Abstract
Purpose of the study:
This study examines urban–rural differences in end-of-life (EOL) quality of care provided to nursing home (NH) residents.
Data and Methods:
We constructed 3 risk-adjusted EOL quality measures (QMs) for long-term decedent residents: in-hospital death, hospice referral before death, and presence of severe pain. We used CY2005-2007 100% Minimum Data Set, Medicare beneficiary file, and inpatient and hospice claims. Logistic regression models were estimated to predict the probability of each outcome conditional on decedents’ risk factors. For each facility, QMs were calculated as the difference between the actual and the expected risk-adjusted outcome rates. We fit multivariate linear regression models, with fixed state effects, for each QM to assess the association with urban–rural location.
Results:
We found urban–rural differences for in-hospital death and hospice QMs, but not for pain. Compared with NHs located in urban areas, facilities in smaller towns and in isolated rural areas have significantly (p < .001) worse EOL quality for in-hospital death and hospice use. Whereas the differences in these QMs are statistically significant between facilities located in large versus small towns, they are not statistically significant between facilities located in small towns and isolated rural areas.
Implications:
This study provides empirical evidence for urban–rural differences in EOL quality of care using a national sample of NHs. Identifying differences is a necessary first step toward improving care for dying NH residents and for bridging the urban–rural gap.
Keywords: Nursing homes, Quality of care, Rural & urban issues
The last two decades have witnessed important changes in the provision of end-of-life (EOL) health services to older Americans. During this time, the proportion of Americans dying in nursing homes (NHs) has increased from 16% in 1990 to 25% in 2001 (Brown Atlas, 2001) and is projected to grow to 40% by 2020 (Christopher, 2000). Research focusing on NH EOL care suggests that residents’ need for pain management (Teno, Bird, & Mor, 2007) as well as their and their families’ expectations about EOL treatments (Hanson, Danis, & Garrett, 1997) often have not been met. For example, only 30% of decedent NH residents had received hospice care and most just briefly prior to death (Miller, Gozalo, & Mor, 2010). At the same time, the risk of in-hospital deaths among NH decedents has continued to increase (Temkin-Greener, Zheng, & Mukamel, 2010). With NHs becoming a major setting in the provision of EOL care, concerns about care quality they provide continue to mount (Huskamp et al., 2010; Meier, Lim, & Carlson, 2010).
In the midst of these changes, geographic variations have remained the only constant as research continues to show that the care EOL patients receive depends largely on where they reside (Goodman, Esty, Fisher, & Chang, 2011). To date, research on urban–rural differences in NHs has been sparse, focusing largely on issues of access and utilization but rarely on facility-level quality differences (Kang, Meng, & Miller, 2011; Phillips, Holan, Sherman, Williams, & Hawes, 2004). Only a handful of studies have focused on urban–rural NH differences at the EOL. Based on a sample of residents with severe dementia, those living in rural NHs had lower intensity of medical care (Gessert, Haller, Kane, & Degenholtz, 2006), including feeding tube use (Gessert & Calkins, 2001) at the EOL. Residents with end-stage disease, living in rural NHs, were more likely to report frequent pain (Bolin, Phillips, & Hawes, 2006). Compared with their urban counterparts, NH residents in rural areas have increased odds of being transferred to acute care hospitals at the EOL (Menec, Nowicki, & Kalischuk, 2010). Much still remains to be learned about urban–rural differences in EOL care provision and quality.
In this study, we examined urban–rural differences in EOL quality of care provided to NH residents. We focused on three risk-adjusted measures of EOL quality: use of hospice, in-hospital death, and presence of severe pain. Hospice enrollment prior to death has been identified as a desirable outcome and a mechanism for improving EOL care quality (Gozalo & Miller, 2007; Teno et al., 2011). Studies have demonstrated that for NH residents, access to hospice may be more influenced by the facility and its location (Zerzan, Stearns, & Hanson, 2000), and by staff members’ ability to recognize terminal decline, knowledge about hospice, and belief in its efficacy (Welch, Miller, Martin, & Nanda, 2008), than by the residents’ treatment preferences. For long-term NH residents, in-hospital deaths are often considered inappropriate and a marker for poor EOL quality of care; almost half of hospitalizations leading to in-hospital deaths are potentially avoidable (Saliba et al., 2000) and are often inconsistent with residents’ preferences (Dobalian, 2004). Pain has long been endorsed as an important and highly prevalent measure for EOL quality of care (Teno et al., 2007), one that is highly dependent on the care provided, and correctable through proper assessment, treatment, and monitoring. With regard to these EOL quality measures (QMs), we addressed the following questions: (a) are there urban–rural differences in risk-adjusted EOL quality of care provided to decedent NH residents? and (b) if differences exist, what facility and environmental (market) characteristics help to explain them?
Conceptual Framework: An Ecological Model
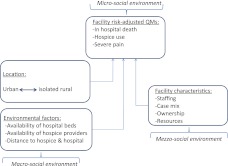
Our conceptual model can be summarized, with regard to each QM, as a function of facility characteristics, location, and environmental factors indicating service supply and distance (Figure 1). This model is derived from an ecological framework (Bronfenbrenner, 1986) and has been previously used to examine how various levels of factors influence EOL care in NHs (Blevins & Deason-Howell, 2002). This framework views individuals within a system of coexisting and interrelated environments (domains) each of which may influence EOL care quality. The individual (micro-social) environment refers to individuals’ characteristics, attitudes, and treatment preferences. The organizational (mezzo-social) environment refers to the characteristics of facilities. The environmental (macro-social) domain refers to the geographic location of facilities and their proximity to and the availability of services. Motivated by this framework, and based on prior research, we included individual resident characteristics in developing EOL QMs and NH and county-level factors in order to examine the relationships between these levels and care quality.
Figure 1.
Conceptual framework: An ecological model.
Quality Measures
Although a set of QMs is now posted quarterly on the Centers for Medicare and Medicaid Services’ (CMS) website, allowing the public to assess NHs on specific quality indicators, none of the QMs available today specifically address EOL quality. Only two measures—pain and depression—focus on symptoms that are also associated with EOL, as identified by the American Geriatrics Society, the Institute of Medicine, and the National Consensus Project for Quality of Palliative Care. However, neither of these quality indicators are measured on or reported specifically for EOL residents.
In selecting EOL QMs we focused on those that (a) address an effect of importance to EOL residents, (b) are affected by clinical care provided, and (c) can account for residents’ risks over which the NH has no control (Mukamel & Brower, 1998). The EOL QMs we examined in this study met these criteria. Numerous studies have documented that recognition and alleviation of pain is clearly a desirable outcome of major importance to NH residents, including those at the EOL (Lorenz, Rosenfeld, & Wenger, 2007). At the EOL, less aggressive treatments that do not result in in-hospital death and greater use of hospice care have been shown to be consistent with better quality of care (Saliba et al., 2005a, 2005b). These two QMs were of particular interest because they may be sensitive to NH location (Gessert et al., 2006). Since raw outcome rates are a function of both the residents’ risks and quality of care provided, quality may only be inferred from risk-adjusted rates (Mukamel et al., 2008a). Therefore, in constructing risk-adjusted QMs, we included information about residents’ relevant demographic and health status factors based on the last health assessment prior to death.
Methods
Study Design and Data Sources
We used individual, facility, and county data for CY2005-2007 (except as noted) obtained from nine national data sources. These included 100% Medicare denominator file, 100% Minimum Data Set (MDS), 100% Medicare Standard Analytical Files for inpatient and hospice claims, the Area Resource File (ARF) for 2007, the Provider of Service (POS) file for the third quarter of 2007, the zip code level Rural–Urban Commuting Area Codes (RUCA), and the Brown University’s Long-Term Care Facts website (http://ltcfocus.org/about.aspx).
The study sample consisted of decedent long-term NH residents aged 65 or older. We focused on long-term residents because typically postacute residents stay in NHs for a short period of time and are expected to return to the community. For them, death is not an expected outcome, and although it does occur, it is viewed as a failure of care. The Medicare denominator files were used to identify beneficiaries who died between January 1, 2005, and December 31, 2007. The MDS was used to select decedents who had an NH stay within 8 days prior to death, that is, who died in an NH or shortly after discharge or transfer to a different care setting. The MDS is a federally mandated process for clinical assessment of residents in Medicare and Medicaid certified NHs. It contains information on residents’ sociodemographics and health status at admission and at predetermined intervals thereafter or when health status significantly changes. The finder file of decedents with a prior NH stay was used to select all of their MDS assessments as well as inpatient hospital and hospice claims.
Facility-level characteristics were obtained from the RUCA files, the POS, and the Brown University Facts website and were linked using the unique Medicare provider number and zip code. County-level characteristics were obtained from the ARF database.
Study Population
We identified 963,313 Medicare eligible, aged 65+, decedent long-term NH residents who died between January 1, 2005, and December 31, 2007, and who resided in a Medicare and/or Medicaid certified facility (n = 15,954). Long-term residents were defined as those whose stay was not Medicare reimbursable or who stayed longer than 90 days.
We excluded 2,748 facilities, which could not be linked to the POS (n = 211); had missing zip codes and/or could not be assigned to a RUCA area (n = 908); were missing a case-mix index (n = 192); or had fewer than 20 decedents (n = 1,437), as QMs based on small sample size may not be reliable (Mukamel et al., 2008a) Our analytical sample consisted of 915,688 decedent long-term residents (95% of all eligible decedents) from 13,206 facilities.
Variables
Outcome Variables.—
We constructed three EOL outcome measures. Place of death (POD), was defined as dichotomous (1 if death occurred in a hospital, zero otherwise). Use of hospice obtained the value of 1 if the decedent used NH hospice within last 100 days of life and zero otherwise. Pain was identified as present (value of 1) if resident experienced moderate pain daily or excruciating pain at any frequency, based on the last MDS assessment prior to death; otherwise pain obtained the value of zero.
Key Variables of Interest.—
Urban–rural NH location was the variable of interest. Each facility was assigned a RUCA code, based on the NH’s zip code and the commuting patterns for the population residing in this zip code. Based on the RUCA codes, NHs were categorized as urban (i.e., city with a population >50,000 and its commuting area), large town (i.e., population of 10,000–49,999 and its commuting area), small town (i.e., population of 2,500–9,999 with some people commuting to an urban cluster), and isolated rural (i.e., fewer than 2,500 residents, primarily commuting to a tract outside an urban area or cluster). These four categories have been commonly used in other health-related studies (http://depts.washington.edu/uwruca/).
Other Control Variables.—
All other control variables were categorized as individual-level risk factors, facility-level characteristics, and environmental factors.
Individual-level risk factors.
We employed individual risk factors previously identified in developing EOL QMs for hospice use, in-hospital death (Mukamel, et al., 2011)., and pain. The risk factors were based on the information available in the MDS (version 2.0), and the following criteria were used in selecting them: (a) a characteristic likely to affect the outcome of interest and (b) a characteristic that is not likely to be influenced by the practice style of the facility (Mukamel, 1997).
All risk factors were identified from the last MDS assessment prior to death. Because the MDS assessments are mandated to occur every 90 days, they are not correlated with the date of death assuring that residents’ risk factors are randomly distributed during the EOL period. In our sample, the median time between the last assessment and death was 34 days; 25th and 75th percentiles were 14 and 61 days, respectively.
MDS assessments may be categorized as full (e.g., admission, annual, change of status) or partial (e.g., quarterly), depending on whether they include all available information on a resident or a subset. When the last MDS assessment was not a full assessment, some variables, for example, those indicating presence of a chronic illness, were imputed from a prior full assessment because such conditions were not likely to change between assessments. However, conditions of a more transient nature (e.g., pneumonia) could not and were not imputed from a prior record. This difference in the availability of information by assessment type required us to estimate separate risk-adjustment models depending on the type of assessment available prior to death. Risk factors that were included in the risk-adjustment models were presented in Supplementary Material.
Facility-level characteristics.
We constructed several variables reflecting facility characteristics that may be associated with the outcomes of interest. Studies have suggested that facility ownership and chain membership may influence EOL care. We defined both of these characteristics as dichotomous variables. It has been shown that the intensity of nurse staffing is directly related to the quality of EOL care in NHs (Temkin-Greener et al., 2009). We defined staffing capacity as the total number of nurse hr/resident/day (of registered nurses—RN, licensed practical nurses—LPN, and certified nurse assistants—CNA) and skilled care mix as the ratio of RN hours to LPN and CNA hours combined. Based on the literature, we included variables representing volume of residents—measured as facility bed size multiplied by its occupancy rate, residents’ acuity—measured by case mix at admission, and a proxy for facility resources—measured by percent of residents with Medicare or Medicaid as a primary payer (Cai, Mukamel, Veazie, Katz, & Temkin-Greener, 2011).
Environmental factors.
The environment within which each NH is located was characterized by the number of hospice providers in the county, number of hospital beds per 100 people aged 65 and older in the county, and distance (spatial distance between the centroids of zip codes) from an NH to closest hospice and hospital.
Analytical Approach
The statistical analyses were performed in three steps. First, we estimated separate risk adjustment models for each outcome (pain, in-hospital death, hospice), with the selected set of risk factors for each assessment type. These risk adjustment models were examined for face, content, and construct validity in our prior work (Mukamel, et al., 2011). Logistic regression models were fit at the individual resident level with random facility effects to account for resident clustering at the facility level. Risk factors with p values at .2 or greater were excluded from the final models, and an F test was used to examine their joint significance. The goodness of fit of the models was assessed by the C statistic. These models were used to predict, for each resident, the probability of each outcome conditional on the individual risk factors.
In the second step, we constructed a QM for each outcome. Each QM was defined as the difference between the actual (observed) facility outcome rate and the expected risk-adjusted outcome rate. The latter was calculated as the average of the predicted probabilities for all residents, given their risk factors and the available assessment type.
In the third step, we fit three multivariable regression models, with state fixed effects, for each QM. In each model, we included urban–rural status, facility-specific factors, and environmental characteristics.
Results
Are There Urban–Rural Differences in EOL QMs?—Descriptive Statistics
Most NHs were located in urban areas (n = 8,915), with 1,938 located in large towns and 1,567 and 1,182 in small towns and isolated rural areas, respectively. Analysis of facility-level characteristics indicated statistically significant (p < .001) differences between urban–rural NHs with regard to each of the three unadjusted outcomes (Table 1). Prevalence of hospice use increased from 19.08% in isolated rural facilities to 21.71% in small town facilities and to 24.32% and 37.15% in large town and urban areas, respectively. On the other hand, prevalence of in-hospital deaths was the highest in small town facilities (19.51%) and lowest in urban areas (16.81%). Similarly, prevalence of severe pain was lowest (13.14%) in urban NHs compared with rural facilities.
Table 1.
Study Population Characteristics (n = 13,206 nursing homes)
| Urban focused |
Large rural focused |
Small rural focused |
Isolated rural |
p Valuea | |||||
|
n = 8,519 |
n = 1,938 |
n = 1,567 |
n = 1,182 |
||||||
| M | SD | M | SD | M | SD | M | SD | ||
| Quality measures (O-E) | |||||||||
| Hospice enrollment | 0.12 | 0.21 | 2.720 × 10−3 | 0.21 | −0.02 | 0.21 | −0.06 | 0.21 | <.001 |
| In-hospital death | 4.192 × 10−3 | 0.09 | 0.02 | 0.09 | 0.04 | 0.10 | 0.02 | 0.10 | <.001 |
| Prevalence of severe pain | 0.01 | 0.08 | 0.02 | 0.09 | 0.02 | 0.09 | 0.02 | 0.09 | <.001 |
| Observed prevalence (%) | |||||||||
| Hospice enrollment | 37.15 | 22.77 | 24.32 | 22.28 | 21.71 | 21.88 | 19.08 | 21.89 | <.001 |
| In-hospital death | 16.81 | 9.74 | 18.23 | 10.10 | 19.51 | 11.14 | 17.41 | 10.51 | <.001 |
| Prevalence of severe pain | 13.14 | 11.13 | 15.01 | 12.41 | 14.68 | 10.49 | 14.44 | 10.04 | <.001 |
| Facility characteristics | |||||||||
| Admission case mix | 1.06 | 0.08 | 1.06 | 0.09 | 1.04 | 0.09 | 1.02 | 0.09 | <.001 |
| RN/CNA + LPN ratio (%) | 9.43 | 6.22 | 9.43 | 5.63 | 8.12 | 5.42 | 9.07 | 5.51 | <.001 |
| Total nurse hr/resident/day | 3.36 | 1.00 | 3.20 | 0.94 | 3.14 | 0.83 | 3.08 | 0.69 | <.001 |
| Beds × Occupancy | 111.32 | 63.74 | 88.14 | 38.09 | 75.54 | 32.29 | 61.26 | 26.79 | <.001 |
| % Medicaid | 61.40 | 19.73 | 65.00 | 14.78 | 66.51 | 14.22 | 64.43 | 14.54 | <.001 |
| % Medicare | 14.48 | 9.53 | 13.34 | 8.77 | 10.89 | 6.63 | 8.85 | 5.82 | <.001 |
| % For-profit (ownership) | 72.54 | 70.85 | 67.43 | 54.17 | <.001 | ||||
| % Chain membership | 55.52 | 57.78 | 55.68 | 48.39 | <.001 | ||||
| No. of hospice providers in the county | 9.60 | 16.84 | 1.40 | 1.46 | 0.79 | 1.06 | 0.55 | 0.92 | <.001 |
| No. of hospital beds per 100 people 65+ | 4.51 | 2.99 | 3.42 | 2.78 | 3.28 | 3.68 | 2.72 | 2.34 | <.001 |
| Distance to a hospice (miles) | 4.41 | 5.19 | 6.13 | 9.81 | 13.98 | 14.11 | 18.92 | 12.37 | <.001 |
| Distance to a hospital (miles) | 2.63 | 3.67 | 1.64 | 3.74 | 2.92 | 5.97 | 8.36 | 8.20 | <.001 |
Note: aBased on analysis of variance. CNA = certified nurse assistants; LPN = licensed practical nurses; O-E = observed-expected outcome rate; RN = registered nurses.
Depending on their location, NHs also differed with regard to other characteristics. For example, facilities in the more urbanized areas had more nursing staff and more highly skilled staff (i.e., higher RN ratio) compared with NHs in more rural areas. Compared with NHs located in isolated rural areas, urban facilities were more likely to be for-profit (72.54% vs. 54.17%), have chain membership (55.52% vs. 48.39%), be larger (111.32 residents vs. 61.26), and had fewer Medicaid residents (61.40% vs. 64.43%). Furthermore, there were significant differences in the number of hospice providers (9.60 vs. 0.55) and in the availability of hospital beds (4.51 vs. 2.72) in urban compared with the isolated rural areas.
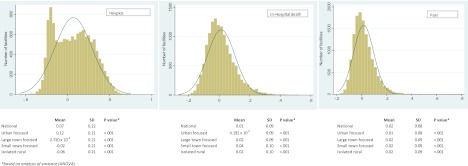
The distribution of risk-adjusted EOL QMs is presented in Figure 2. The risk adjustment models for all outcomes and all assessment types are presented in Supplementary Material. All models had similar goodness of fit, with C statistics ranging between 0.634 and 0.716, values that are typical for risk adjustment models of NH outcomes based on the MDS (Mukamel, 1997).
Figure 2.
Distribution of facility-level end-of-life quality measures.
The left-most panel in Figure 2 shows the distribution of hospice QM. With the average of 0.07 and the standard deviation of 0.22, this QM showed substantial variations across NHs. Since risk-adjusted hospice enrollment is considered to be a desirable outcome, QM values greater than zero indicate better quality. Compared with the national average, facilities located in the urban areas had significantly higher quality with regard to EOL hospice use (average = 0.12; SD = 0.21), whereas facilities in more rural areas had increasingly poorer quality (e.g., average for isolated rural facilities is −0.06; SD = 0.21). Differences in this QM were statistically significant (p < .001) in facilities located in urban compared with rural areas.
In the remaining two panels (Figure 2), we depicted the distribution of QMs for in-hospital death and presence of severe pain. The national average of the in-hospital death QM was 0.01 and of pain was 0.02. With standard deviations of 0.09 and 0.08, respectively, in-hospital death and pain QMs showed less variability across facilities than hospice QM. Unlike hospice, in-hospital death and presence of severe pain are considered to be undesirable outcomes; thus, QM values lower than zero indicate better quality. There were statistically significant (p < .001) differences in these QMs based on facility location.
What Facility/Environmental Factors Explain Urban–Rural Differences in EOL QMs?
The results of the multivariable regression model indicated that NHs located in more rural areas had significantly worse hospice QM compared with facilities in the urban areas (Table 2), even after adjusting for the effects of other covariates and the state fixed effects. For example, compared with urban NHs, facilities in large towns had significantly worse hospice QM (lower use; β = −.108), controlling for other conditions. The same statistically significant and monotonic relationship was observed when facilities located in small towns (β = −.132) and in isolated rural areas (β = −.143) were compared with NHs in urban areas. Although the difference in the hospice QM was not statistically significant between NHs in the isolated rural areas and those in small towns (p = .173), it was significant between facilities located in small and large towns.
Table 2.
Characteristics Predicting Quality Measures for Hospice, In-hospital Death, and Severe Pain: Multivariable Models With State Fixed Effects
| Hospice O-E |
In-hospital death O-E |
Severe pain O-E |
||||
| β | p Value | β | p Value | β | p Value | |
| Ref = urban focused | ||||||
| Large rural focused | −.108 | <.001 | .013 | <.001 | .001 | .707 |
| Small rural focused | −.132 | <.001a | .026 | <.001b | .002 | .434c |
| Isolated rural | −.143 | <.001d | .023 | <.001e | .001 | .792f |
| Admission case mix | −.173 | <.001 | .040 | <.001 | −.014 | .150 |
| RN/CNA + LPN ratio (per 10% increase) | −.014 | <.001 | −.005 | .001 | −.001 | .379 |
| Total nurse hours per resident per day | −.005 | .007 | .001 | .392 | 2.449 × 10−4 | .770 |
| Beds × Occupancy (per 10 increase) | .001 | .005 | 1.932 × 10−4 | .184 | −.001 | <.001 |
| % Medicaid (per 10% increase) | −.018 | <.001 | .011 | <.001 | .001 | .034 |
| % Medicare (per 10% increase) | −.007 | .004 | .007 | <.001 | .007 | <.001 |
| % For-profit (ownership) | .034 | <.001 | .007 | <.001 | −.006 | <.001 |
| % Chain membership | .027 | <.001 | −.008 | <.001 | −.003 | .051 |
| No. of hospice providers in the county | .001 | <.001 | .973 × 10−4 | .124 | −.001 | <.001 |
| No. of hospital beds per 100 people 65+ | 2.023 × 10−4 | .726 | 4.911 × 10−4 | .053 | 1.678 × 10−4 | .494 |
| Distance to a hospice (per 10 mile increase) | .001 | .626 | .001 | .570 | .001 | .162 |
| Distance to a hospital (per 10 mile increase) | .006 | .129 | −.010 | <.001 | .002 | .203 |
| Observations (n) | 13,067 | 13,065 | 13,067 | |||
Notes: Hospice is a desirable outcome, whereas in-hospital death and pain are not desirable. CNA = certified nurse assistants; LPN = licensed practical nurses; O-E=observed-expected outcome rate; RN = registered nurses.
Difference between small rural and large rural is significant (p < .001).
Difference between small rural and large rural is significant (p < .001).
Difference between small rural and large rural is not significant (p = .678).
Difference between isolated rural and small rural is not significant (p = .173).
Difference between isolated rural and small rural is not significant (p = .438).
Difference between isolated rural and small rural is not significant (p = .718).
With regard to in-hospital deaths, NHs in less-urbanized areas showed poorer QMs (Table 2). Compared with urban-based facilities, those located in large (β = .013), small (β = .026), and isolated rural (β = .023) areas had significantly worse hospital QMs (more in-hospital deaths), controlling for all other conditions. Although differences in QMs between large and small towns were statistically significant, the difference between isolated rural and small town areas was not.
We found no statistically significant differences across facilities in severe pain QM based on location.
Several facility and environmental factors were statistically significant predictors of the QMs of interest. Regardless of the location, NHs with higher admission case mix had significantly lower use of hospice (β = −.173) and were more likely to have residents who died in a hospital (β = .040). Facilities with higher skilled care mix (RN/CNA + LPN ratio) were less likely to use hospice (β = −.014) but more likely to have had fewer residents dying in hospitals (β = −.005). We found significant relationship between the proportion of residents who were Medicaid and Medicare and all three QMs, but these associations, although statistically significant, tended to have very small coefficients. Overall, for-profit facilities appeared to use more hospice (β = .034) and had fewer residents with severe pain (β = −.006; better QMs) and more in-hospital deaths (β = .007; worse QM) compared with not-for-profit NHs. Facilities with chain membership were similar to the for-profits with regard to hospice and pain QMs but had better in-hospital death QMs (β = −.008).
Several environmental factors were independently associated with EOL QMs. NHs located in counties with higher number of hospice providers had better hospice QMs (β = .001) and better pain QMs (β = −.001). NHs in counties with higher availability of hospital beds had worse hospital death QMs (β = .001). When distance between an NH and a hospital was longer, in-hospital death QMs were better (fewer in-hospital deaths; β = −.010).
Discussion
Our findings suggest that geography may, in some instances, indeed be destiny as the quality of EOL care in NHs depends to a very large extent on where the facility is located. Adjusting for residents’ health status and preferences at the EOL, we explored the interrelationships between three EOL QMs and facility location, its structural and organizational characteristics, and the environmental/market factors. We found that of the three QMs only one, pain, showed no difference with regard to locality, whereas the other two measures, hospice enrollment and in-hospital death, suggested better quality in urban NHs. Providing explanations for this phenomenon is beyond the scope of this paper and would require additional and a different type of research, but we offer several scenarios that might shed some light on the differences we observed and help guide future research.
We detected no statistically significant differences between rural and urban NHs in the pain QM. Research has demonstrated that NH workers are largely not sufficiently trained to recognize EOL symptoms, including pain, or to provide palliative/EOL care (Zimmerman, Sloan, Hanson, Mitchell, & Shy, 2003). Our findings suggest that any deficiencies in such training may be similarly distributed across the urban–rural continuum. We also note that the pain QM reported in the NH Compare web-based report card published by CMS, was one of the few QM showing improvement following publication (Mukamel, Weimer, Spector, Ladd, & Zinn, 2008b). The attention paid to this outcome and the overall improvement in it might explain why we did not observe any urban–rural differential as facilities, regardless of locality, may have similar opportunities to improve this QM.
The in-hospital death QM suggests that rural NHs may have been more likely to hospitalize their residents prior to death compared with their urban counterparts, even after controlling for other facility and resident characteristics, including the presence of the do-not-hospitalize orders. This finding appears to be partially consistent with prior research. Gessert and colleagues (2006) reported that NH decedents with cognitive impairment, living in rural facilities, were more likely to be hospitalized at the EOL than their urban counterparts and received higher intensity of medical care.
Like the pain QM, the decision to hospitalize is to a large degree under the control of the facility and is thought to reflect ingrained practice styles (Grabowski, Stewart, Broderick, & Coots, 2008). The systematic differences between rural and urban NHs, with regard to this QM, may be attributed to lower staffing, particularly of RNs, in rural facilities (although we controlled for this in our model, there might still be second order effects that remain unaccounted) or possibly a lower availability of physicians, which we did not explicitly control for in our analysis (due to lack of data).
The hospice QM is different from the pain and place of death QMs because it is not completely under the control of the NH but also depends on residents’ and their family members’ preferences and on the availability of this service in the community. For example, some have suggested that black residents are less likely to use hospice because they (or their family members) prefer more aggressive EOL treatment (Kwak, Haley, & Chiriboga, 2008). However, it has also been shown that although within the same facility, there may be no Black–White differentials with regard to hospice use, facilities that disproportionately serve minority residents do have lower rates of hospice use compared with the more racially balanced NHs (Zheng, Mukamel, Cai, & Temkin-Greener, 2011); a pattern that may also reflect differences in NH practice styles.
With respect to service availability, urban areas are likely to have a larger supply of hospice providers and closer proximity of those provides to NHs, thus decreasing potential barriers to hospice utilization. Indeed, we found that residents in urban NHs were more likely to enroll in hospice, even after controlling for all other individual and facility characteristics.
We are not aware of other studies that specifically examined urban–rural differences in hospice use in NHs. However, rates of hospice use by Medicare beneficiaries at large have been previously shown to be significantly lower (by 56%) in isolated rural compared with urban areas (Virnig, Moscovice, Durham, & Casey, 2004). Most studies have attributed such differences to limited access to hospice in rural compared with urban areas (Virnig, Hartman, Moscovice, & Carlin, 2006). But the more recent studies, which better reflect the substantial growth among hospice providers, suggest that although variations in access continue to exist, geographic access to hospice care is now widespread throughout the United States (Carlson, Bradley, Du, & Morrison, 2010). Although NHs in rural areas tend to have a significantly longer distance to hospice, we did not find distance to be a statistically significant factor in explaining hospice enrollment. However, it is possible that in rural locations our measure of distance was not sufficiently sensitive to detect barriers that location and availability of providers may present. It is not clear to what extent differences in NH hospice use reflect differentials in practice patterns or access to hospice services that are specific to facility location.
Overall, research comparing the quality of care in urban–rural NHs has been scarce. A study by Phillips and colleagues (2004) employed several measures of quality among long-term residents and concluded “one sees arguably better outcomes of care for residents in homes located in isolated areas.” With regard to EOL quality of care, our findings demonstrated the opposite, that is, rural NHs perform significantly worse than urban NHs on QMs indicating more aggressive treatment. This apparent contradiction is not, however, totally unexpected as lack of correlation between performance measures for different outcomes has been well documented in NHs as well as in other long-term care settings.
The differences we observed in urban–rural practice patterns may well be a function of resources and organizational relationships, which nursing facilities maintain, by choice or by necessity, with other health care providers. Several factors, such as lower staffing, lower physician availability, and greater reliance on Medicaid revenues (indicating fewer resources) may reduce the capacity of rural NHs to provide care for residents whose health is failing, thus lowering the threshold for transferring residents to hospitals where they subsequently die. It has been suggested that rural NHs are more likely than urban to have administrative relationships with acute care hospitals (Shah, Fennell, & Mor, 2001). Such affiliations may provide increased opportunities for or pressures on rural NHs to hospitalize their residents, including at the EOL. These may be more pronounced when the two types of facilities are colocated or within a relatively short distance of each other. Indeed, as our findings suggest, as distance between NHs and hospitals increased the in-hospital death QM significantly decreased.
Hospice requires physician referral, and while this is not unique to rural areas, physician shortage and high physician turnover are. Thus, rural NHs may face additional obstacles referring their residents to hospice. NHs’ relationship with hospice may be further complicated in rural areas as Medicare hospice payments there are substantially lower than in urban hospices, adjusting for wage differences (MedPac, 2004). However, these payments are not adjusted for other factors such as higher transportation costs that may be faced by rural hospice providers. Furthermore, compared with urban hospices, rural hospices are more likely to be smaller, hospital-based, and owned (Virnig et al., 2004). They may have less capacity to provide on-site care in NHs, thus requiring rural NHs to transfer their failing residents out of the facility for hospice care.
Several limitations of this study should be acknowledged. As always, there is a threat of omitted variable bias. However, we included many important predictors of NH quality at the individual, facility, and county levels. We accounted for the number of hospice providers and distance but not for their capacity to provide care. It is possible that rural hospices are smaller, rely more heavily on part-time employees, face greater challenges recruiting and retaining staff and may thus have lower capacity to provide sufficient care to nearby NHs. Although we included as risk adjustors information on residents’ preferences for hospital treatment and for cardiopulmonary resuscitation, we could not account in this study for preferences of the residents’ family members, which are well known to often supersede the residents’ wishes, especially when advance directives are not clearly written and are subject to interpretation.
In conclusion, our analysis suggests that compared with residents of urban NHs, individuals residing in rural facilities may be receiving poorer EOL quality of care with regard to hospice use and in-hospital death. Further research is needed to identify the reasons behind these differentials in order to develop specific strategies for bridging these gaps.
Funding
We gratefully acknowledge funding support from the National Institute of Nursing Research grant NR010727.
Supplementary Material
Supplementary material can be found at: http://gerontologist.oxfordjournals.org.
References
- Blevins D, Deason-Howell L. End-of-life care in nursing homes: The interface of policy, research, and practice. Behavioral Sciences and the Law. 2002;20:271–286. doi: 10.1002/bsl.486. [DOI] [PubMed] [Google Scholar]
- Bolin JN, Phillips CD, Hawes C. Urban and rural differences in end-of-life pain treatment status on admission to a nursing facility. American Journal of Hospice & Palliative Care. 2006;23:51–57. doi: 10.1177/104990910602300109. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U. Ecology of the family as a context for human development: Research perspective. Developmental Psychology. 1986;22:726–742. [Google Scholar]
- Brown Atlas. Facts on dying. 2001. Retrieved from http://www.chcr.brown.edu/dying/FACTSONDYING.HTM. [Google Scholar]
- Cai S, Mukamel D, Veazie P, Katz P, Temkin-Greener H. Hospitalizations in nursing homes: Does payer source matter—Evidence from New York State. Medical Care Research and Review. 2011;68:559–578. doi: 10.1177/1077558711399581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M, Bradley E, Du Q, Morrison RS. Geographic access to hospice in the United States. Journal of Palliative Medicine. 2010;13:1331–1338. doi: 10.1089/jpm.2010.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher M. Benchmarks to improve end of life care. Kansas City, MO: Midwest Bioethics Center; 2000. [Google Scholar]
- Dobalian A. Nursing facility compliance with do-not-hospitalize orders. The Gerontologist. 2004;44:159–165. doi: 10.1093/geront/44.2.159. [DOI] [PubMed] [Google Scholar]
- Gessert C, Calkins D. Rural-urban differences in end-of-life care: The use of feeding tubes. Journal of Rural Health. 2001;17:16–24. doi: 10.1111/j.1748-0361.2001.tb00250.x. [DOI] [PubMed] [Google Scholar]
- Gessert C, Haller I, Kane R, Degenholtz H. Rural-urban differences in medical care for NH residents with severe dementia at the end of life. Journal of the American Geriatrics Society. 2006;54:1199–1205. doi: 10.1111/j.1532-5415.2006.00824.x. [DOI] [PubMed] [Google Scholar]
- Goodman DC, Esty AR, Fisher E, Chang C. Trends and variation in end-of-life care for Medicare beneficiaries with severe chronic illness. The Dartmouth Insitute; 2011. http://www.dartmouthatlas.org/downloads/reports/EOL_Trend_Report_0411.pdf. [PubMed] [Google Scholar]
- Gozalo P, Miller SC. Hospice enrollment and evaluation of its causal effect on hospitaliation of dying NH residents. Health Services Research. 2007;42:587–610. doi: 10.1111/j.1475-6773.2006.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski D, Stewart A, Broderick S, Coots L. Predictors of NH hospitalization: A review of the literature. Medical Care Research and Review. 2008;65:3–39. doi: 10.1177/1077558707308754. [DOI] [PubMed] [Google Scholar]
- Hanson LC, Danis M, Garrett J. What is wrong with end-of-life care? Opinions of bereaved family members. Journal of the American Geriatrics Society. 1997;45:1339–1344. doi: 10.1111/j.1532-5415.1997.tb02933.x. [DOI] [PubMed] [Google Scholar]
- Huskamp HA, Stevenson DG, Chernew ME, Newhouse JP. A new medicare end-of-life benefit for nursing home residents. Health Affairs. 2010;29:130–135. doi: 10.1377/hlthaff.2009.0523. [DOI] [PubMed] [Google Scholar]
- Kang Y, Meng H, Miller NA. Rurality and nursing home quality: Evidence from the 2004 National Nursing Home Survey. The Gerontologist. 2011 doi: 10.1093/geront/gnr065. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Kwak J, Haley WE, Chiriboga DA. Racial differences in hospice use and in-hospital death among Medicare and Medicaid dual-eligible nursing home residents. The Gerontologist. 2008;48:32–41. doi: 10.1093/geront/48.1.32. [DOI] [PubMed] [Google Scholar]
- Lorenz K, Rosenfeld K, Wenger N. Quality indicators for palliative and end-of-life care in vulnerable elders. Journal of the American Geriatrics Society. 2007;55(Suppl. 2):S318–S326. doi: 10.1111/j.1532-5415.2007.01338.x. [DOI] [PubMed] [Google Scholar]
- MedPac. Report to congress: New approaches in Medicare. Washington, DC: 2004. http://www.medpac.gov/documents/June04_Entire_Report.pdf. [Google Scholar]
- Meier DE, Lim B, Carlson M. Raising the standard: Palliative care in nursing homes. Health Affairs. 2010;29:136–140. doi: 10.1377/hlthaff.2009.0912. [DOI] [PubMed] [Google Scholar]
- Menec V, Nowicki S, Kalischuk A. Transfer to acute care hospitals at the end of life: do rural/remote regions differ from urban regions? Rural and Remote Health. 2010;10:1281. http://www.rrh.org.au. [PubMed] [Google Scholar]
- Miller S, Lima J, Gozalo PL, Mor V. The growth of hospice care in U.S. nursing homes. Journal of the American Geriatrics Society. 2010;58:1481–1488. doi: 10.1111/j.1532-5415.2010.02968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamel DB. Risk adjusted outcome measures and quality of care in nursing homes. Medical Care. 1997;35:367–385. doi: 10.1097/00005650-199704000-00007. [DOI] [PubMed] [Google Scholar]
- Mukamel DB, Brower CA. The influence of risk adjustment methods on conclusions about quality of care in nursing homes based on outcome measures. The Gerontologist. 1998;38:695–703. doi: 10.1093/geront/38.6.695. [DOI] [PubMed] [Google Scholar]
- Mukamel DB, Caprio T, Ahn R, Zheng NT, Norton S, Quill T, et al. End-of-life quality of care measures for nursing homes: Place of death and hospice. Journal of Palliative Medicine, 2011. in press. [DOI] [PMC free article] [PubMed]
- Mukamel DB, Glance LG, Li Y, Spector WD, Zinn JS, Mosqueda L. Does risk adjustment of the CMS quality measures for nursing homes matter? Medical Care. 2008a;46:532–541. doi: 10.1097/MLR.0b013e31816099c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamel DB, Weimer D, Spector W, Ladd H, Zinn JS. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Services Research. 2008b;43:1244–1262. doi: 10.1111/j.1475-6773.2007.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C, Holan S, Sherman M, Williams M, Hawes C. Rurality and nursing home quality: Results from a national sample of nursing home admissions. American Journal of Public Health. 2004;94:1717–1722. doi: 10.2105/ajph.94.10.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba D, Kington R, Buchanan J, Bell R, Wang M, Lee M, et al. Appropriateness of the decision to transfer nursing facility residents to the hospital. Journal of the American Geriatrics Society. 2000;48:154–163. doi: 10.1111/j.1532-5415.2000.tb03906.x. [DOI] [PubMed] [Google Scholar]
- Saliba D, Solomon D, Rubenstein L, Young R, Schnelle J, Roth C, et al. Feasibility of quality indicators for the measurement of geriatric syndromes in nursing home residents. Journal of the American Medical Directors Association. 2005a;6(Suppl.):50–59. doi: 10.1016/j.jamda.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Saliba D, Solomon D, Rubenstein L, Young R, Schnelle J, Roth C, et al. Quality indicators for the management of medical conditions in nursing home residents. Journal of the American Medical Directors Association. 2005b;6(Suppl.):36–48. doi: 10.1016/j.jamda.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Shah A, Fennell M, Mor V. Hospital diversification into long-term care. Health Care Management and Review. 2001;26:86–100. doi: 10.1097/00004010-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Temkin-Greener H, Zheng N, Norton S, Quill T, Ladwig S, Veazie P. Measuring end-of-life care processes in nursing homes. The Gerontologist. 2009;49:803–815. doi: 10.1093/geront/gnp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin-Greener H, Zheng TN, Mukamel DB. Where do nursing home residents die: A National Study CY2003-2007. 2010, June. Podium Presentation at the Academy Health Annual Research Meeting, Boston, MA. [Google Scholar]
- Teno J, Bird C, Mor V. The prevalence and treatment of pain in US nursing homes. Providence, RI: Center for Gerontology and Health Care Research at brown University; 2007. [Google Scholar]
- Teno J, Gozalo P, Lee I, Kuo S, Spence C, Connor S, et al. Does hospice improve quality of care for persons dying from dementia? Journal of the American Geriatrics Society. 2011;59:1531–1536. doi: 10.1111/j.1532-5415.2011.03505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virnig BA, Hartman L, Moscovice I, Carlin B. Access to home-based hospice care for rural populations: Identification of areas lacking service. Journal of Palliative Medicine. 2006;9:1292–1299. doi: 10.1089/jpm.2006.9.1292. [DOI] [PubMed] [Google Scholar]
- Virnig BA, Moscovice I, Durham S, Casey M. Do rural elders have limited access to Medicare hospice services? Journal of the American Geriatrics Society. 2004;52:731–735. doi: 10.1111/j.1532-5415.2004.52213.x. [DOI] [PubMed] [Google Scholar]
- Welch LC, Miller SC, Martin EW, Nanda A. Referral and timing of referral to hospice care in nursing homes: The significant role of staff members. The Gerontologist. 2008;48:477–484. doi: 10.1093/geront/48.4.477. [DOI] [PubMed] [Google Scholar]
- Zerzan J, Stearns S, Hanson L. Access to palliative care and hospice in nursing homes. Journal of the American Medical Association. 2000;284:2489–2494. doi: 10.1001/jama.284.19.2489. [DOI] [PubMed] [Google Scholar]
- Zheng N, Mukamel D, Cai S, Temkin-Greener H. Racial dispartities in in-hospital death and hospice use among nursing home residents. Medical Care. 2011;49:992–998. doi: 10.1097/MLR.0b013e318236384e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S, Sloan PD, Hanson L, Mitchell CM, Shy A. Staff perceptions of end-of-life care in long-term care. Journal of the American Medical Directors Association. 2003;4:23–26. doi: 10.1097/01.JAM.0000046935.64053.54. [DOI] [PubMed] [Google Scholar]