA diblock copolymer resulted in Pt(IV) loaded nanoparticles (33). The nanoparticles were of sub-100 nm size and exhibited a cisplatin loading yield of 1.1 wt%. Due to the acid-labile hydrazone bond, the system showed acid-responsive drug release kinetics. The ability to kill ovarian cancer cells was enhanced when compared to that of cisplatin [113].
A silica shell coating of coordination polymer nanoparticles based on Pt(succ)2 increased half-release time for Pt by factor 9 - from 1 h for the uncoated Pt loaded polymer to 9 h. Cytotoxicity was similar to that of cisplatin in breast cancer cells. Upon conjugation of integrin-targeting peptides c(RGDfK), in vitro toxicity against colon cancer cells could be slightly increased in comparison to cisplatin [114].
Lastly, ethoxysuccinato Pt(IV), the prodrug also used for coupling to SWNT in 28, was allowed to react postsynthetically with a dispersion of amino functionalized iron-carboxylate nanoscale metal-organic frameworks. Once Pt was loaded into the framework, the particles were coated with silica. The resulting material was then evaluated for its cytotoxicity in HT-29 cells and found to be slightly less cytotoxic than cisplatin. In order to increase the cytotoxicity, the silica shell was functionalized with a silyl derivative of c(RGDfK) (34). As in the other examples described above, it was thereby armed for targeting integrins, over-expressed in many anigiogenic tumors. Cytotoxicity was thus increased, approaching that of cisplatin [115].
6. Conclusion
Therapeutically active complexes of Pt and Ru demonstrate that metal complexes can play an important role in the treatment of cancer. It has been estimated that approx-imately half the patients being treated for cancer today by chemotherapy receive a platinum compound. The motivation for seeking other metal complexes with therapeutic potential and for developing new metal-based drugs comes especially from the success of the anticancer drug cisplatin. Also bioorganometallic chemistry, a relatively new branch of medicinal inorganic chemistry, has contributed to the field, as exemplified by the tamoxifen analog ferrocifen.
In such complexes the tunable redox properties of the metal ion and ligand modifications can be exploited to control biological action. Ligand loss during reduction in hypoxic cancer tissue can activate metal complexes for binding to target molecules. Furthermore, as for Co and Cu, the loss of the ligand in a reductive environment can also trigger the release of an active species, a molecule that would have shown systemic toxicity if not bound to the deactivating metal center.
In the future, the design of metal drugs should focus on molecular targeting agents to provide greater selectivity and more effective drug administration. The activation by reduction strategy presented in this review is one strategy by which this goal can be approached. Metal complexes exhibiting targeting, both to cancer cells as well as to sub-cellular targets therein, and selective activation properties can reduce side effects in therapy and potentially cure a wider range of cancers by circumventing resistance. The many opportunities that metal complexes have by comparison to organic molecules, especially their versatile redox chemistry, should be exploited for creating more efficient anticancer drugs.
Acknowledgments
N.G. thanks DAAD (German Academic Exchange Service) for a fellowship and a reintegration grant. This work was supported by grant CA034992 from the National Cancer Institute.
Abbreviations
- acac
acetylacetonato
- cyclam
1,4,8,11-tetraazacyclotetradecane
- cyclen
1,4,7,10-tetraazacyclododecane
- DCE
N,N-bis(2-chloroethyl)ethylenediamine
- eddp
ethylene-diamine-N,N′-di-3-propionic acid
- en
ethylenediamine
- EPR
enhanced permeability and retention
- EXAFS
extended X-ray absorption fine structure
- hex
hexanoato
- HMGB
high-mobility group box
- Im
1H-imidazole
- In
1H-indazole
- levul
levulinato (4-oxopentanoato)
- MMP
matrix metalloproteinase
- MW
molecular weight
- NAMI-A
New Anti-tumor Metastasis Inhibitor, -A means that this is the first of a series
- NHE
normal hydrogen electrode
- PSMA
prostate-specific membrane antigen
- QSAR
quantitative structure-activity relationship
- salen
N,N′-bis(salicylidene)-ethane-1,2-diimine
- SRIXE
synchrotron radiation-induced X-ray emission
- succ
succinato (butanedioato)
- SWNT
single-walled nanotube
- tacn
1,4,7-triazacyclononane
- tpa
tris(2-methylpyridyl)-amine
- XANES
X-ray Absorption Near Edge Structure
- XAS
X-ray absorption spectroscopy
References
- 1.Hambley TW. Chemistry - Metal-based therapeutics. Science. 2007;318:1392–1393. doi: 10.1126/science.1150504. [DOI] [PubMed] [Google Scholar]
- 2.Bruijnincx PCA, Sadler PJ. New trends for metal complexes with anticancer activity. Curr Opin Chem Biol. 2008;12:197–206. doi: 10.1016/j.cbpa.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cisplatin . Chemistry and Biochemistry of a Leading Anticancer Drug. Wiley-VCH; 1999. [Google Scholar]
- 4.Hambley TW. Developing new metal-based therapeutics: challenges and opportunities. Dalton Trans. 2007:4929–4937. doi: 10.1039/b706075k. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez-Cano C, Hannon MJ. Novel and emerging approaches for the delivery of metallo-drugs. Dalton Trans. 2009:10702–10711. doi: 10.1039/b912708a. [DOI] [PubMed] [Google Scholar]
- 6.Reisner E, Arion VB, Keppler BK, Pombeiro AJL. Electron-transfer activated metal-based anticancer drugs. Inorg Chim Acta. 2008;361:1569–1583. [Google Scholar]
- 7.Teicher BA. Hypoxia and drug resistance. Cancer Metastasis Rev. 1994;13:139–168. doi: 10.1007/BF00689633. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Hu L. Design of Anticancer Prodrugs for Reductive Activation. Med Res Rev. 2009;29:29–64. doi: 10.1002/med.20137. [DOI] [PubMed] [Google Scholar]
- 9.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 10.Galanski M, Jakupec MA, Keppler BK. Update of the preclinical situation of anticancer platinum complexes: novel design strategies and innovative analytical approaches. Curr Med Chem. 2005;12:2075–2094. doi: 10.2174/0929867054637626. [DOI] [PubMed] [Google Scholar]
- 11.Hall MD, Dillon CT, Zhang M, Beale P, Cai Z, Lai B, Stampfl APJ, Hambley TW. The cellular distribution and oxidation state of platinum(II) and platinum(IV) antitumour complexes in cancer cells. J Biol Inorg Chem. 2003;8:726–732. doi: 10.1007/s00775-003-0471-6. [DOI] [PubMed] [Google Scholar]
- 12.Hall MD, Alderden RA, Zhang M, Beale PJ, Cai Z, Lai B, Stampfl AP, Hambley TW. The fate of platinum(II) and platinum(IV) anti-cancer agents in cancer cells and tumours. J Struct Biol. 2006;155:38–44. doi: 10.1016/j.jsb.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Khokhar AR, Deng Y, Kido Y, Siddik ZH. Preparation, characterization, and antitumor activity of new ethylenediamine platinum(IV) complexes containing mixed carboxylate ligands. J Inorg Biochem. 1993;50:79–87. doi: 10.1016/0162-0134(93)80015-2. [DOI] [PubMed] [Google Scholar]
- 14.Hall MD, Hambley TW. Platinum(IV) antitumour compounds: their bioinorganic chemistry. Coord Chem Rev. 2002;232:49–67. [Google Scholar]
- 15.Hall MD, Amjadi S, Zhang M, Beale PJ, Hambley TW. The mechanism of action of platinum(IV) complexes in ovarian cancer cell lines. J Inorg Biochem. 2004;98:1614–1624. doi: 10.1016/j.jinorgbio.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Gibson D. The mechanism of action of platinum anticancer agents-what do we really know about it? Dalton Trans. 2009:10681–10689. doi: 10.1039/b918871c. [DOI] [PubMed] [Google Scholar]
- 17.Choi S, Filotto C, Bisanzo M, Delaney S, Lagasee D, Whitworth JL, Jusko A, Li C, Wood NA, Willingham J, Schwenker A, Spaulding K. Reduction and anticancer activity of platinum(IV) complexes. Inorg Chem. 1998;37:2500–2504. [Google Scholar]
- 18.Ellis LT, Er HM, Hambley TW. The Influence of the Axial Ligands of a Series of Platinum(IV) Anticancer Complexes on Their Reduction to Platinum(II) and Reaction with DNA. Aust J Chem. 1995;48:793–806. [Google Scholar]
- 19.Battle AR, Deacon GB, Dolman RC, Hambley TW. Electrochemistry, protein binding and crystal structures of platinum(II) and platinum(IV) carboxylato complexes. Aust J Chem. 2002;55:699–704. [Google Scholar]
- 20.Kido Y, Khokhar AR, Siddik ZH. Glutathione-mediated modulation of tetraplatin activity against sensitive and resistant tumor cells. Biochem Pharmacol. 1994;47:1635–1642. doi: 10.1016/0006-2952(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 21.Gibbons GR, Wyrick S, Chaney SG. Rapid reduction of tetrachloro(D,L-trans)1,2-diaminocyclohexaneplatinum(IV) (tetraplatin) in RPMI 1640 tissue culture medium. Cancer Res. 1989;49:1402–1407. [PubMed] [Google Scholar]
- 22.Eastman A. Glutathione-mediated activation of anticancer platinum(IV) complexes. Biochem Pharmacol. 1987;36:4177–4178. doi: 10.1016/0006-2952(87)90581-8. [DOI] [PubMed] [Google Scholar]
- 23.Rabenstein DL, Guevremont R, Evans CA. In: Metal Ions in Biological Systems. Sigel H, editor. CRC Press; 1979. pp. 103–141. [Google Scholar]
- 24.Vanderveer JL, Peters AR, Reedijk J. Reaction-Products from Platinum(IV) Amine Compounds and 5′-GMP Are Mainly Bis(5′-GMP)Platinum(II) Amine Adducts. J Inorg Biochem. 1986;26:137–142. doi: 10.1016/0162-0134(86)80006-x. [DOI] [PubMed] [Google Scholar]
- 25.Zhong W, Zhang Q, Yan Y, Yue S, Zhang B, Tang W. Reaction of a platinum(IV) complex with native Cd,Zn-metallothionein in vitro. J Inorg Biochem. 1997;66:179–185. doi: 10.1016/s0162-0134(96)00201-2. [DOI] [PubMed] [Google Scholar]
- 26.Hong R, Han G, Fernández JM, Kim B-j, Forbes NS, Rotello VM. Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J Am Chem Soc. 2006;128:1078–1079. doi: 10.1021/ja056726i. [DOI] [PubMed] [Google Scholar]
- 27.Pendyala L, Cowens JW, Chheda GB, Dutta SP, Creaven PJ. Identification of cis-dichloro-bis-isopropylamine platinum(II) as a major metabolite of iproplatin in humans. Cancer Res. 1988;48:3533–3536. [PubMed] [Google Scholar]
- 28.Talman EG, Kidani Y, Mohrmann L, Reedijk J. Can Pt(IV)-amine complexes act as ‘prodrugs’? Inorg Chim Acta. 1998;283:251–255. [Google Scholar]
- 29.Roat RM, Reedijk J. Reaction of mer-Trichloro(diethylenetriamine)-platinum(IV) Chloride, (mer-[Pt(dien)Cl3]Cl), with Purine Nucleosides and Nucleotides Results in Formation of Platinum(II) as Well as Platinum(IV) Complexes. J Inorg Biochem. 1993;52:263–274. [Google Scholar]
- 30.Nováková O, Vrána O, Kiseleva VI, Brabec V. DNA Interactions of Antitumor Platinum(IV) Complexes. Eur J Biochem. 1995;228:616–624. doi: 10.1111/j.1432-1033.1995.tb20301.x. [DOI] [PubMed] [Google Scholar]
- 31.Gramatica P, Papa E, Luini M, Monti E, Gariboldi MB, Ravera M, Gabano E, Gaviglio L, Osella D. Antiproliferative Pt(IV) complexes: synthesis, biological activity, and quantitative structure-activity relationship modeling. J Biol Inorg Chem. 2010;15:1157–1169. doi: 10.1007/s00775-010-0676-4. [DOI] [PubMed] [Google Scholar]
- 32.Hambley TW, Battle AR, Deacon GB, Lawrenz ET, Fallon GD, Gatehouse BM, Webster LK, Rainone S. Modifying the properties of platinum(IV) complexes in order to increase biological effectiveness. J Inorg Biochem. 1999;77:3–12. doi: 10.1016/s0162-0134(99)00133-6. [DOI] [PubMed] [Google Scholar]
- 33.Mellor HR, Snelling S, Hall MD, Modok S, Jaffar M, Hambley TW, Callaghan R. The influence of tumour microenvironmental factors on the efficacy of cisplatin and novel platinum(IV) complexes. Biochem Pharmacol. 2005;70:1137–1146. doi: 10.1016/j.bcp.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 34.Barnes KR, Kutikov A, Lippard SJ. Synthesis, characterization, and cytotoxicity of a series of estrogen-tethered platinum(IV) complexes. Chem Biol. 2004;11:557–564. doi: 10.1016/j.chembiol.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 35.He Q, Liang CH, Lippard SJ. Steroid hormones induce HMG1 overexpression and sensitize breast cancer cells to cisplatin and carboplatin. Proc Natl Acad Sci. 2000;97:5768–5772. doi: 10.1073/pnas.100108697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ang WH, Khalaila I, Allardyce CS, Juillerat-Jeanneret L, Dyson PJ. Rational design of platinum(IV) compounds to overcome glutathione-S-transferase mediated drug resistance. J Am Chem Soc. 2005;127:1382–1383. doi: 10.1021/ja0432618. [DOI] [PubMed] [Google Scholar]
- 37.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 38.Dhar S, Lippard SJ. Mitaplatin, a potent fusion of cisplatin and the orphan drug dichloroacetate. Proc Natl Acad Sci. 2009;106:22199–22204. doi: 10.1073/pnas.0912276106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukhopadhyay S, Barnes CM, Haskel A, Short SM, Barnes KR, Lippard SJ. Conjugated platinum(IV)-peptide complexes for targeting angiogenic tumor vasculature. Bioconjugate Chem. 2008;19:39–49. doi: 10.1021/bc070031k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stocker BL, Hoberg JO. Synthesis of platinacyclobutanes bearing biological components for targeted, cisplatin prodrugs. Organometallics. 2006;25:4537–4541. [Google Scholar]
- 41.Harper BW, Krause-Heuer AM, Grant MP, Manohar M, Garbutcheon-Singh KB, Aldrich-Wright JR. Advances in Platinum Chemotherapeutics. Chem Eur J. 2010;16:7064–7077. doi: 10.1002/chem.201000148. [DOI] [PubMed] [Google Scholar]
- 42.Wheate NJ, Walker S, Craig GE, Oun R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010;39:8113–8127. doi: 10.1039/c0dt00292e. [DOI] [PubMed] [Google Scholar]
- 43.Choy H, Park C, Yao M. Current status and future prospects for satraplatin, an oral platinum analogue. Clin Cancer Res. 2008;14:1633–1638. doi: 10.1158/1078-0432.CCR-07-2176. [DOI] [PubMed] [Google Scholar]
- 44.Nemirovski A, Vinograd I, Takrouri K, Mijovilovich A, Rompel A, Gibson D. New reduction pathways for ctc-[PtCl2(CH3CO2)2(NH3)(Am)] anticancer prodrugs. Chem Commun. 2010;46:1842–1844. doi: 10.1039/b925721g. [DOI] [PubMed] [Google Scholar]
- 45.Carr JL, Tingle MD, McKeage MJ. Satraplatin activation by haemoglobin, cytochrome C and liver microsomes in vitro. Cancer Chemother Pharmacol. 2006;57:483–490. doi: 10.1007/s00280-005-0069-5. [DOI] [PubMed] [Google Scholar]
- 46.Nemirovski A, Kasherman Y, Tzaraf Y, Gibson D. Reduction of cis,trans,cis-[PtCl2(OCOCH3)2(NH3)2] by aqueous extracts of cancer cells. J Med Chem. 2007;50:5554–5556. doi: 10.1021/jm070740j. [DOI] [PubMed] [Google Scholar]
- 47.Kaluderović GN, Kommera H, Schwieger S, Paethanom A, Kunze M, Schmidt H, Paschke R, Steinborn D. Synthesis, characterization, in vitro antitumoral investigations and interaction with plasmid pBR322 DNA of R2eddp-platinum(IV) complexes (R = Et, n-Pr) Dalton Trans. 2009:10720–10726. doi: 10.1039/b911597h. [DOI] [PubMed] [Google Scholar]
- 48.Dwyer FP, Gyarfas EC, Rogers WP, Koch JH. Biological Activity of Complex Ions. Nature. 1952;170:190–191. doi: 10.1038/170190a0. [DOI] [PubMed] [Google Scholar]
- 49.Clarke MJ. Oncological implications of the chemistry of ruthenium. In: Sigel H, editor. Metal Ions in Biological Systems. Marcel Dekker; 1980. pp. 231–283. [Google Scholar]
- 50.Bergamo A, Gava B, Alessio E, Mestroni G, Serli B, Cocchietto M, Zorzet S, Sava G. Ruthenium-based NAMI-A type complexes with in vivo selective metastasis reduction and in vitro invasion inhibition unrelated to cell cytotoxicity. Int J Oncol. 2002;21:1331–1338. [PubMed] [Google Scholar]
- 51.Pluim D, van Waardenburg RCAM, Beijnen JH, Schellens JHM. Cytotoxicity of the organic ruthenium anticancer drug NAMI-A is correlated with DNA binding in four different human tumor cell lines. Cancer Chemother Pharmacol. 2004;54:71–78. doi: 10.1007/s00280-004-0773-6. [DOI] [PubMed] [Google Scholar]
- 52.Harris TV, Szilagyi RK, McFarlane Holman KL. Electronic structural investigations of ruthenium compounds and anticancer prodrugs. J Biol Inorg Chem. 2009;14:891–898. doi: 10.1007/s00775-009-0501-0. [DOI] [PubMed] [Google Scholar]
- 53.Hartinger CG, Jakupec MA, Zorbas-Seifried S, Groessl M, Egger A, Berger W, Zorbas H, Dyson PJ, Keppler BK. KP1019, A New Redox-Active Anticancer Agent -Preclinical Development and Results of a Clinical Phase I Study in Tumor Patients. Chem Biodivers. 2008;5:2140–2155. doi: 10.1002/cbdv.200890195. [DOI] [PubMed] [Google Scholar]
- 54.Levina A, Mitra A, Lay PA. Recent developments in ruthenium anticancer drugs. Metallomics. 2009;1:458–470. doi: 10.1039/b904071d. [DOI] [PubMed] [Google Scholar]
- 55.Clarke MJ. Ruthenium metallopharmaceuticals. Coord Chem Rev. 2003;236:209–233. [Google Scholar]
- 56.Hartinger CG, Zorbas-Seifried S, Jakupec MA, Kynast B, Zorbas H, Keppler BK. From bench to bedside - preclinical and early clinical development of the anticancer agent indazolium trans-[tetrachlorobis(1H-indazole)ruthenate(III)] (KP1019 or FFC14A) J Inorg Biochem. 2006;100:891–904. doi: 10.1016/j.jinorgbio.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 57.Kostova I. Ruthenium complexes as anticancer agents. Curr Med Chem. 2006;13:1085–1107. doi: 10.2174/092986706776360941. [DOI] [PubMed] [Google Scholar]
- 58.Alessio E, Mestroni G, Bergamo A, Sava G. Ruthenium antimetastatic agents. Curr Top Med Chem. 2004;4:1525–1535. doi: 10.2174/1568026043387421. [DOI] [PubMed] [Google Scholar]
- 59.Galanski M, Arion VB, Jakupec MA, Keppler BK. Recent developments in the field of tumor-inhibiting metal complexes. Curr Pharm Des. 2003;9:2078–2089. doi: 10.2174/1381612033454180. [DOI] [PubMed] [Google Scholar]
- 60.Alessio E, Mestroni G, Bergamo A, Sava G. Ruthenium Anticancer Drugs. In: Sigel H, editor. Metal Ions in Biological Systems. CRC Press; 2004. pp. 323–351. [PubMed] [Google Scholar]
- 61.Malina J, Novakova O, Keppler BK, Alessio E, Brabec V. Biophysical analysis of natural, double-helical DNA modified by anticancer heterocyclic complexes of ruthenium(lll) in cell-free media. J Biol Inorg Chem. 2001;6:435–445. doi: 10.1007/s007750100223. [DOI] [PubMed] [Google Scholar]
- 62.Ott I, Gust R. Non platinum metal complexes as anti-cancer drugs. Arch Pharm Chem Life Sci. 2007;340:117–126. doi: 10.1002/ardp.200600151. [DOI] [PubMed] [Google Scholar]
- 63.Lindauer E, Holler E. Cellular distribution and cellular reactivity of platinum(II) complexes. Biochem Pharmacol. 1996;52:7–14. doi: 10.1016/0006-2952(96)00106-2. [DOI] [PubMed] [Google Scholar]
- 64.Pongratz M, Schluga P, Jakupec MA, Arion VB, Hartinger CG, Allmaier G, Keppler BK. Transferrin binding and transferrin-mediated cellular uptake of the ruthenium coordination compound KP1019, studied by means of AAS, ESI-MS and CD spectroscopy. J Anal At Spectrom. 2004;19:46–51. [Google Scholar]
- 65.Jakupec MA, Reisner E, Eichinger A, Pongratz M, Arion VB, Galanski M, Hartinger CG, Keppler BK. Redox-active antineoplastic ruthenium complexes with indazole: Correlation of in vitro potency and reduction potential. J Med Chem. 2005;48:2831–2837. doi: 10.1021/jm0490742. [DOI] [PubMed] [Google Scholar]
- 66.Schluga P, Hartinger CG, Egger A, Reisner E, Galanski M, Jakupec MA, Keppler BK. Redox behavior of tumor-inhibiting ruthenium(III) complexes and effects of physiological reductants on their binding to GMP. Dalton Trans. 2006:1796–1802. doi: 10.1039/b511792e. [DOI] [PubMed] [Google Scholar]
- 67.Sava G, Bergamo A, Zorzet S, Gava B, Casarsa C, Cocchietto M, Furlani A, Scarcia V, Serli B, Iengo E, Alessio E, Mestroni G. Influence of chemical stability on the activity of the antimetastasis ruthenium compound NAMI-A. Eur J Cancer. 2002;38:427–435. doi: 10.1016/s0959-8049(01)00389-6. [DOI] [PubMed] [Google Scholar]
- 68.Frasca D, Ciampa J, Emerson J, Umans RS, Clarke MJ. Effects of hypoxia and transferrin on toxicity and DNA binding of ruthenium antitumor agents in HeLa cells. Met-Based Drugs. 1996;3:197–209. doi: 10.1155/MBD.1996.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Rijn JA, Marqués-Gallego P, Reedijk J, Lutz M, Spek AL, Bouwman E. A novel ruthenium(III) complex with a tridentate dianionic P,O,O-ligand showing high cytotoxic activity. Dalton Trans. 2009:10727–10730. doi: 10.1039/b909520a. [DOI] [PubMed] [Google Scholar]
- 70.Gianferrara T, Bratsos I, Iengo E, Milani B, Oštric A, Spagnul C, Zangrando E, Alessio E. Synthetic strategies towards ruthenium-porphyrin conjugates for anticancer activity. Dalton Trans. 2009:10742–10756. doi: 10.1039/b911393b. [DOI] [PubMed] [Google Scholar]
- 71.Schatzschneider U, Metzler-Nolte N. New principles in medicinal organometallic chemistry. Angew Chem Int Ed. 2006;45:1504–1507. doi: 10.1002/anie.200504604. [DOI] [PubMed] [Google Scholar]
- 72.Pigeon P, Top S, Vessières A, Huché M, Hillard EA, Salomon E, Jaouen G. Selective estrogen receptor modulators in the ruthenocene series. Synthesis and biological behavior. J Med Chem. 2005;48:2814–2821. doi: 10.1021/jm049268h. [DOI] [PubMed] [Google Scholar]
- 73.Allardyce CS, Dorcier A, Scolaro C, Dyson PJ. Development of organometallic (organo-transition metal) pharmaceuticals. Appl Organomet Chem. 2005;19:1–10. [Google Scholar]
- 74.Dorcier A, Ang WH, Bolaño S, Gonsalvi L, Juillerat-Jeannerat L, Laurenczy G, Peruzzini M, Phillips AD, Zanobini F, Dyson PJ. In vitro evaluation of rhodium and osmium RAPTA analogues: The case for organometallic anticancer drugs not based on ruthenium. Organometallics. 2006;25:4090–4096. [Google Scholar]
- 75.Peacock AFA, Habtemariam A, Fernández R, Walland V, Fabbiani FPA, Parsons S, Aird RE, Jodrell DI, Sadler PJ. Tuning the reactivity of osmium(II) and ruthenium(II) arene complexes under physiological conditions. J Am Chem Soc. 2006;128:1739–1748. doi: 10.1021/ja055886r. [DOI] [PubMed] [Google Scholar]
- 76.Egger A, Cebrián-Losantos B, Stepanenko IN, Krokhin AA, Eichinger R, Jakupec MA, Arion VB, Keppler BK. Hydrolysis and cytotoxic properties of osmium(II)/(III)-DMSO-azole complexes. Chem Biodivers. 2008;5:1588–1593. doi: 10.1002/cbdv.200890146. [DOI] [PubMed] [Google Scholar]
- 77.Cebrián-Losantos B, Krokhin AA, Stepanenko IN, Eichinger R, Jakupec MA, Arion VB, Keppler BK. Osmium NAMI-A analogues: synthesis, structural and spectroscopic characterization, and antiproliferative properties. Inorg Chem. 2007;46:5023–5033. doi: 10.1021/ic700405y. [DOI] [PubMed] [Google Scholar]
- 78.Hillard E, Vessières A, Thouin L, Jaouen G, Amatore C. Ferrocene-mediated proton-coupled electron transfer in a series of ferrocifen-type breast-cancer drug candidates. Angew Chem Int Ed. 2006;45:285–290. doi: 10.1002/anie.200502925. [DOI] [PubMed] [Google Scholar]
- 79.Osella D, Ferrali M, Zanello P, Laschi F, Fontani M, Nervi C, Cavigiolio G. On the mechanism of the antitumor activity of ferrocenium derivatives. Inorg Chim Acta. 2000;306:42–48. [Google Scholar]
- 80.Duivenvoorden WCM, Liu Y-n, Schatte G, Kraatz H-B. Synthesis of redox-active ferrocene pyrazole conjugates and their cytotoxicity in human mammary adenocarcinoma MCF-7 cells. Inorg Chim Acta. 2005;358:3183–3189. [Google Scholar]
- 81.Failes TW, Cullinane C, Diakos CI, Yamamoto N, Lyons JG, Hambley TW. Studies of a cobalt(III) complex of the MMP inhibitor marimastat: A potential hypoxia-activated prodrug. Chem Eur J. 2007;13:2974–2982. doi: 10.1002/chem.200601137. [DOI] [PubMed] [Google Scholar]
- 82.Failes TW, Hambley TW. Towards bioreductively activated prodrugs: Fe(III) complexes of hydroxamic acids and the MMP inhibitor marimastat. J Inorg Biochem. 2007;101:396–403. doi: 10.1016/j.jinorgbio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 83.Fricker SP. Medical Uses of Gold Compounds: Past, Present and Future. Gold bulletin. 1996;29:53–60. [Google Scholar]
- 84.Failes TW, Diakos CI, Underwood CK, Hambley TW, Cullinane CM, Lyons JG. Can metal complexes serve as hypoxia activated prodrugs? Investigations of a Co(III) complex of the MMP inhibitor marimastat. J Inorg Biochem. 2003;96:128–128. [Google Scholar]
- 85.Anderson RF, Denny WA, Ware DC, Wilson WR. Pulse radiolysis studies on the hypoxia-selective toxicity of a colbalt-mustard complex. Br J Cancer Suppl. 1996;27:S48–S51. [PMC free article] [PubMed] [Google Scholar]
- 86.Denny WA. Prodrug strategies in cancer therapy. Eur J Med Chem. 2001;36:577–595. doi: 10.1016/s0223-5234(01)01253-3. [DOI] [PubMed] [Google Scholar]
- 87.Denny WA, Wilson WR. Bioreducible mustards: a paradigm for hypoxia-selective prodrugs of diffusible cytotoxins (HPDCs) Cancer Metastasis Rev. 1993;12:135–151. doi: 10.1007/BF00689806. [DOI] [PubMed] [Google Scholar]
- 88.Ware DC, Palmer HR, Brothers PJ, Rickard CEF, Wilson WR, Denny WA. Bis-tropolonato derivatives of cobalt(III) complexes of bidentate aliphatic nitrogen mustards as potential hypoxia-selective cytotoxins. J Inorg Biochem. 1997;68:215–224. doi: 10.1016/s0162-0134(97)00090-1. [DOI] [PubMed] [Google Scholar]
- 89.Parker LL, Lacy SM, Farrugia LJ, Evans C, Robins DJ, O’Hare CC, Hartley JA, Jaffar M, Stratford IJ. A novel design strategy for stable metal complexes of nitrogen mustards as bioreductive prodrugs. J Med Chem. 2004;47:5683–5689. doi: 10.1021/jm049866w. [DOI] [PubMed] [Google Scholar]
- 90.Craig PR, Brothers PJ, Clark GR, Wilson WR, Denny WA, Ware DC. Anionic carbonato and oxalato cobalt(III) nitrogen mustard complexes. Dalton Trans. 2004:611–618. doi: 10.1039/b311091e. [DOI] [PubMed] [Google Scholar]
- 91.Ware DC, Brothers PJ, Clark GR, Denny WA, Palmer BD, Wilson WR. Synthesis, structures and hypoxia-selective cytotoxicity of cobalt(III) complexes containing tridentate amine and nitrogen mustard ligands. Dalton Trans. 2000:925–932. [Google Scholar]
- 92.Ahn G-O, Botting KJ, Patterson AV, Ware DC, Tercel M, Wilson WR. Radiolytic and cellular reduction of a novel hypoxia-activated cobalt(III) prodrug of a chloromethylbenzindoline DNA minor groove alkylator. Biochem Pharmacol. 2006;71:1683–1694. doi: 10.1016/j.bcp.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 93.Failes TW, Hambley TW. Models of hypoxia activated prodrugs: Co(III) complexes of hydroxamic acids. Dalton Trans. 2006:1895–1901. doi: 10.1039/b512322d. [DOI] [PubMed] [Google Scholar]
- 94.Souza ET, Castro LC, Castro FAV, do Canto Visentin L, Pinheiro CB, Pereira MD, de Paula Machado S, Scarpellini M. Synthesis, characterization and biological activities of mononuclear Co(III) complexes as potential bioreductively activated prodrugs. J Inorg Biochem. 2009;103:1355–1365. doi: 10.1016/j.jinorgbio.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 95.Ware DC, Wilson WR, Denny WA, Rickard CEF. Design and Synthesis of Cobalt(III) Nitrogen-Mustard Complexes as Hypoxia Selective Cytotoxins. The X-Ray Crystal-Structure of Bis(3-chloropentane-2,4-dionato)(RS-N,N′-bis(2-chloroethyl)ethylenediamine)cobalt(III) Perchlorate, [Co(Clacac)2(bce)]ClO4. Chem Commun. 1991:1171–1173. [Google Scholar]
- 96.Ware DC, Palmer BD, Wilson WR, Denny WA. Hypoxia-Selective Antitumor Agents. 7. Metal-Complexes of Aliphatic Mustards as a New Class of Hypoxia-Selective Cytotoxins - Synthesis and Evaluation of Cobalt(III) Complexes of Bidentate Mustards. J Med Chem. 1993;36:1839–1846. doi: 10.1021/jm00065a006. [DOI] [PubMed] [Google Scholar]
- 97.Blower PJ, Dilworth JR, Maurer RI, Mullen GD, Reynolds CA, Zheng Y. Towards new transition metal-based hypoxic selective agents for therapy and imaging. J Inorg Biochem. 2001;85:15–22. doi: 10.1016/s0162-0134(00)00228-2. [DOI] [PubMed] [Google Scholar]
- 98.Lin P-S, Ho K-C. CuTira brachytherapy: A new combination of radioactive copper isotopes and the hypoxic cytotoxin, tirapazamine, for targeted tumor therapy. J Nucl Med. 1998;39:677–678. [PubMed] [Google Scholar]
- 99.Dearling JLJ, Lewis JS, Mullen GED, Rae MT, Zweit J, Blower PJ. Design of hypoxia-targeting radiopharmaceuticals: selective uptake of copper-64 complexes in hypoxic cells in vitro. Eur J Nucl Med. 1998;25:788–792. doi: 10.1007/s002590050283. [DOI] [PubMed] [Google Scholar]
- 100.Maurer RI, Blower PJ, Dilworth JR, Reynolds CA, Zheng Y, Mullen GED. Studies on the mechanism of hypoxic selectivity in copper bis(thiosemicarbazone) radiopharmaceuticals. J Med Chem. 2002;45:1420–1431. doi: 10.1021/jm0104217. [DOI] [PubMed] [Google Scholar]
- 101.Torre MH, Gambino D, Araujo J, Cerecetto H, González B, Lavaggi ML, Azqueta A, de Cerain AL, Vega AM, Abram U, Costa-Filho AJ. Novel Cu(II) quinoxaline N1,N4-dioxide as selective hypoxic cytotoxins. Eur J Med Chem. 2005;40:473–480. doi: 10.1016/j.ejmech.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 102.Urquiola C, Gambino D, Cabrera M, Lavaggi ML, Cerecetto H, González M, de Cerain AL, Monge A, Costa-Filho AJ, Torre MH. New copper-based complexes with quinoxaline N1,N4-dioxide derivatives, potential antitumoral agents. J Inorg Biochem. 2008;102:119–126. doi: 10.1016/j.jinorgbio.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 103.Monge A, Palop JA, de Ceráin AL, Senador V, Martínez-Crespo FJ, Sainz Y, Narro S, García E, de Miguel C, González M, Hamilton E, Barker AJ, Clarke ED, Greenhow DT. Hypoxia-Selective Agents Derived from Quinoxaline 1,4-Di-N-Oxides. J Med Chem. 1995;38:1786–1792. doi: 10.1021/jm00010a023. [DOI] [PubMed] [Google Scholar]
- 104.Vieites M, Noblía P, Torre MH, Cerecetto H, Lavaggi ML, Costa-Filho AJ, Azqueta A, de Cerain AL, Monge A, Parajón-Costa B, González M, Gambino D. Selective hypoxia-cytotoxins based on vanadyl complexes with 3-aminoquinoxaline-2-carbonitrile-N1,N4-dioxide derivatives. J Inorg Biochem. 2006;100:1358–1367. doi: 10.1016/j.jinorgbio.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 105.Ikotun OF, Higbee EM, Ouellette W, Doyle RP. Pyrophosphate-bridged complexes with picomolar toxicity. J Inorg Biochem. 2009;103:1254–1264. doi: 10.1016/j.jinorgbio.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 106.Feazell RP, Nakayama-Ratchford N, Dai H, Lippard SJ. Soluble single-walled carbon nanotubes as longboat delivery systems for Platinum(IV) anticancer drug design. J Am Chem Soc. 2007;129:8438–8439. doi: 10.1021/ja073231f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dhar S, Liu Z, Thomale J, Dai H, Lippard SJ. Targeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing device. J Am Chem Soc. 2008;130:11467–11476. doi: 10.1021/ja803036e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dhar S, Daniel WL, Giljohann DA, Mirkin CA, Lippard SJ. Polyvalent Oligonucleotide Gold Nanoparticle Conjugates as Delivery Vehicles for Platinum(IV) Warheads. J Am Chem Soc. 2009;131:14652–14653. doi: 10.1021/ja9071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Min Y, Mao C, Xu D, Wang J, Liu Y. Gold nanorods for platinum based prodrug delivery. Chem Commun. 2010;46:8424–8426. doi: 10.1039/c0cc03108a. [DOI] [PubMed] [Google Scholar]
- 110.Avgoustakis K, Beletsi A, Panagi Z, Klepetsanis P, Karydas AG, Ithakissios DS. PLGA-mPEG nanoparticles of cisplatin: in vitro nanoparticle degradation, in vitro drug release and in Vivo drug residence in blood properties. J Controlled Release. 2002;79:123–135. doi: 10.1016/s0168-3659(01)00530-2. [DOI] [PubMed] [Google Scholar]
- 111.Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc Natl Acad Sci. 2008;105:17356–17361. doi: 10.1073/pnas.0809154105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc Natl Acad Sci. 2011;108:1850–1855. doi: 10.1073/pnas.1011379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aryal S, Hu C-MJ, Zhang L. Polymer-Cisplatin Conjugate Nanoparticles for Acid-Responsive Drug Delivery. ACS Nano. 2010;4:251–258. doi: 10.1021/nn9014032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rieter WJ, Pott KM, Taylor KML, Lin W. Nanoscale coordination polymers for platinum-based anticancer drug delivery. J Am Chem Soc. 2008;130:11584–11585. doi: 10.1021/ja803383k. [DOI] [PubMed] [Google Scholar]
- 115.Taylor-Pashow KML, Della Rocca J, Xie Z, Tran S, Lin W. Postsynthetic Modifications of Iron-Carboxylate Nanoscale Metal-Organic Frameworks for Imaging and Drug Delivery. J Am Chem Soc. 2009;131:14261–14263. doi: 10.1021/ja906198y. [DOI] [PMC free article] [PubMed] [Google Scholar]