Abstract
Background:
Animal models suggest that immunomodulatory properties of macrolide antibiotics have therapeutic value for patients with acute lung injury (ALI). We investigated the association between receipt of macrolide antibiotics and clinical outcomes in patients with ALI.
Methods:
Secondary analysis of multicenter, randomized controlled trial data from the Acute Respiratory Distress Syndrome Network Lisofylline and Respiratory Management of Acute Lung Injury Trial, which collected detailed data regarding antibiotic use among participants with ALI.
Results:
Forty-seven of 235 participants (20%) received a macrolide antibiotic within 24 h of trial enrollment. Among patients who received a macrolide, erythromycin was the most common (57%), followed by azithromycin (40%). The median duration of macrolide use after study enrollment was 4 days (interquartile range, 2-8 days). Eleven of the 47 (23%) patients who received macrolides died, compared with 67 of the 188 (36%) who did not receive a macrolide (P = .11). Participants administered macrolides were more likely to have pneumonia as an ALI risk factor, were less likely to have nonpulmonary sepsis or to be randomized to low tidal volume ventilation, and had a shorter length of stay prior to trial enrollment. After adjusting for potentially confounding covariates, use of macrolide was associated with lower 180-day mortality (hazard ratio [HR], 0.46; 95% CI, 0.23-0.92; P = .028) and shorter time to successful discontinuation of mechanical ventilation (HR, 1.93; 95% CI, 1.18-3.17; P = .009). In contrast, fluoroquinolone (n = 90) and cephalosporin antibiotics (n = 93) were not associated with improved outcomes.
Conclusions:
Receipt of macrolide antibiotics was associated with improved outcomes in patients with ALI.
Acute lung injury (ALI) is a syndrome of acute inflammatory pulmonary edema estimated to affect 200,000 people in the United States yearly, with a mortality rate of 30% to 40%.1 Thus far, only a low tidal volume lung-protective ventilation strategy has been demonstrated to reduce mortality in ALI.2 Although numerous pharmacologic interventions for ALI have been studied, none has been shown to reduce mortality.3‐8
Macrolide antibiotics have pulmonary antiinflammatory actions beyond their antimicrobial activity,9‐11 and have potential clinical benefit in chronic lung diseases such as panbronchiolitis12 and cystic fibrosis.13 Animal models suggest a therapeutic role for macrolide antibiotics in ALI.14‐20
We sought to investigate the association between macrolide antibiotics and mortality in patients with ALI using data from the Acute Respiratory Distress Syndrome Network (ARDSNet) Lisofylline and Respiratory Management of Acute Lung Injury (LARMA) trial.8 We hypothesized that use of macrolide antibiotics would be associated with reduced mortality in patients with ALI.
Materials and Methods
Data Source
We used deidentified, open-access data from subjects previously enrolled in the ARDSNet LARMA trial, during which antibiotic type and timing was recorded in detail for all participants. Details of LARMA have been published previously.8 Briefly, participants were enrolled from 21 hospitals across the United States within 36 h of meeting ALI consensus criteria21 and were randomized via 2 × 2 factorial design to receive 6 mL or 12 mL/kg tidal volumes (as part of the Respiratory Management of ALI [ARMA] trial) and lisofylline or placebo.All study procedures were approved by the Boston University School of Medicine Institutional Review Board (H-31026) with a data use agreement through the National Heart, Lung and Blood Institute’s Biologic Specimen and Data Repository Information Coordinating Center.
Subject Characteristics
We recorded prerandomization subject characteristics, including demographics, comorbidities, lung injury risk factors, mechanical ventilation parameters, randomization group, and duration of inpatient care prior to study enrollment. We calculated Simplified Acute Physiology II Score (SAPS II)22 and Brussels Organ Failure Scores23 for all subjects.
Antibiotic Exposures
Our primary area of interest was receipt of a macrolide antibiotic (azithromycin, clarithromycin, or erythromycin) within the first 24 h of trial enrollment (ie, the first 60 h of ALI). We chose the 24-h time window in order to reduce immortal time bias that might be associated with macrolide use later in a participant’s course.24
In order to investigate a potential class effect of any antibiotic effective against atypical pathogens, we performed a secondary analysis investigating the association between receipt of a fluoroquinolone and survival. We performed additional analyses for any antibiotic coadministered in > 50% of patients who received a macrolide.
Outcomes
Our a priori primary outcome was multivariable-adjusted 180-day survival. As a secondary outcome, we evaluated time to successful discontinuation of mechanical ventilation. Time to successful discontinuation of mechanical ventilation, an analog of ventilator-free days,25 was defined as the time to achieve 48 h of unassisted breathing, censored at day 28. Because death biases toward fewer ventilator days, participants who died prior to day 28 were assigned as having 28 ventilator days.25
Statistical Methods
We analyzed differences in continuous and categorical variables with Wilcoxon rank sum and Fisher exact tests, respectively. Given the likelihood of imbalanced covariates among antibiotic exposure groups, we used multivariable-adjusted Cox proportional hazards models as our a priori primary analysis to evaluate adjusted differences in survival and time to successful discontinuation of ventilation among antibiotic groups. We included the a-priori-defined covariates age and SAPS II, and covariates imbalanced with P < .10 among antibiotic exposure groups. Stepwise regression was used to select among collinear covariates for inclusion in the multivariable models (eg, between durations of hospital stay and ICU stay prior to trial enrollment). We evaluated the proportional hazards assumption with log-log plots and tests of interactions among time, macrolide exposure, and survival. We used unadjusted and adjusted Kaplan-Meier survival plots to visually demonstrate survival differences among the antibiotic groups.
As a sensitivity analysis, we used propensity scores to adjust for potential confounding by indication for each antibiotic in Cox proportional hazards models. Nonparsimonious propensity scores for use of each antibiotic were calculated using multivariable logistic regression models, including all measured covariates with < 20% missing data.26,27 Propensity score model discrimination was measured via the area under the receiver operating curve (C statistic).
Because macrolides are often used to treat pneumonia, we evaluated the interaction between pneumonia diagnosis and the association between macrolide and mortality. In addition, we tested for interaction between randomization to the 6 mL/kg or 12 mL/kg group and the association between macrolide and mortality. We chose an α level of 0.05. SAS software, version 9.1 was used for all statistical analyses; survival plots were generated with PASW statistics 18.0.
Results
The characteristics of the 235 study participants stratified by macrolide exposure are shown in Table 1. Analogous results for fluoroquinolone use are shown in Table 2.
Table 1.
—Baseline Characteristics of Study Cohort Stratified by Macrolide Exposure
| Variable | Macrolide (n = 47) | No Macrolide (n = 188) | P Value |
| Age, y | 51 ± 16 | 51 ± 17 | .88 |
| Sex, male | 30 (64) | 115 (62) | .87 |
| Race, white | 36 (77) | 143 (76) | .47 |
| BMI, kg/m2 (n = 228) | 27.5 ± 7.3 | 28.3 ± 7.6 | .57 |
| Primary ALI risk factor | |||
| Pneumonia | 36 (77) | 48 (26) | < .001a |
| Nonpulmonary sepsis | 4 (8.5) | 54 (29) | .004a |
| Aspiration | 12 (13) | 26 (18) | .47 |
| Trauma | 5 (6) | 17 (12) | .17 |
| Multiple transfusions | 0 | 6 (4) | .18 |
| Other | 8 (10) | 19 (12) | .83 |
| SAPS II | 45.8 ± 14.5 | 48.0 ± 15.1 | .61 |
| Brussels Organ Failure Score | 1.72 ± 0.85 | 1.86 ± 1.06 | .60 |
| Comorbidities | |||
| Diabetes | 10 (21) | 32 (17) | .42 |
| AIDS | 4 (9) | 10 (5) | .49 |
| Metastatic or hematologic malignancy | 2 (4) | 12 (6) | .74 |
| End-stage renal failure/dialysis | 0 | 4 (2) | .59 |
| Radiographic lung injury score (n = 232) | 3.7 ± 0.6 | 3.7 ± 0.6 | … |
| Tidal volume, mg/kg of IBW (n = 157) | 9.7 ± 2.0 | 10.4 ± 2.0 | .14 |
| Pao2/Fio2 (n = 221) | 147 ± 69 | 141 ± 54 | .96 |
| PEEP, cm H2O | 10.2 ± 5.2 | 8.7 ± 3.8 | .11 |
| Pplat, cm H2O (n = 185) | 31 ± 8.1 | 30 ± 8.8 | .52 |
| Peak pressure, cm H2O (n = 213) | 38 ± 11 | 37 ± 10 | .59 |
| Paco2, mm Hg (n = 221) | 36 ± 9.5 | 36 ± 8.5 | .83 |
| Arterial pH | 7.41 ± 0.08 | 7.40 ± 0.07 | .58 |
| Minute ventilation, L/min | 13 ± 4.5 | 13 ± 3.8 | .74 |
| Duration of intubation prior to enrollment, median (IQR), d | 1 (1-1) | 1 (1-2) | .13 |
| Duration of ICU stay prior to enrollment, median (IQR), d | 1 (0-1) | 1 (1-2) | .0004a |
| Duration of hospitalization prior to enrollment, median (IQR), d | 1 (1-2) | 2 (1-5) | .01a |
| Randomized to low tidal volume | 20 (43) | 115 (61) | .03a |
| Randomized to lisofylline | 23 (49) | 93 (49) | 1.0 |
Continuous variables are presented as mean ± SD and categorical variables are presented as No. (%) unless indicated otherwise. N = 235, unless otherwise noted. ALI = acute lung injury; IBW = ideal body weight; IQR = interquartile range; PEEP = positive end-expiratory pressure; Pplat = plateau pressure; SAPS II = Simplified Acute Physiology II Score.
P < .1 and consideration for inclusion in multivariable model.
Table 2.
—Baseline Characteristics of Study Cohort Stratified by Fluoroquinolone Exposure
| Variable | Fluroquinolone (n = 90) | No Fluoroquinolone (n = 145) | P Value |
| Age, y | 51 ± 17 | 51 ± 17 | .98 |
| Sex, male | 47 (52) | 98 (68) | .02a |
| Race, white | 71 (79) | 108 (75) | .53 |
| BMI, kg/m2 | 28.6 ± 8.3 | 27.9 ± 7.1 | .88 |
| Primary ALI risk factor | |||
| Pneumonia | 39 (43) | 45 (31) | .07a |
| Sepsis | 25 (28) | 33 (23) | .43 |
| Aspiration | 12 (13) | 26 (18) | .47 |
| Trauma | 5 (6) | 17 (12) | .17 |
| Multiple transfusions | 1 (1) | 5 (3.5) | .41 |
| Other | 8 (9) | 19 (13) | .40 |
| SAPS II | 50.7 ± 15.7 | 45.6 ± 14.2 | .02a |
| Brussels Organ Failure Score | 2.0 ± 1.11 | 1.70 ± 0.94 | .05a |
| Comorbidities | |||
| Diabetes | 15 (17) | 27 (19) | .73 |
| AIDS | 5 (6) | 9 (6) | 1.0 |
| Metastatic or hematologic malignancy | 7 (8) | 7 (5) | .40 |
| End-stage renal failure/dialysis | 3 (3) | 1 (0.7) | .16 |
| Radiographic lung injury score | 3.71 ± 0.55 | 3.70 ± 0.61 | |
| Tidal volume, mg/kg of IBW | 10.4 ± 2 | 10 ± 2 | .19 |
| Pao2/Fio2 | 144 ± 73 | 146 ± 62 | .36 |
| PEEP, cm H2O | 8.8 ± 4.3 | 9.1 ± 4.0 | .38 |
| Pplat, cm H2O | 30 ± 10 | 30 ± 7.8 | .48 |
| Peak pressure, cm H2O | 37 ± 12 | 36.8 ± 8.8 | .85 |
| Paco2, mm Hg | 35 ± 10 | 37 ± 8.1 | .14 |
| Arterial pH | 7.39 ± 0.09 | 7.41 ± 0.07 | .31 |
| Minute ventilation, L/min | 13.5 ± 4.2 | 12.7 ± 3.72 | .18 |
| Duration of intubation prior to enrollment, median (IQR), d | 1 (0-1) | 1 (1-2) | .02a |
| Duration of ICU stay prior to enrollment, median (IQR), d | 1 (1-2) | 1 (1-2) | .05a |
| Duration of hospitalization prior to enrollment, median (IQR), d | 2 (1-5) | 2 (1-4) | .32 |
| Randomized to low tidal volume | 52 (58) | 83 (57) | 1.0 |
| Randomized to lisofylline | 47 (52) | 69 (48) | .51 |
Continuous variables are presented as mean ± SD and categorical variables are presented as No. (%) unless indicated otherwise. See Table 1 legend for expansion of abbreviations.
P < .1 and consideration for inclusion in multivariable model.
An antibiotic was administered to 232 of 235 trial participants (99%) within 24 h of enrollment. Macrolide antibiotics were used in 47 trial participants (20%). The most common macrolide received was erythromycin (n = 27, 57%), followed by azithromycin (n = 19, 40%); one participant received clarithromycin. The median duration of macrolide use after trial enrollment was 4 days (interquartile range, 2-8 days).
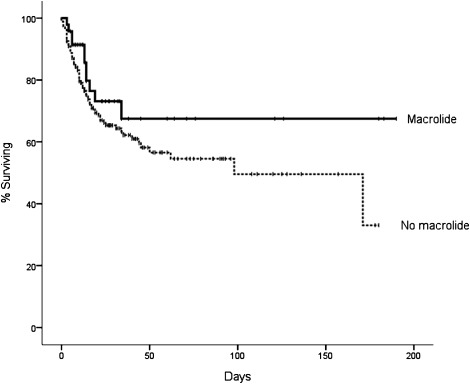
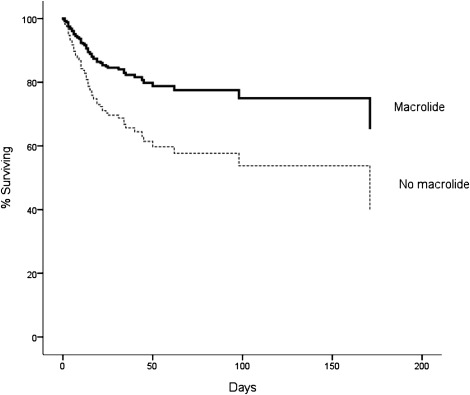
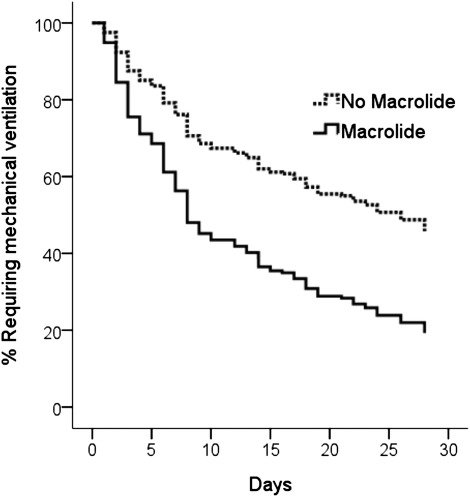
Seventy-eight participants in the trial died prior to the 180-day outcome assessment: 11 of 47 (23%) who received macrolides died, compared with 67 of the 188 (36%) who did not receive macrolide (P = .11) (Fig 1). After adjustment for confounding covariates, mortality was significantly lower in participants who did receive macrolide antibiotics compared with those who did not (Fig 2, Table 3). In addition, the time to successful discontinuation of mechanical ventilation was shorter in participants given macrolides, compared with participants not receiving macrolides (adjusted hazard ratio [HR] for successful ventilator discontinuation, 1.93; 95% CI, 1.18-3.17; P = .009) (Fig 3). We did not identify mortality differences between participants who received fluoroquinolones vs those who did not (Table 3). Cephalosporins (n = 93) were coadministered to 24 patients (51%) receiving a macrolide, but administration of cephalosporins was not associated with mortality (Table 3). We did not identify a statistically significant interaction between macrolides and mortality for pneumonia status (HR with pneumonia, 0.70 [n = 84] vs HR without pneumonia, 0.16 [n = 151]; P for interaction = .19) or tidal volume randomization group (HR, 6 mL/kg 0.26 [n = 135] vs HR, 12 mL/kg 0.66 [n = 100]; P for interaction = .18).
Figure 1.
Unadjusted Kaplan-Meier survival plots for the association between macrolide use and 180-day mortality. Log rank test, P = .15.
Figure 2.
Survival curves for the association between macrolide use and 180-day mortality from the Cox proportional hazards model, adjusted for age, Simplified Acute Physiology II Score, duration of ICU stay prior to study enrollment, tidal volume randomization group, presence of pneumonia, and presence of sepsis. Use of a macrolide antibiotic was associated with decreased mortality (relative risk, 0.46; 95% CI, 0.23-0.92; P = .028).
Table 3.
—Cox Proportional Hazards Model Results for Antibiotics and Mortality
| Macrolide (n = 47) |
Fluoroquinolone (n = 90) |
Cephalosporin (n = 93) |
|||||||
| Model | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value |
| Unadjusted (n = 235) | 0.63 | 0.33-1.19 | .15 | 1.18 | 0.75-1.85 | .48 | 0.86 | 0.54-1.36 | .51 |
| Multivariable adjusted (n = 235) | 0.46 | 0.23-0.92 | .028a | 0.97 | 0.61-1.55 | .90b | 0.76 | 0.47-1.25 | .28c |
| Propensity adjustedd (n = 193) | 0.37 | 0.16-0.88 | .024 | 0.84 | 0.45-1.57 | .58 | 0.93 | 0.53-1.63 | .80 |
See Table 1 legend for expansion of abbreviations.
Adjusted for age, SAPS II, and covariates imbalanced with P < .1: duration of ICU stay prior to study enrollment, tidal volume randomization group, presence of pneumonia, and presence of sepsis.
Adjusted for age, SAPS II, and covariates imbalanced with P < .1: sex, duration of intubation prior to study enrollment, and presence of pneumonia.
Adjusted for age, SAPS II, and covariates imbalanced with P < .1: duration of hospitalization prior to study enrollment, and presence of trauma.
C statistics for propensity score regression models were as follows: macrolide 0.87, fluoroquinolone 0.80, cephalosporin 0.67.
Figure 3.
Survival curves for the association between macrolide use and successful discontinuation of mechanical ventilation at 28 days. Cox proportional hazards model adjusted for age, Simplified Acute Physiology II Score, duration of ICU stay prior to study enrollment, tidal volume randomization group, presence of pneumonia, and presence of sepsis. Use of a macrolide antibiotic was associated with a significant increase in the likelihood of achieving successful discontinuation of mechanical ventilation (hazard ratio, 1.93; 95% CI, 1.18-3.17; P = .009).
Discussion
The analysis suggests an association between macrolide antibiotic use and improved outcomes in ALI. We observed increased survival and decreased time to successful discontinuation of mechanical ventilation associated with receipt of a macrolide early during the course of ALI. Our analyses did not demonstrate an association between fluoroquinolone or cephalosporin use and ALI survival. These findings suggest that macrolide antibiotics hold promise as a potential therapy early in the course of ALI.
We identified several factors associated with macrolide use. Patients with pneumonia as an ALI risk factor were more likely to receive macrolides. In contrast, patients with nonpulmonary sepsis were less likely to receive macrolides. Patients with greater ICU length of stay prior to ALI onset were also less likely to receive a macrolide. We speculate that suspected nosocomial infections were less likely to be treated with a macrolide. Patients who received a macrolide were less likely to be randomized to the low tidal volume strategy previously shown to result in lower mortality,2 an observation that makes the improved outcomes associated with the use of macrolides even more striking. We did not find evidence to support differences in the association between macrolides and improved outcomes based on whether patients were randomized to low tidal volume or whether patients had pneumonia as an ALI risk factor. After adjusting for observed imbalances in clinical characteristics based on macrolide use using both multivariable-adjusted and propensity-score-adjusted regression models, we observed a significant association between use of macrolides and important clinical outcomes, including survival and successful discontinuation of mechanical ventilation.
Several mechanisms may explain a potential benefit of macrolides in ALI. Macrolide antibiotics may treat bacterial organisms not covered by other antibiotics, such as Legionella species. However, broader-spectrum antibiotic coverage is a less plausible explanation because similar associations with survival were not seen with fluoroquinolone antibiotics, which have similar atypical pathogen activity. Alternatively, macrolide antibiotics have been shown to have antiinflammatory effects, which may decrease the inflammation associated with ALI.28 These antiinflammatory effects may be responsible for the improved outcomes seen with the use of macrolide therapy in community-acquired pneumonia,11 ventilator-associated pneumonia,29 diffuse panbronchiolitis,12 and cystic fibrosis.13 Specifically, the pleiotropic antiinflammatory activities of macrolides include attenuation of pulmonary epithelial cell nuclear factor-κB activity,30 decreased survival31 and oxidative burst32 of activated neutrophils, reduced endotoxin-induced goblet cell hypersecretion,18 and inhibition of immune-complex-induced lung injury.17 The potential therapeutic value of the antiinflammatory effects of macrolides is supported by observations in murine models of ALI caused by endotoxin,16,19 influenza,14 or bleomycin,15,20 which have demonstrated less severe injury and increased survival with macrolide treatment.
Our study has several limitations. First, we investigated macrolide use within 24 h of trial enrollment and did not have information on medication use prior to trial enrollment. Whether macrolide use later in ALI is associated with benefit is unclear. Second, our study was underpowered to detect differences in interaction analyses, and larger studies are needed to confirm our findings. Whereas the number of patients who received macrolides was relatively small in the LARMA trial, the ARDSNet open-access database is uniquely suited to investigate the association between macrolide use and outcomes of patients with ALI because of the specific inclusion of patients with ALI and detailed covariate information. In contrast, administrative data sets that rely on International Classification of Diseases, Ninth Revision codes do not reliably identify patients with ALI/ARDS.33 Third, it is possible that residual confounding by indication for macrolide or by unmeasured covariates may have biased our results if patients who received a macrolide were less ill than those not receiving macrolides. However, the lack of any survival benefit associated with other antibiotics of similar spectrum (eg, fluoroquinolones) and the similar baseline SAPS II and number of organ failures in the macrolide and nonmacrolide groups decrease the likelihood of strong confounding by indication. Finally, the ARDSNet LARMA data set used for this study is now more than 10 years old; whether changes in ALI practice patterns over time (such as the use of lung protective ventilation) would modify the potential benefit of macrolides is unknown. However, we did not detect an interaction between lung protective ventilation and macrolides that influenced mortality.
Conclusions
In conclusion, this analysis suggests a novel association between macrolide use and increased survival in patients with ALI. Further studies to investigate potential therapeutic benefits for macrolides in ALI are warranted.
Acknowledgments
Author contributions: Dr Walkey had full access to the data and takes full responsibility for the contents of this manuscript.Dr Walkey: contributed to the study concept, statistical analyses, results interpretation, and drafting of the manuscript.Dr Wiener: contributed to the results interpretation and drafting of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: We acknowledge the work of the ARDSNet investigators, without which this study would not have been possible.
Abbreviations
- ALI
acute lung injury
- ARDSNet
Acute Respiratory Distress Syndrome Network
- HR
hazard ratio
- LARMA
Lisofylline and Respiratory Management of Acute Lung Injury
- SAPS II
Simplified Acute Physiology II Score
Footnotes
For editorial comment see page 1131
Funding/Support: Dr Wiener is supported by a career development award through the National Cancer Institute [K07 CA138772] and by the Department of Veterans Affairs.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.The ARDS Network Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2000;283(15):1995–2002. doi: 10.1001/jama.283.15.1995. [DOI] [PubMed] [Google Scholar]
- 4.Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ. 2007;334(7597):779. doi: 10.1136/bmj.39139.716794.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberg KP, Hudson LD, Goodman RB, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354(16):1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 6.Perkins GD, McAuley DF, Thickett DR, Gao F. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med. 2006;173(3):281–287. doi: 10.1164/rccm.200508-1302OC. [DOI] [PubMed] [Google Scholar]
- 7.Liu KD, Levitt J, Zhuo H, et al. Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am J Respir Crit Care Med. 2008;178(6):618–623. doi: 10.1164/rccm.200803-419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2002;30(1):1–6. doi: 10.1097/00003246-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 9.López-Boado YS, Rubin BK. Macrolides as immunomodulatory medications for the therapy of chronic lung diseases. Curr Opin Pharmacol. 2008;8(3):286–291. doi: 10.1016/j.coph.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Altenburg J, de Graaff CS, van der Werf TS, Boersma WG. Immunomodulatory effects of macrolide antibiotics–part 1: biological mechanisms. Respiration. 2011;81(1):67–74. doi: 10.1159/000320319. [DOI] [PubMed] [Google Scholar]
- 11.Wunderink RG. Adjunctive therapy in community-acquired pneumonia. Semin Respir Crit Care Med. 2009;30(2):146–153. doi: 10.1055/s-0029-1202933. [DOI] [PubMed] [Google Scholar]
- 12.Akira M, Higashihara T, Sakatani M, Hara H. Diffuse panbronchiolitis: follow-up CT examination. Radiology. 1993;189(2):559–562. doi: 10.1148/radiology.189.2.8210390. [DOI] [PubMed] [Google Scholar]
- 13.Wolter J, Seeney S, Bell S, Bowler S, Masel P, McCormack J. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax. 2002;57(3):212–216. doi: 10.1136/thorax.57.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato K, Suga M, Akaike T, et al. Therapeutic effect of erythromycin on influenza virus-induced lung injury in mice. Am J Respir Crit Care Med. 1998;157(3 Pt 1):853–857. doi: 10.1164/ajrccm.157.3.9703098. [DOI] [PubMed] [Google Scholar]
- 15.Kawashima M, Yatsunami J, Fukuno Y, Nagata M, Tominaga M, Hayashi S. Inhibitory effects of 14-membered ring macrolide antibiotics on bleomycin-induced acute lung injury. Lung. 2002;180(2):73–89. doi: 10.1007/pl00021246. [DOI] [PubMed] [Google Scholar]
- 16.Leiva M, Ruiz-Bravo A, Jimenez-Valera M. Effects of telithromycin in in vitro and in vivo models of lipopolysaccharide-induced airway inflammation. Chest. 2008;134(1):20–29. doi: 10.1378/chest.07-3056. [DOI] [PubMed] [Google Scholar]
- 17.Tamaoki J, Kondo M, Kohri K, Aoshiba K, Tagaya E, Nagai A. Macrolide antibiotics protect against immune complex-induced lung injury in rats: role of nitric oxide from alveolar macrophages. J Immunol. 1999;163(5):2909–2915. [PubMed] [Google Scholar]
- 18.Tamaoki J, Takeyama K, Yamawaki I, Kondo M, Konno K. Lipopolysaccharide-induced goblet cell hypersecretion in the guinea pig trachea: inhibition by macrolides. Am J Physiol. 1997;272(1 Pt 1):L15–L19. doi: 10.1152/ajplung.1997.272.1.L15. [DOI] [PubMed] [Google Scholar]
- 19.Tamaoki J, Tagaya E, Yamawaki I, Sakai N, Nagai A, Konno K. Effect of erythromycin on endotoxin-induced microvascular leakage in the rat trachea and lungs. Am J Respir Crit Care Med. 1995;151(5):1582–1588. doi: 10.1164/ajrccm.151.5.7735618. [DOI] [PubMed] [Google Scholar]
- 20.Azuma A, Furuta T, Enomoto T, et al. Preventive effect of erythromycin on experimental bleomycin-induced acute lung injury in rats. Thorax. 1998;53(3):186–189. doi: 10.1136/thx.53.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernard GR, Artigas A, Brigham KL, et al. The Consensus Committee Report of the American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. Intensive Care Med. 1994;20(3):225–232. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- 22.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 23.Bernard GR. The Brussels Score. Sepsis. 1997;1(1):43–44. [Google Scholar]
- 24.Suissa S. Effectiveness of inhaled corticosteroids in chronic obstructive pulmonary disease: immortal time bias in observational studies. Am J Respir Crit Care Med. 2003;168(1):49–53. doi: 10.1164/rccm.200210-1231OC. [DOI] [PubMed] [Google Scholar]
- 25.Schoenfeld DA, Bernard GR. ARDS Network Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30(8):1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 27.Zhehui Luo, Gardiner JC, Bradley CJ. Applying propensity score methods in medical research: pitfalls and prospects. Med Care Res Rev. 2010;67(5):528–554. doi: 10.1177/1077558710361486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamaoki J. The effects of macrolides on inflammatory cells. Chest. 2004;125(suppl 2):41S–50S. doi: 10.1378/chest.125.2_suppl.41s. quiz 51S. [DOI] [PubMed] [Google Scholar]
- 29.Giamarellos-Bourboulis EJ, Pechère JC, Routsi C, et al. Effect of clarithromycin in patients with sepsis and ventilator-associated pneumonia. Clin Infect Dis. 2008;46(8):1157–1164. doi: 10.1086/529439. [DOI] [PubMed] [Google Scholar]
- 30.Ichiyama T, Nishikawa M, Yoshitomi T, et al. Clarithromycin inhibits NF-kappaB activation in human peripheral blood mononuclear cells and pulmonary epithelial cells. Antimicrob Agents Chemother. 2001;45(1):44–47. doi: 10.1128/AAC.45.1.44-47.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamasawa H, Oshikawa K, Ohno S, Sugiyama Y. Macrolides inhibit epithelial cell-mediated neutrophil survival by modulating granulocyte macrophage colony-stimulating factor release. Am J Respir Cell Mol Biol. 2004;30(4):569–575. doi: 10.1165/rcmb.2003-0105OC. [DOI] [PubMed] [Google Scholar]
- 32.Labro MT, el Benna J, Babin-Chevaye C. Comparison of the in-vitro effect of several macrolides on the oxidative burst of human neutrophils. J Antimicrob Chemother. 1989;24(4):561–572. doi: 10.1093/jac/24.4.561. [DOI] [PubMed] [Google Scholar]
- 33.Howard AE, Courtney-Shapiro C, Kelso LA, Goltz M, Morris PE. Comparison of 3 methods of detecting acute respiratory distress syndrome: clinical screening, chart review, and diagnostic coding. Am J Crit Care. 2004;13(1):59–64. [PubMed] [Google Scholar]