Abstract
Background
Collagenous colitis is a chronic inflammatory bowel disease of unknown etiology. It is fairly common in adult humans, but rare in infants, and has been associated with autoimmune disorders.
Case Reports
We report four infant baboons (age 7–12 months) that had received a transplant at three months of age and subsequent immunosuppressive therapy for periods of 4–10 months. All presented identical symptoms within a period of four weeks, including weight loss associated with chronic watery diarrhea that was unresponsive to standard antimicrobial treatment. Clinical chemistry evaluations were within normal ranges, viral causes were ruled out, and fecal and blood cultures were repeatedly negative. At necropsy, two infant baboons were found to have a form of collagenous colitis. In the remaining two baboons that had identical clinical features, immunosuppressive therapy was discontinued and treatment with budesonide was initiated. Both baboons recovered and remained well on no medication until the end of follow-up (24 months).
Conclusions
Collagenous colitis has occasionally been reported in patients with organ transplants. It has been reported only once previously in baboons. The four cases reported here strongly suggest that (i) clinical features as well as histopathological findings of collagenous colitis in baboons are very similar to those in human patients; (ii) it was associated with the immunocompromised state of the baboons, as two non-immunosuppressed age-matched baboons in close proximity did not develop the condition, and (iii) it may have had an infectious origin as all four cases developed within a four week period of time.
Keywords: Baboon; Colitis, collagenous; Diarrhea; Infant; Transplantation
INTRODUCTION
Collagenous colitis and lymphocytic colitis are two types of what is known as microscopic colitis (1). Both are chronic inflammatory bowel diseases, characterized by a normal macroscopic appearance of the mucosa of the colon, but distinct histopathological changes upon microscopic examination. Clinical symptoms are chronic non-bloody diarrhea, abdominal cramping, and weight loss. Biopsy is diagnostic and differentiates between collagenous and lymphocytic colitis. In humans, the incidence varies from 4–15 cases/100,000 population, and is higher in the elderly and in women (2, 3). The development of collagenous colitis in children is rare (4, 5). It is not commonly associated with an immunocompromised state, although cases of microscopic colitis have been reported in patients with organ transplants who were receiving immunosuppressive therapy (IS) (6).
There is almost no information on the occurrence of collagenous colitis in nonhuman primates. In baboons, only one case has been reported in a large cohort of 132 baboons presenting with chronic colitis (7, 8). We report four cases (two of which were confirmed histologically) which occurred in a group of six infant baboons in our animal facility. All four were receiving IS therapy and developed clinical features of colitis within the same period of four weeks.
CASE REPORTS
Study Population: six infant baboons
The six baboons in this study were obtained from the Oklahoma University Health Sciences Center, Oklahoma City, OK. All animal care procedures were in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication No. 86-23, revised 1985). All protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Four infant baboons, ranging from 3–4 months of age and 1.3–2.3 kg in weight, received artery patch grafts into the wall of the infra-renal abdominal aorta (Table 1, described in detail in Dons EM, et al, submitted). The grafts were either from a blood group A/B-incompatible baboon (AB-I, n=2) or from a wild-type (i.e., genetically-unmodified) pig (pig, n=2). All four received IS therapy from the time of the transplant (Table 2). This consisted of induction with two doses of anti-human thymocyte globulin (ATG) and maintenance with a combination of an anti-human CD154 monoclonal antibody (anti-CD154 mAb) and mycophenolate mofetil (MMF). Details of the IS regimen and of supportive medical therapy are provided in Table 2.
Table 1.
Clinical Details of 6 Infant Baboons
| Animal number | Sex | Age at arrival in our facility (days) | Age at Tx (days) | Start of clinical features (age, days) | Period of IS (age, days) | Survival after Tx (days) | Comments |
|---|---|---|---|---|---|---|---|
| B4908 | M | 70 | - | - | - | - | No Tx or IS No diarrhea Alive |
| B5108 | M | 64 | - | - | - | - | No Tx or IS No diarrhea Alive |
| B7507 | M | 67 | 98 | 300 | Anti-CD154 (317) MMF (307) |
324 (Died) | Diarrhea Dehydration Collagenous colitis diagnosed at necropsy |
| B5508 | M | 57 | 98 | 125 | Anti-CD154 (137) MMF (132) |
146 (Euthanized) | Diarrhea Dehydration Collagenous colitis diagnosed at necropsy |
| B5008 | M | 65 | 107 | 130 | Anti-CD154 (139) MMF (134) |
> (euthanized at 24m, end of follow-up) | Diarrhea Colon biopsy (non-diagnostic) Treatment with budesonide |
| B5708 | F | 43 | 87 | 127 | Anti-CD154 (145) MMF (131) |
> (euthanized at 24m, end of follow-up) | Diarrhea Treatment with budesonide |
Anti-CD154 mAb = anti-CD154 monoclonal antibody
IS = immunosuppressive therapy
MMF = mycophenolate mofetil
Tx = transplantation
Table 2.
Summary of Drugs Administered to Experimental Baboons
| Induction Therapy | Dose | Duration |
|---|---|---|
| Thymoglobulin | 2.0–2.5mg/kg i.v. | Day -3 and -1 |
| Methylprednisolone | 5 mg/kg i.v. | Before each dose of ATG and on day 0 The dose was then reduced by 1 mg/kg/day, and discontinued on day 5 |
| Maintenance Therapy | Dose | Duration |
| Anti-human CD154 mAb | 20–25 mg/kg i.v. | Days −1, 0, 4, 7, 10, 14, then every 7 days (to maintain a trough level of 400–800 ng/ml) |
| Mycophenolate mofetil | 20–150 mg/kg/d p.o. ×2 daily | From day -2 (to maintain a trough level of 3–6 ug/ml) |
| Supportive Therapy | Dose | Duration |
| Cefazolin | 25mg/kg bid i.v. | For 3 days after surgery |
| Famotidine | 0.25 mg/kg bid i.v. | From day -3 until day 14 |
| Ganciclovir | 5 mg/kg/ i.v. | From day -4 until day 21 |
| Ketorolac | 0.5 mg/kg i.v. | Before every dose of anti-CD154 |
| Buprenorphine | 0.01 mg/kg bid i.v. | For 3 days after surgery |
As the experiments were aimed towards documenting the development of anti-AB or anti-pig antibodies during infancy (9) in the presence of an AB-incompatible allograft or pig xenograft, two other baboons, matched for age and weight, were followed as controls (Table 1). These did not receive either an allograft or xenograft and did not receive any IS therapy or other drugs on a regular basis, but simply were maintained in the same room, received the same diet and had blood drawn at the same time intervals as the four experimental baboons.
At the time of entry to our facility, all six baboons were between 43 and 70 days (median 64 days) or approximately 2 months of age. However, one of them had been born earlier than the others and was admitted to our facility and received a transplant and IS therapy approximately 5 months before the others.
The artery patch grafts were carried out when the four baboons were aged 87–107 days (median 98 days) or approximately 3 months of age. They received IS therapy continuously for periods ranging from as long as 10 months to as short as 5 months, at which time they all developed diarrhea and either died (B7507) or were euthanized (B5508) or had IS therapy discontinued (B5008 and B5708) (Table 1).
Clinical Features
All four immunosuppressed infant baboons developed severe unexplained watery diarrhea within the same time-period extending over four weeks (Figure 1). This was associated with either actual weight loss or discontinuation of the normal weight gain seen in infant baboons (Figure 2).
Figure 1. Time line of disease onset and progress.
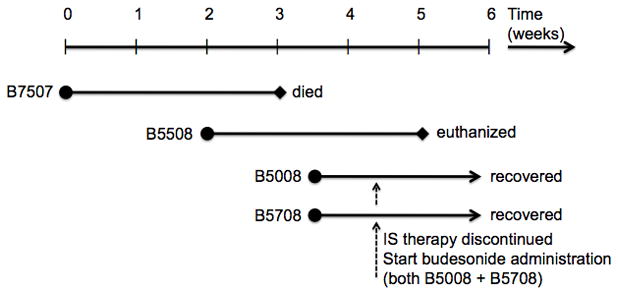
Time line of disease onset and progress in 4 immunosuppressed and 2 age-matched infant baboons. All 4 immunosuppressed infants developed the same symptoms within the same period of 4 weeks.
Figure 2. Weight changes of immunosuppressed baboons and controls.
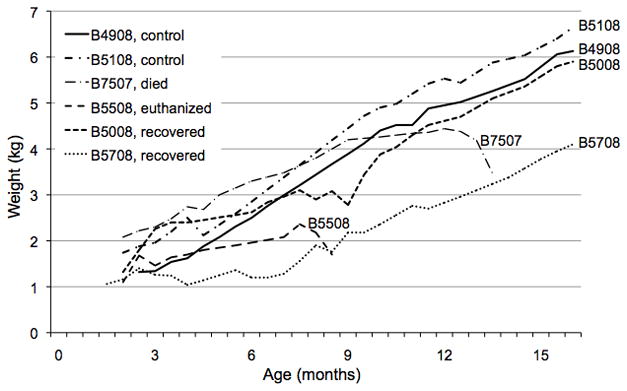
Weight changes of the 4 experimental and 2 control baboons over the course of the study. Onset of weight loss associated with diarrhea - 7507 at age 12 months; 5508 at age 7.5 months; 5008 and 5708 at age 8 months.
Diarrhea is not uncommon in humans or baboons receiving MMF (10–12), particularly if the blood levels of the drug are higher than planned. However, the blood levels were within the therapeutic range in all cases. Nevertheless, when diarrhea persisted for >1 week, MMF was discontinued to reduce possible MMF toxicity and to improve the baboons’ immunocompetency. In the two baboons already affected by the condition, persistent diarrhea, dehydration and decreasing weight (Figure 2) necessitated discontinuation of the anti-CD154mAb subsequently. In the remaining two infants, all immunosuppressive therapy was discontinued immediately upon manifestation of similar features.
After onset of diarrhea, all infants showed clinical signs of dehydration (decreased skin turgor, weight loss). Clinical chemistry evaluations were within the normal range except for mild features of dehydration. Multiple fecal cultures proved negative for standard enteric pathogens, although an overgrowth of yeast was seen on one sample. Testing for occult blood in the stool was negative, and blood cultures were also negative in all 4 baboons. Polymerase chain reaction (PCR) against cytomegalovirus (CMV) and norovirus as well as serology for anti-adeno and anti-CMV antibodies (all carried out by BioReliance, Rockville, MD) were tested at time of clinical features and were found negative in all four infants.
The diarrhea was unresponsive to treatment with metronidazole (0.5ml p.o. daily, administered for 8–14 days; Flagyl, Pfizer, New York, NY) or subsequently enrofloxacin (0.5ml i.v. daily, Baytril, Bayer, Pittsburgh, PA). Additionally, all infants received daily oral fluid support (Pedialyte, Abbott Laboratories, Abbott Park, IL) and loperamide (Imodium, Jansen-Cilag, Beerse, Belgium) for 2–3 weeks.
Despite these measures, in two infants, the condition could not be alleviated. One infant (B7507, age 13 months) died overnight due to severe dehydration, and the other (B5508, age 8 months) was euthanized in view of progressive dehydration that did not improve with medical treatment and fluid replacement. Necropsy was performed on both baboons; histological examination of the colon demonstrated a form of collagenous colitis in both cases (see below).
When the third and fourth infants showed similar clinical progression, colonoscopy and colon biopsies were performed in one (B5008, age 7.5 months). Macroscopically, no lesions or abnormalities were seen; the biopsies did not include layers deeper than the mucosa, and therefore histopathology was inconclusive. However, in the light of the diagnosis in the previous two baboons, treatment with budesonide (at 2.5mg/kg p.o., Entocort, AstraZeneca, Prometheus Laboratories, San Diego, CA) was initiated in both infants. Over the course of 2 weeks, this treatment resulted in cessation of the diarrhea and a return of normal weight gain (Figure 2). Immunosuppressive therapy was not restarted. Budesonide was continued for 7 months and then discontinued. Total follow-up was for 16 months after initiation of budesonide therapy; no further diarrhea occurred in either baboon, and both followed a normal growth curve (Figure 2).
Gross Pathology Findings
Two baboons underwent necropsy at the time of death or euthanasia, with clinical features of colitis (B7507 and B5508), the other two were euthanized 16 months after all clinical features of colitis had disappeared. In all four animals, the gastrointestinal tract (stomach, jejunum, ileum, and colon) was grossly normal. Additionally, no abnormalities were noted in any thoracic or abdominal organs.
Histopathological Findings
The most consistent histopathologic change was an increased amount of fibrous connective tissue within the colon of all four baboons (Figure 3). Increased collagen deposition was found primarily within the submucosa and, to a lesser degree, within the lamina propria. The most striking changes were found in B7507 (Figure 3A).
Figure 3. Colon H&E and collagen deposition.
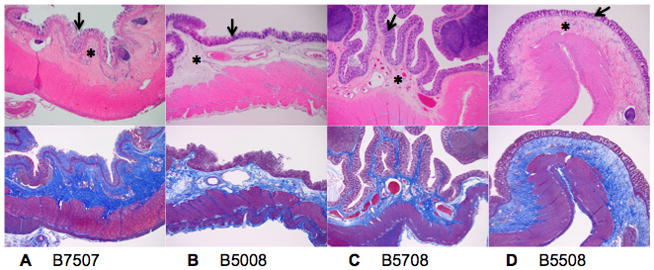
H&E staining (top) and collagen staining (Trichrome, bottom) of the colon. B7507 (A), B5508 (B), B5008 (C) and B5708 (D) (magnification ×4). A moderate to extensive increase in collagen can be seen within the submucosa (asterix, top figures) and is illustrated by blue staining by Trichrome (bottom figures). Mild to extensive collagen deposition is also present between the glands within mucosa (arrow). The most severe changes are illustrated in B7507 with a thick layer of dense submucosal fibrous connective tissue and regions of complete mucosal epithelial cell loss secondary to collagen deposition.
Within small intestine, marked villous atrophy and enterocyte necrosis were found in B7507 and B5508 (Figure 4A and 4B). These animals also had extensive collagen deposition within the submucosa and lesser amounts within the lamina propria that mirrors the deposition found in the large intestine. Lesions within the small intestine of B5008 and B5708 (Figure 4C and 4D) were limited to mild villous blunting in B5008 and mild multifocal subacute enteritis. Within all intestinal sections of B7507 and B5508, occasional eosinophilic intranuclear inclusion bodies were present.
Figure 4. Small intestine H&E and collagen deposition.
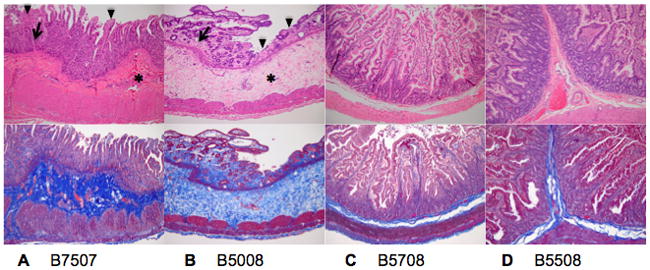
H&E staining (top) and collagen staining (Trichrome, bottom) of the small intestine of B7507 (A), B5508 (B), B5008 (C) and B5708 (D) (magnification ×10). A marked increase in collagen is found in both the lamina propria (arrow) and submucosa (asterix) of B7507 and B5508 in addition to moderate (B7507) to severe (B5508) blunting and loss of villi with necrosis and loss of surface enterocytes (arrowhead). Mild villous blunting is present in B5508. The small intestine of B5008 and B5708is has a normal appearance.
Colon, small intestine, stomach and liver of all 4 animals were stained with Gram (bacteria), periodic acid Schiff (fungae), congo red (amyloid; NHP may develop amyloid secondary to infection or inflammation) and trichrome (connective tissue). These stains demonstrated no active infection with microorganisms or amyloid deposition in any of the infant baboons. A generic herpesvirus PCR on formalin fixed and paraffin embedded small intestinal tissues from B5508 was performed since the inclusion bodies found in some intestinal sections suggested a potential viral etiology. Test results were negative.
DISCUSSION AND REVIEW OF THE LITERATURE
We report four baboons in which a diagnosis of a form of collagenous colitis was made on the basis of clinical features and, particularly, histopathologic appearances in two out of the four animals. The rapid recovery in response to discontinuation of IS therapy and initiation of budesonide, in combination with unresponsiveness to other treatments and negative bacterial and viral findings, adds further support for the diagnosis in the other two cases.
Collagenous colitis in humans
Collagenous colitis is a chronic inflammatory bowel disease that was first described by Lindström in 1976 (13). Clinical features are chronic watery diarrhea that is often of sudden onset, mimicking infectious diarrhea, accompanied by abdominal pain and weight loss. Dehydration is rarely seen in humans. The disease is mainly localized to the colon and rectum, and is characterized by a normal appearance of the mucosa on endoscopy and radiology, but with distinct histopathological changes seen on microscopic examination. Biopsy is therefore essential in making the diagnosis. Histopathological features include (i) a diffuse non-continuous thickening of a subepithelial collagen layer throughout the colon, (ii) inflammation of the lamina propria, dominated by lymphocytes and plasma cells, and (iii) flattening and vacuolization of the epithelial cells and detachment of the surface epithelium (14, 15).
The incidence of collagenous colitis would appear to be increasing (or at least being more frequently recognized). Pardi et al (16) reported an increased incidence from 0.3 to 6.2 per million between 1985 and 2001, and others reported an increase from 0.8 to 6.1 per million between 1984 and 1998 (3, 17). The peak incidence is around 65 years of age, and the female:male ratio is reported to be between 6:1 (17) and 9:1 (18).
The etiology and pathogenesis of collagenous colitis remain unknown, although an association with autoimmune diseases is generally accepted (19, 20). Treatment, therefore, remains mainly empirical. Currently, budesonide, a topical intestinal steriod that has efficacy in the terminal ileum and colon, is the drug of choice; it has been studied in several double-blind placebo-controlled trials, and has been demonstrated to significantly improve the patient’s condition within 2–4 weeks, although short-term treatment is associated with an approximate 60–80% relapse rate (21, 22). If treatment is continued for >8 weeks, the response is maintained for >6 months. We elected to maintain budesonide for 7 months to allow sufficient time for the baboons to return to a normal or near-normal rate of growth, similar to age-matched controls. The long-term prognosis of successfully treated collagenous colitis is good. Although the course of the disease is chronic with relapses in the majority of patients, symptoms can be controlled by maintenance treatment to maintain a good quality of life in the majority of patients (23). Serious complications, such as perforation of the colon, are rarely seen. Mortality is not increased compared to healthy subjects (24–26).
Of considerable interest in relation to our presented cases is a recent report indicating an increased risk of microscopic colitis in patients with solid organ transplants (kidney, kidney and pancreas, or liver) (6). The incidence of microscopic colitis was 8.8/1000 patients; three of seven patients were diagnosed with collagenous colitis, which is an approximately 1,000-fold higher incidence than in the general population (16, 18). The seven patients were receiving different immunosuppressive regimens that included drugs such as cyclosporine, tacrolimus, prednisone, sirolimus, MMF, and azathioprine. Six of the seven patients were given budesonide, and all responded well. The causative factors for the development of collagenous colitis in these patients remain uncertain, but the state of immunosuppression is very likely to be important.
Collagenous colitis in nonhuman primates
There are very few reports of collagenous or microscopic colitis in the literature. In a study at the Southwest Foundation for Biomedical Research (San Antonio, TX, USA) involving 3,200 baboons of <20 years of age, 132 died with chronic diarrhea. All were infants, juveniles, or young adults (i.e., <8 years of age). Diarrhea persisted for several months to >1 year. All were infected with endemic bacterial pathogens, particularly Campylobacter species and/or Balantidium coli, although these microorganisms were also found in healthy animals. Microscopic examination of the bowel was carried out in 86 of the baboons that died, and in only a single case were the appearances consistent with collagenous colitis. The histopathology was similar to that reported in humans, with a thick hyaline band underlying the superficial epithelium and the presence of chronic inflammatory cells (7). The same group also reported a study on 1,600 cynomolgus monkeys (Macaca fascicularis); 90 died with chronic diarrhea, but in no case was a diagnosis of collagenous colitis made (8). We conclude that collagenous colitis is very rare in naïve populations of nonhuman primates.
Diagnosis of collagenous colitis: comparison of findings in baboons and humans
Collagenous colitis was a primary differential diagnosis in all four baboons, although there were some differences from the appearances reported in humans (Table 3). First, the deposition of collagenous tissue in the baboons appeared to be deeper within, and involving a substantially greater portion of, the submucosal layer than described in humans, where it is confined to a region just beneath the surface epithelium. Second, there was no general increase in inflammatory cells in any layer of the large and small intestine except to a minimal degree in the small intestines of B5008 and B5708. However, the combination of clinical features, blood and stool cultures that were repeatedly negative for enteric pathogens, serum tests that were negative for viral pathogens, the normal macroscopic appearance of the intestinal mucosa, and the distinct deposition of abundant collagen in the colonic mucosa were all comparable to the clinical presentation in humans (Table 3), supporting a diagnosis of a variant form of collagenous colitis. Additionally, there is an association between collagenous colitis and microscopic findings of small intestinal villous necrosis and blunting (27, 28). Interestingly, this change was found in the small intestine of both the baboons B7507 and B5508, which were examined at time of active disease (Table 3, Figure 4).
Table 3.
Collagenous Colitis in Humans and Baboons: Comparison of Pathology
| Feature | Humans | Baboons |
|---|---|---|
| Gross intestinal lesions | No | No |
| Pathogens associated with disease onset | None | None |
| Collagen deposition | Extensive in colon | Extensive in colon |
| Small intestine involvement | Villous blunting and necrosis | Villous blunting and necrosis |
| Location of collagen deposition | Primarily subepithelial | Primarily submucosal |
| Inflammatory cell infiltrates | Yes; lymphocytes, plasma cells | Minimal |
Comments
In recent years, preclinical models for research in the field of organ and cell Tx have increasingly used nonhuman primates. These species are particularly important in xenotransplantation research as Old World nonhuman primates, e.g., baboons and Old World monkeys, are the only mammals (other than humans) to make antibodies directed to the important Galα1,3Gal antigens expressed in pigs (reviewed by Kobayashi and Cooper (29)). In almost all of these studies, the recipient nonhuman primate is immunosuppressed, which can be associated with complications similar to those in humans, particularly infection (30–32). One fairly common problem is diarrhea, often associated with the drugs the animal is receiving. If drug-induced, it can frequently be reversed by reduction or cessation of drug therapy. However, we have not knowingly seen collagenous colitis previously in >100 immunosuppressed baboons and cynomolgus monkeys, even when the period of immunosuppression extended for several months. Several of these baboons and monkeys -though almost all of these were adults or young adults rather than infants- developed diarrhea. However, the pathogenic microorganism was usually identified and it was generally easily controlled by empirical agents such as loperamide, anti-microbial agents, and/or reduction in immunosuppressive therapy.
Our own experience of collagenous colitis reported here is in some respects in contrast to that in humans, but in other respects similar. Our baboons were very young, rather than elderly, as in humans (3, 16), although there have been rare cases reported in children (4, 5, 33). Three of the four affected animals were male, rather than the predominance of females (as in humans). It is likely to be important that our baboons were immunosuppressed, as collagenous colitis is associated with an autoimmune state in humans. In the two baboons treated with budesonide, this drug rapidly induced remission (as in humans) and, in the absence of continuing immunosuppressive therapy, no relapse occurred either during the 7-month period of therapy or after cessation of therapy.
The four baboons had received immunosuppressive as well as supportive medication for periods ranging between 4–10 months (Tables 1 and 2). A review of the literature suggests an association between microscopic colitis and certain drugs, though not specifically between collagenous colitis and these drugs. Because of the small numbers of patients in these studies, discrimination between collagenous and lymphocytic colitis is often not made. However, drugs that have a high likelihood of inducing microscopic colitis include acarbose, aspirin, lansoprazole, non-steroidal anti-inflammatory agents, ranitidine, sertraline, and ticlopidine (34). The infant baboons in our study received none of these agents, except for the non-steroidal anti-inflammatory agent, ketoprofen, which all four baboons had received only once weekly at low-dose prior to anti-CD154 mAb administration. Olesen et al (3) suggested a weak association between ketoprofen and lymphocytic colitis, but not with collagenous colitis.
Importantly, once the diagnosis was confirmed or suspected, two of the baboons survived as a result of aggressive fluid replacement and treatment with budesonide. We would recommend that collagenous colitis should be suspected and investigated in any baboon, particularly if immunosuppressed, that develops diarrhea and weight loss that does not respond to conventional investigation and therapeutic measures. Awareness of this disease could prevent unnecessary morbidity and mortality in valuable nonhuman primates involved in experimental studies.
A potentially important observation from our own study was the occurrence of all 4 cases within a time span of 4 weeks (Figure 1), despite the fact that the period of time for which the baboons had been receiving immunosuppressive therapy ranged from 4–10 months. The outbreak of collagenous colitis in 4 baboons, housed in the same room, over such a short period of the study suggests to us an infectious etiology. One possible explanation for this could be that the two healthy controls had a subclinical infection to a common viral cause that did not lead to any symptoms, while the state of immunosuppression allowed the 4 experimental animals in the same room to suffer from an opportunistic infection. This contention is also strengthened by the presence of concurrent active inflammatory lesions as well as more chronic change (i.e., villous atrophy) in the small intestine of B7507 and B5508. It should be noted that cases of collagenous colitis associated with small intestinal villous atrophy are reported in the human literature (27, 28).
Whether the infectious agent was introduced to each individual baboon by an animal handler or other vector, or whether one infected baboon passed on the condition to others by direct or close contact remains unclear, but a common infectious agent leading to a pan enterocolitis with consequential severe colonic mucosal fibrosis cannot be ruled out. We would suggest that efforts to identify infectious agents as a causative factor in patients with proven collagenous colitis should be intensified.
Acknowledgments
EMD, MD is the recipient of fellowships from the Ter Meulen Fund of the Royal Netherlands Academy of Arts and Sciences and the Stichting Professor Michael van Vloten Fund, The Netherlands. GJE, MD, is the recipient of the Shelly Patrick Research Fellowship of the Thomas E. Starzl Transplantation Institute. Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is supported in part by NIH grants # U01 AI068642, # R21 A1074844 and # U19 AI090959, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA. The baboons were provided by the Oklahoma University Health Sciences Center, Division of Animal Resources, which is supported in part by NIH P40 sponsored grant RR012317-09.
ABBREVIATIONS
- Anti-CD154 mAb
anti-human CD154 monoclonal antibody
- IS
immunosuppressive therapy
- NHP
Non-human primate
- MMF
mycophenolate mofetil
- Tx
transplantation
References
- 1.Tangri V, Chande N. Microscopic colitis: an update. J Clin Gastroenterol. 2009;43(4):293–296. doi: 10.1097/MCG.0b013e31818f50ce. [DOI] [PubMed] [Google Scholar]
- 2.Nyhlin N, Bohr J, Eriksson S, et al. Systematic review: microscopic colitis. Aliment Pharmacol Ther. 2006;23(11):1525–1534. doi: 10.1111/j.1365-2036.2006.02913.x. [DOI] [PubMed] [Google Scholar]
- 3.Olesen M, Eriksson S, Bohr J, et al. Lymphocytic colitis: a retrospective clinical study of 199 Swedish patients. Gut. 2004;53(4):536–541. doi: 10.1136/gut.2003.023440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camarero C, Leon F, Colino E, et al. Collagenous colitis in children: clinicopathologic, microbiologic, and immunologic features. J Pediatr Gastroenterol Nutr. 2003;37(4):508–513. doi: 10.1097/00005176-200310000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Meunier S, Villard F, Bouvier R, et al. Collagen gastritis, an unusual cause of anemia in children. Report of 2 cases. Arch Pediatr. 2001;8(1):47–50. doi: 10.1016/s0929-693x(00)00166-4. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan GG, Seminowich S, Williams J, et al. The risk of microscopic colitis in solid-organ transplantation patients: a population-based study. Transplantation. 2008;85(1):48–54. doi: 10.1097/01.tp.0000298001.66377.a2. [DOI] [PubMed] [Google Scholar]
- 7.Rubio CA, Hubbard GB. Chronic colitis in baboons: similarities with chronic colitis in humans. In Vivo. 2001;15(1):109–116. [PubMed] [Google Scholar]
- 8.Rubio CA, Hubbard GB. Chronic colitis in Macaca fascicularis: similarities with chronic colitis in humans. In Vivo. 2002;16(3):191–195. [PubMed] [Google Scholar]
- 9.Rood PP, Tai HC, Hara H, et al. Late onset of development of natural anti-nonGal antibodies in infant humans and baboons: implications for xenotransplantation in infants. Transpl Int. 2007;20(12):1050–1058. doi: 10.1111/j.1432-2277.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 10.Selbst MK, Ahrens WA, Robert ME, et al. Spectrum of histologic changes in colonic biopsies in patients treated with mycophenolate mofetil. Mod Pathol. 2009;22(6):737–743. doi: 10.1038/modpathol.2009.44. [DOI] [PubMed] [Google Scholar]
- 11.Ducloux D, Ottignon Y, Semhoun-Ducloux S, et al. Mycophenolate mofetil-induced villous atrophy. Transplantation. 1998;66(8):1115–1116. doi: 10.1097/00007890-199810270-00027. [DOI] [PubMed] [Google Scholar]
- 12.Papadimitriou JC, Cangro CB, Lustberg A, et al. Histologic features of mycophenolate mofetil-related colitis: a graft-versus-host disease-like pattern. Int J Surg Pathol. 2003;11(4):295–302. doi: 10.1177/106689690301100406. [DOI] [PubMed] [Google Scholar]
- 13.Lindstrom CG. ‘Collagenous colitis’ with watery diarrhoea--a new entity? Pathol Eur. 1976;11(1):87–89. [PubMed] [Google Scholar]
- 14.Bohr J, Olesen M, Tysk C, et al. Collagenous and lymphocytic colitis: a clinical and histopathological review. Can J Gastroenterol. 2000;14(11):943–947. doi: 10.1155/2000/317573. [DOI] [PubMed] [Google Scholar]
- 15.Warren BF, Edwards CM, Travis SP. ‘Microscopic colitis’: classification and terminology. Histopathology. 2002;40(4):374–376. doi: 10.1046/j.1365-2559.2002.01341.x. [DOI] [PubMed] [Google Scholar]
- 16.Pardi DS. Microscopic colitis: an update. Inflamm Bowel Dis. 2004;10(6):860–870. doi: 10.1097/00054725-200411000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Bohr J, Tysk C, Eriksson S, et al. Collagenous colitis in Orebro, Sweden, an epidemiological study 1984–1993. Gut. 1995;37(3):394–397. doi: 10.1136/gut.37.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez-Banares F, Salas A, Forne M, et al. Incidence of collagenous and lymphocytic colitis: a 5-year population-based study. Am J Gastroenterol. 1999;94(2):418–423. doi: 10.1111/j.1572-0241.1999.00870.x. [DOI] [PubMed] [Google Scholar]
- 19.Olesen M, Eriksson S, Bohr J, et al. Microscopic colitis: a common diarrhoeal disease. An epidemiological study in Orebro, Sweden, 1993–1998. Gut. 2004;53(3):346–350. doi: 10.1136/gut.2003.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chande N, McDonald JW, Macdonald JK. Interventions for treating lymphocytic colitis. Cochrane Database Syst Rev. 2008;(2):CD006096. doi: 10.1002/14651858.CD006096.pub3. [DOI] [PubMed] [Google Scholar]
- 21.Miehlke S, Madisch A, Bethke B, et al. Oral budesonide for maintenance treatment of collagenous colitis: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2008;135(5):1510–1516. doi: 10.1053/j.gastro.2008.07.081. [DOI] [PubMed] [Google Scholar]
- 22.Bonderup OK, Hansen JB, Teglbjaerg PS, et al. Long-term budesonide treatment of collagenous colitis: a randomised, double-blind, placebo-controlled trial. Gut. 2009;58(1):68–72. doi: 10.1136/gut.2008.156513. [DOI] [PubMed] [Google Scholar]
- 23.Hjortswang H, Tysk C, Bohr J, et al. Defining clinical criteria for clinical remission and disease activity in collagenous colitis. Inflamm Bowel Dis. 2009 doi: 10.1002/ibd.20977. [DOI] [PubMed] [Google Scholar]
- 24.Bonner GF, Petras RE, Cheong DM, et al. Short- and long-term follow-up of treatment for lymphocytic and collagenous colitis. Inflamm Bowel Dis. 2000;6(2):85–91. doi: 10.1002/ibd.3780060204. [DOI] [PubMed] [Google Scholar]
- 25.Freeman HJ. Complications of collagenous colitis. World J Gastroenterol. 2008;14(11):1643–1645. doi: 10.3748/wjg.14.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allende DS, Taylor SL, Bronner MP. Colonic perforation as a complication of collagenous colitis in a series of 12 patients. Am J Gastroenterol. 2008;103(10):2598–2604. doi: 10.1111/j.1572-0241.2008.01998.x. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton I, Sanders S, Hopwood D, et al. Collagenous colitis associated with small intestinal villous atrophy. Gut. 1986;27(11):1394–1398. doi: 10.1136/gut.27.11.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maguire AA, Greenson JK, Lauwers GY, et al. Collagenous sprue: a clinicopathologic study of 12 cases. Am J Surg Pathol. 2009;33(10):1440–1449. doi: 10.1097/PAS.0b013e3181ae2545. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi T, Cooper DK. Anti-Gal, alpha-Gal epitopes, and xenotransplantation. Subcell Biochem. 1999;32:229–257. doi: 10.1007/978-1-4615-4771-6_10. [DOI] [PubMed] [Google Scholar]
- 30.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 31.Fishman JA. Infection in renal transplant recipients. Semin Nephrol. 2007;27(4):445–461. doi: 10.1016/j.semnephrol.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Fishman JA. Transplantation microbiology: an evolving pillar of transplant care. Am J Transplant. 2009;9(2):249–250. doi: 10.1111/j.1600-6143.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 33.Benchimol EI, Kirsch R, Viero S, et al. Collagenous colitis and eosinophilic gastritis in a 4-year old girl: a case report and review of the literature. Acta Paediatr. 2007;96(9):1365–1367. doi: 10.1111/j.1651-2227.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- 34.Beaugerie L, Pardi DS. Review article: drug-induced microscopic colitis -proposal for a scoring system and review of the literature. Aliment Pharmacol Ther. 2005;22(4):277–284. doi: 10.1111/j.1365-2036.2005.02561.x. [DOI] [PubMed] [Google Scholar]


