Abstract
Immunoglobulin heavy chain (IgH) variable region exons are assembled from VH, D and JH gene segments in developing B lymphocytes. Within the 2.7 megabase (Mb) mouse IgH locus (IgH), V(D)J recombination is regulated to ensure specific and diverse antibody repertoires. Herein, we report a key IgH V(D)J recombination regulatory region, termed InterGenic Control Region-1 (IGCR1), that lies between the VH and D clusters. Functionally, IGCR1 employs CTCF looping/insulator factor binding elements and, correspondingly, mediates IgH loops containing distant enhancers. IGCR1 promotes normal B cell development and balances antibody repertoires by inhibiting transcription and rearrangement of DH-proximal VHs and promoting rearrangement of distal VHs. IGCR1 maintains ordered and lineage-specific VH(D)JH recombination, respectively, by suppressing VH joining to Ds not joined to JHs and VH to DJH joins in thymocytes. IGCR1 also is required to allow feedback regulation and allelic exclusion of proximal VH to DJH recombination. Our studies elucidate a long-sought IgH V(D)J recombination control region and implicate a new role for the generally expressed CTCF protein.
The variable region exons of IgH, Ig light (IgL), and T cell receptor genes are assembled during B or T cell development from variable (V), diversity (D) and joining (J) gene segments1. The V(D)J recombination reaction is initiated by RAG endonuclease1, which cleaves paired gene segments flanked by complementary recombination signals (RSs) referred to as 12RSs and 23RSs1. The cleaved segments are then fused via classical non-homologous end-joining (C-NHEJ)2. The mouse IgH contains hundreds of VHs within a several Mb region, followed downstream by a 100 kilobase (kb) “intergenic” region separating the most downstream VH (VH7183.a2.3, referred to as “VH81X”)3 from DFL16.1, the first of 13 clustered DHs. The most downstream D (DQ52) lies upstream of 4 JHs4. VHs and JHs are flanked by 23RSs and Ds are flanked on both sides by 12RSs, ensuring that VHDJH assembly involves joining VHs and JHs, respectively, to upstream and downstream sides of a DH4. The IgH constant region exons (CHs) lie in the 200kb region downstream of the JHs; RNA splicing fuses productively assembled VHDJH and CH exons during IgH mRNA formation.
IgH V(D)J recombination in developing B cells is regulated to be highly “ordered” and “stage” specific; thus, DH to JH joining developmentally occurs first on both alleles in “pre”-progenitor (pro)-B cells followed by appendage of a VH to a DJH complex in pro-B cells4-6. Direct joining of a VH to an un-rearranged DH does not occur, even though theoretically permitted by the 12/23 rule3,7. The VH to DJH joining step also is regulated to achieve “lineage specificity”; thus, while developing T cells generate DJH joins, they do not form complete VH DJH exons7,8. At the pro-B stage, V(D)J recombination is regulated in the context of “allelic exclusion”, with a signal from a productive (i.e. μ IgH protein-encoding) VHDJH rearrangement inhibiting VH to DJH joining on the other IgH allele, if it is in the DJH configuration5. Expression of the μ chain also signals development to the precursor (pre)-B cell stage and IgL V(D)J recombination9. To generate such signals in pro-B cells, μ IgH chains must pair with surrogate IgL chains10. Subsequently, μ chains must pair with IgL chains in pre-B cells to mediate the pre-B to IgM+ B cell transition. Finally, IgH V(D)J recombination is regulated to ensure utilization of VHs across the large VH locus. However, proximal VHs, particularly VH81X, are rearranged more frequently than distal VHs, leading to over-representation in primary VHDJH repertoires3. Repertoire “normalization” for distal VHs in mature B cells relies on cellular selection3,11, promoted, in part, by inability of certain proximal VHs, including VH81X, to pair with surrogate IgL chains and IgL chains12,13.
V(D)J recombination at all antigen receptor loci is effected by the common V(D)J recombinase comprised of RAG and C-NHEJ components. Regulation of IgH V(D)J recombination in the context of order/stage, lineage and allelic exclusion is achieved via modulation of substrate V, D, and J accessibility14,15. Correlates of such accessibility include transcription of un-rearranged gene segments and certain DNA and histone modifications4,14-21. IgH locus contraction and looping also may mediate higher order regulation of V(D)J recombination, for example by bringing distant VHs into proximity with the DJH8,22-24. Until now, cis elements that control order, lineage-specificity, allelic exclusion, and/or differential VH utilization have been elusive16,19. The only known long-range IgH regulatory elements are a transcriptional enhancer (termed iEμ) in the intron between the JHs and CHs and a set of long-range enhancers (termed 3′IgH regulatory region or 3′IgHRR) downstream of the CHs18,25. The iEμ is required for efficient IgH V(D)J recombination, particularly VH to DJH joining26-28, although mechanisms by which it influences this process are unknown18. To date, the 3′IgHRR has not been implicated in V(D)J recombination25. As most critical aspects of IgH V(D)J recombination are regulated at the VH to DJH step7, 8, relevant regulatory elements may reside in the 100 kb “intergenic” region separating VHs and DHs (See Supp. Discussion)7,16,17,19,29,30.
Role in Normal B Cell Development
The region several kb upstream of DFL16.1 harbors chromatin modifications29,31,32 and two CTCF binding elements (“CBEs”)29,31-33 suggestive of a potential regulatory region (Supp. Fig. 1). CTCF is an 11-zinc finger nuclear protein implicated in transcriptional insulation, chromatin boundary formation, transcriptional activation/repression, and chromosome looping34-36. There are several other potential cis-elements closely linked to these CBEs including potential PU.131 and YY1-binding sites (based on JASPAR data base). We refer to this cluster of factor binding sites as IGCR1 (Fig. 1). To test for a role in IgH V(D)J recombination, we generated an “IGCR1-deleted” 129SV allele in which 4.1 kb DNA fragment that contains both CBEs and other binding sites was deleted in the mouse germline (Fig. 1a; Supp. Fig. 2). To test for specific roles of the CBEs, we generated mice in which both were replaced with scrambled sequences that do not bind CTCF (Supp. Figs. 1, 3). Mice heterozygous or homozygous for the IGCR1 deletion are referred to, respectively, as IGCR1+/- and IGCR1-/-and mice heterozygous or homozygous for the dual CBE-mutation are referred to, respectively, as IGCR1/CBE+/- or IGCR1/CBE-/-. Because generation of mutant alleles involved loxP insertion, we generated control lines heterozygous or homozygous for the loxP insertion referred to, respectively, as loxP+/I or loxPI/I (Fig. 1a). As wild-type (WT), loxP+/I, and loxPI/I mice gave essentially identical results, we refer to them collectively as “controls”. As a further control, we deleted an approximately 2 kb DNA fragment downstream of the DH-proximal end of IGCR1 and found no obvious phenotype (Supp. Fig. 10).
Figure 1. Mutation of IGCR1 CBEs impairs B cell development.
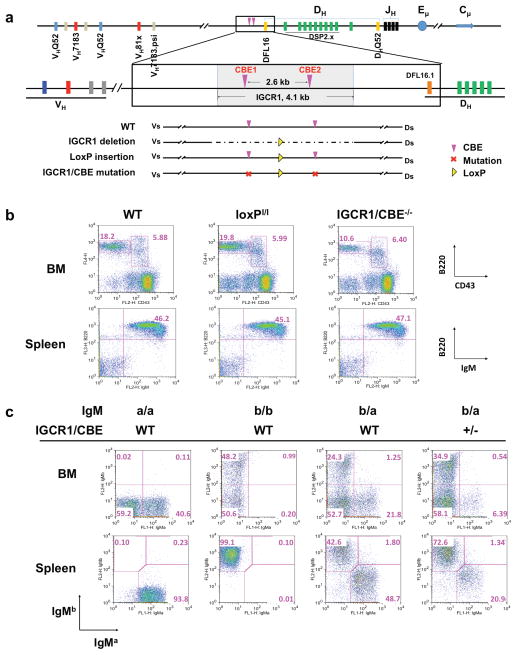
(a) Murine 129SV IgH locus (accession number: AJ851868) schematic showing 4.1 kb IGCR1 region in WT compared to IGCR1 deleted, loxP inserted, or CBE mutated configuration. (b) Flow cytometry analysis of IgM- bone marrow (BM) and IgM+ splenic B cell populations in WT, loxPI/I, and IGCR1/CBE-/- mice. In BM the B220intCD43+ pro-B and B220+CD43- pre-B cell populations are indicated. (c) Expression of IgMa and IgMb allotypic markers in BM and spleen from WT IgMa/IgMa (pure 129SV), WT IgMb/IgMb (pure C57BL/6), WT F1 (IgMa/IgMb), and heterozygous mutant IGCR1/CBE- IgMa/ WT IgMb mice.
129SV IGCR1/CBE+/- or IGCR1/CBE-/- mice had similar splenic IgM+ B cell numbers as controls (Fig. 1b; Supp. Fig. 5a). However, IGCR1/CBE+/- and, more so, IGCR1/CBE-/- mice had a substantial diminution in bone marrow (BM) pre-B cell numbers (Fig. 1b). As the pro-B to pre-B transition is signaled by a productive VHDJH in pro-B cells, this developmental defect suggests an IgH V(D)J recombination defect. As a more sensitive test for roles of IGCR1 in B cell development, we bred 129SV IGCR1/CBE+/- mice with C57BL/6 WT mice to generate F1 mice with a WT IgMb allele and a CBE-mutated IgMa allele and assayed B cells for surface IgMa and IgMb expression. Remarkably, while normal F1 mice, as expected, have roughly equal numbers of IgMa or IgMb B cells (but not both due to IgH allelic exclusion), most IgM+ BM and splenic B cells in F1 mice carrying the IGCR1 CBE-mutant IgMa allele express IgMb (Fig. 1c; Supp. Fig. 5b). Thus, mutation of the IGCR1 CBEs renders an IgH allele ineffective in supporting B cell development when competing against a WT IgH allele. We found identical B cell developmental defects in IGCR1+/- and IGCR1-/- mice (Supp. Figs. 4b, 4c, 5c, 5d).
Mediation of Diverse IgH Repertoires
We employed a PCR approach (Supp. Fig. 6a) to assay for DJH and VHDJH rearrangements in purified control, IGCR1/CBE+/-, IGCR1/CBE-/-, IGCR1+/-, and IGCR1-/- BM pro-B and pre-B cells, and in splenic B cells. We assayed for rearrangements of the two most DH-proximal VH families (VH7183 and VHQ52) and the most distal VH family (VHJ558). IgL Vκ to Jκ joins were assayed as a stage-specific control and the mouse DLG5 gene as a loading control. Levels of DJH and VκJκ rearrangements did not vary markedly among different populations or genotypes; thus, V(D)J recombination in general was not affected by the mutations (Fig. 2a; Supp. Fig. 6). However, relative levels of proximal VH7183DJH rearrangements were dramatically increased and those of distal VHJ558DJH rearrangements dramatically reduced in IGCR1/CBE-/- and IGCR1-/- pro-B cells, with both being intermediate in IGCR1/CBE+/- and IGCR1+/- pro-B cells (Fig. 2a; Supp. Fig. 6). Within the two proximal VH families, VH usage was even more skewed towards the most D proximal members in IGCR1-/- pro-B cells (Supp. Fig. 6c). Together, these findings are consistent with IGCR1 mutations resulting in cis-acting increases and cis-acting decreases, respectively, in proximal and distal VH rearrangement. Given that the proximal VHs contribute to a substantial fraction of VHDJH rearrangements (about 40%) in normal pro-B cells3,11, increased VH7183 joins in IGCR1/CBE+/- and IGCR1+/- pro-B cells indicates that the absolute level of VH to DJH rearrangements on mutant alleles, while even more biased towards proximal VHs than normal, is not decreased. In the various IGCR1 mutant pre-B cells and splenic IgM+ B cells repertoire bias remained; although the extent was progressively moderated (Supp. Fig. 6), likely due to cellular selection for VH repertoire normalization.
Figure 2. IGCR1 mutations alter VH usage, germline transcription and rearrangement order.
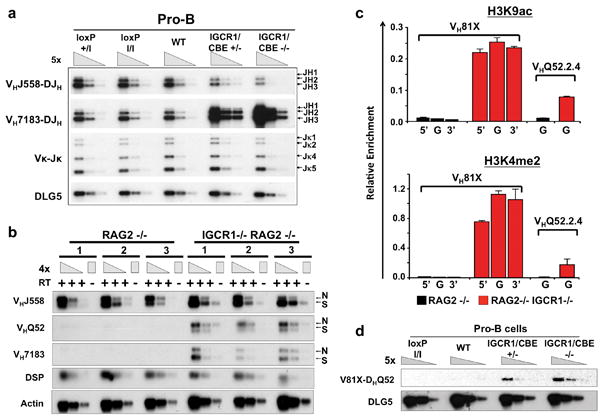
(a) PCR analyses of indicated VH family rearrangements in pro-B cells from indicated mice compared to a DLG5 loading control. Results are typical of four experiments. Bands corresponding to rearrangements to various JHs are indicated on right. (b) RT-PCR analysis of indicated germline VH transcripts in three independent WT and IGCR1-/- A-MuLV virus transformed RAG2-/- pro-B cell lines. N=nonspliced sense/antisense and S=spliced sense. (c) ChIP-qPCR analyses of H3K4me2 and H3K9ac histone modifications at indicated VHs in 129SV Rag2−/− (black) and Rag2−/− IGCR1−/− (red) A-MuLV-transformed pro-B lines. The 5′ region (5′), body (G) and 3′ region (3′) of VH81X and body (G) of VHQ52.2.4 were analyzed. Average values and standard deviations of three experiments with one line shown are representative of results from both. (d) Semi-quantitative PCR analyses of direct VH-D rearrangements in sorted pro-B cells from indicated mice. The PCR assays utilized for panels a, b, and d are diagrammed in Supp. Figs. 6a, 7a, and 8a.
Regulation of Germline VH Transcription
To measure germline VH transcripts, we generated RAG2-deficient A-MuLV-transformed WT, IGCR1+/- and IGCR1-/- pro-B lines. RAG2-deficient lines have unrearranged IgH alleles; thus, any detected VH transcripts are germline. RNA was assayed via RT-PCR for VH expression, utilizing one primer from the VH leader sequence and another from downstream of the RS (Supp. Fig. 7a). Based on size, the PCR assay detects both unspliced germline VH transcripts (sense or antisense) and slightly smaller, spliced sense germline VH transcripts (Fig. 2b). RAG2-/- pro-B lines had robust DH transcripts and spliced and un-spliced VHJ558 transcripts, but lacked readily detectable VHQ52 or VH7183 transcripts (Fig. 2b). However, RAG2-/-IGCR1+/- and, more so, RAG2-/-IGCR1-/- pro-B lines showed dramatic upregulation of spliced and unspliced VHQ52 and VH7183 transcripts with normal levels of VHJ558 and DH transcripts (Fig. 2b; Supp. Fig. 7d). We even detected by Northern blotting an ∼3.5 kb VH81X-hybridizing transcript in RNA from RAG2-/-IGCR1-/- lines, but not in WT RAG2-/- lines, (Supp. Fig. 7f). Primary RAG2-/-IGCR1-/- pro-B cells also strongly up-regulated germline VH7183 transcripts (Supp. Fig. 7e). Finally, ChIP-seq and ChIP-qPCR analyses revealed that deletion of IGCR1 led to a dramatic increase in active histone marks over VH81X (VH7183.a2.3) and the adjacent VHQ52.a2.4 germline gene segments (Fig. 2c; Supp. Figs. 7b, 7c). Thus, IGCR1 suppresses activation of germline VHs over distances of at least 100 kb.
Role in Order and Lineage-specificity
We assayed for VH81X to germline DQ52 joins via PCR with a forward VH81X-specific primer and a reverse primer from sequences between DQ52 and JH1 (Supp. Fig. 8). While we did not detect direct VH81X to DQ52 joins in control pro-B cells; we readily detected them in IGCR1/CBE+/-, IGCR1/CBE-/-, IGCR1+/- and IGCR1-/- pro-B cells (Fig. 2d; Supp. Fig. 8). Sequences of 133 independent direct VH7183DQ52 joins revealed that 120 involved VH81X, 12 involved the downstream pseudo-VH7183, and one involved the next VH7183 upstream of VH81X (Supp. Table 2). Therefore, integrity of the IGCR1 CBEs is required for ordered IgH V(D)J recombination in pro-B cells, at least for proximal VH segments.
To examine potential IGCR1 roles in lineage-specific IgH V(D)J recombination, we assayed for D to JH, VH to DJH and Vκ to Jκ rearrangements in DNA from CD4+/CD8+ (“DP”) thymocytes from control and IGCR1/CBE+/-, IGCR1/CBE-/-, IGCR1+/- and IGCR1-/- mice (Fig. 3a; Supp. Fig. 9). We detected DQ52JH rearrangements in all mice (Fig. 3a; Supp. Fig. 9). However, while there were no VHDJH rearrangements in controls, we readily detected VHDJH rearrangements of proximal VH7183 and VHQ52 segments, but not distal VHJ558 segments, in mutant DP thymocytes (Fig. 3a; Supp. Fig. 9). Lack of VκJκ rearrangements confirmed absence of B cell contamination. Cloning and sequencing of VH7183 and VHQ52 to DJH rearrangements from IGCR1-/- DP thymocytes revealed predominant utilization of the most proximal VHs (VH81X and VHQ52.a2.4; Supp. Table 3). We also assayed for direct VH81X to germline DQ52 joins in DP thymocytes (Fig. 3b; Supp. Fig. 8). As expected, controls lacked detectable direct VH to D joins; but such joins were readily apparent in mutant thymocytes (Fig. 3b; Supp. Fig. 8). Nucleotide sequencing of 32 VHD joins revealed 29 utilized VH81X and the rest utilized the downstream pseudo-VH7183 (Supp. Table 2). Thus, IGCR1 CBEs are required for lineage-specific IgH VH to DJH recombination.
Figure 3. IGCR1/CBE Mutations lead to VHDJH and VHD rearrangements in thymocytes.
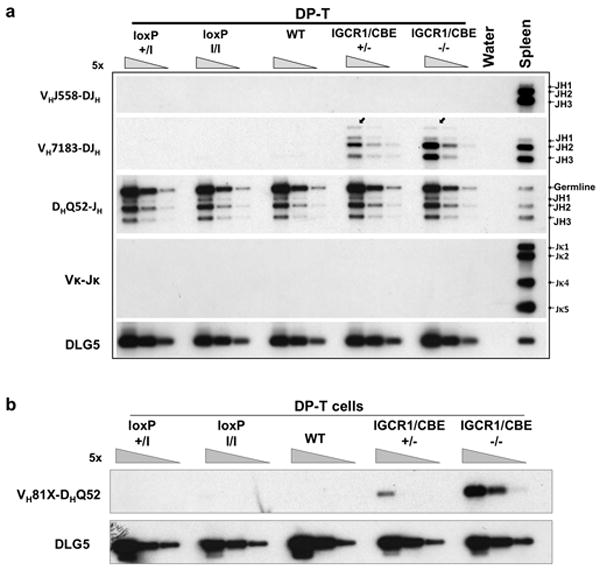
(a) PCR analyses of VH family rearrangements in sorted DP thymocytes and total splenic B cells from indicated mice with DLG5 as a loading control. Bands corresponding to rearrangements to various JHs are indicated on right. Igκ rearrangement (VκJκ) served as a control for B cell contamination. (b) Semi-quantitative PCR analyses of direct VH-D rearrangements in sorted DP-T cells from indicated mice. Assays are diagrammed in Supp. Figs. 6a and 8a.
Role in Proximal VH Feedback Regulation
Surface staining of splenic B cells heterozygous for the IGCR1-deleted IgMa allele and a WT IgMb allele did not reveal allelic inclusion (Supp. Fig. 4c). Likewise, no IgMa/IgMb double expressers were found in nearly 900 individual IGCR1+/- F1 splenic B cells by cytoplasmic staining (Supp. Fig. 11a). Hybridoma analyses showed that about 60% of WT B cells had a productive VHDJH on one allele and a DJH on the other (i.e. VH(D)JH+/DJH configuration) and about 40% had VH(D)JH rearrangements on both alleles (i.e. VH(D)JH+ /VH(D)JH- configuration) (Fig. 4a). This “60/40” ratio reflects feedback regulation of VH to DJH joining from productive rearrangements5,6. In IGCR1+/- B cells, this ratio inverted to 30/70, demonstrating that heterozygous IGCR1 deletion markedly increases B cells with VH(D)JH joins on both alleles, despite allelic exclusion at the protein level. Analyses of 39 VHDJH/VHDJH IGCR1+/- B cell hybridomas revealed that most had a VH(D)JH+ that utilized a distal VH and a VH(D)JH- that used VH81X or a nearby proximal VH (Supp. Table 6). The skewed VH(D)JH+/VH(D)JH- ratio in IGCR1+/- B cells can be explained by frequent early formation of VH81XDJH rearrangements on the mutant allele. Thus, VH81XDJH+ rearrangements would exclude rearrangement of the WT allele but would be lost developmentally; leading to most peripheral B cells deriving from progenitors that formed productive VH(D)JH rearrangements on the WT allele subsequent to VH81X(D)JH- rearrangements on the mutant allele (Supp. Fig. 11d).
Figure 4. IGCR1 is required to allow feedback regulation of proximal VH to DJH recombination.
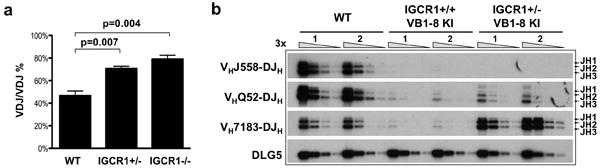
(a) Mean percentage of splenic B cells with VHDJH rearrangements on both IgH alleles as determined by analyses of hybridomas from three independent sets of WT, IGCR1+/-, and IGCR1-/- mice (Supp. Table 5). Error bars represent standard deviation. p-values were calculated by student t-test. (b) IgH VHDJH rearrangements in splenic B cells from two independent WT and VB1-8 knock-in mice carrying either a WT (IGCR1+/+ VB1-8 KI) or an IGCR1 deleted (IGCR1+/- VB1-8 KI) second allele. Bands corresponding to rearrangements to various JHs are indicated on right. DLG5 is the loading control.
The extremely high representation of proximal VHs (e.g. VH81X) rearranged on the IGCR1-deleted allele might mask allelic inclusion because productive VH81X rearrangements are selected against cellularly10,12,13. Therefore, to further examine potential effects of IGCR1-deletion on allelic exclusion, we assayed the VH(D)JH+/DJH, versus VH(D)JH+/VHDJH- ratio of IGCR1-/- hybridomas. Because both IgH alleles would be similarly biased for proximal VH rearrangements in IGCR1-/- B cells, one still would expect the 60/40 ratio if VH to DJH recombination was feedback regulated (Supp. Fig. 11e). However, we found an inverted ratio of 20/80 in IGCR1-/- hybridomas (Fig. 4a), strongly suggesting that IGCR1 deleted alleles escape feedback regulation, at least for proximal VHs (Supp. Fig. 11e). Because of the ambiguities of cellular selection against VH81X and the lack of allotypically marked IGCR1-deleted alleles, we tested for escape from feedback inhibition by assaying for endogenous rearrangements in peripheral B cells from mice with a productive VH(D)JH knock-in IgH allele (“VB1-8 KI”) that was IGCR1+ and a second allele that was IGCR1+ or IGCR1-. Strikingly, IGCR1+/- VB1-8 KI B cells had a more than 20-fold increased level of VH7183 rearrangements compared to IGCR1+/+ VB1-8 KI B cells, but little if any change in the very low level rearrangement of distal VHs (Fig. 4b). Moreover, most rearrangements in IGCR1+/- VB1-8 KI B cells were non-productive VH81X rearrangements (Supp. Fig. 11f), consistent with lack of substantial allelic inclusion at the protein level in IGCR1+/- F1 splenic B cells resulting from selection against VH81X expression (Supp. Figs. 4c, 11a). We conclude that IGCR1 is required to allow feedback regulation of the most proximal VHs.
IGCR1 mediates chromosomal IgH loops
We considered that IGCR1 might mediate IgH loops that would include iEμ and thereby modulate V(D)J recombination. The next CBEs downstream of IGCR1 are a set of 10, about 5 kb downstream of the 3′IgHRR (“3′IgHCBEs”). To test for interactions between the IGCR1 and 3′IgHCBEs, we performed quantitative chromosome conformation capture (3C) assays on 129SV RAG2-/-IGCR1+/+ and RAG2-/-IGCR1-/- A-MuLV transformed pro-B lines. These analyses revealed interaction between the IGCR1 and 3′IgHCBE locales in RAG2-/-IGCR1+/+ pro-B lines (Fig. 5a; Supp. Fig. 12a), as found in another study37. We also found this interaction in DP thymocytes (Supp. Fig. 13). Notably, this interaction was eliminated in RAG2-/-IGCR1-/- pro-B lines (Fig. 5a; Supp. Fig. 12a). We also found interactions between the iEμ locale and the IGCR1 and 3′IgHCBE locales in RAG2-/-IGCR1+/+ A-MuLV transformed pro-B cells that were diminished in RAG2-/-IGCR1-/- pro-B lines (Fig. 5b; Supp. Fig. 12b). Finally, we found strong interactions between the iEμ and 3′IgHRR locales, as reported for mature B cells38; but these were not diminished by IGCR1 deletion (Fig. 5b). These studies demonstrate that IGCR1 mediates formation of 300 kb iEμ-containing IgH loops to the 3′IgHCBE locale in pro-B lines, with iEμ also being directly juxtaposed to the IGCR1 locale in an IGCR1-dependent manner, likely within the larger loop. As iEμ lacks CBEs, its interactions with the IGCR1 locale likely are mediated, at least in part, by factors other than CTCF.
Figure 5. IGCR1 mediates long distance IgH chromosomal loops.
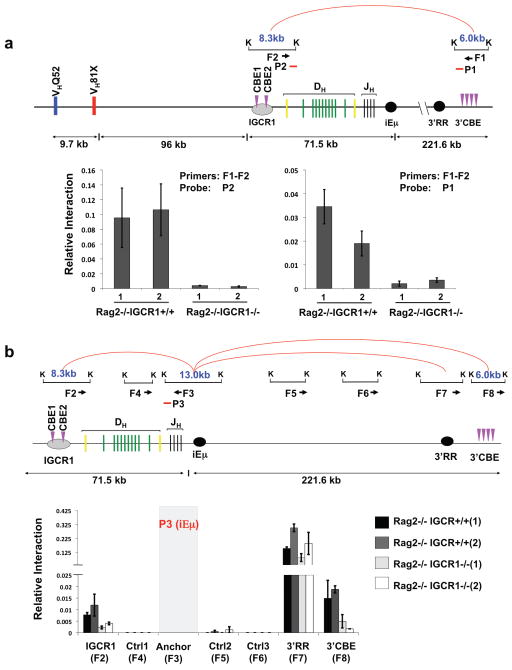
(a) Schematic of chromosome interactions between IGCR1-containing and 3′IgHCBE-containing KpnI restriction fragments in 3C assays. Interactions between IGCR1 and 3′IgHCBE locales in 129SV RAG2-/- and RAG2-/-IGCR1-/- A-MuLV transformed pro-B cells were quantified by real time PCR (Taqman) using probe (P2) (left) and a probe (P1) (right). (b) Schematic of chromosome interactions between iEμ-containing KpnI restriction fragment and indicated KpnI restriction fragments in other IgH locales. Interactions between iEμ and IGCR1, iEμ and 3′IgHRR locales, iEμ and 3′IgHCBE locales in RAG2-/- and RAG2-/-IGCR1-/- A-MuLV transformed pro-B cells were quantified by real time PCR using a probe (P3) from the iEμ locale. F1-F8 indicate primers used for PCR. K indicates KpnI sites. Red arcs indicate interactions detected in RAG2-/- cells. The average association frequency of three independent 3C experiments with 2 independent A-MuLV transformed lines from each genotype is shown with standard deviation indicated.
Discussion
IGCR1, through its CBEs, mediates ordered and lineage-specific VH to DJH recombination and balances proximal versus distal VH rearrangement. Indeed, IGCR1 functions are required for an IgH allele to efficiently generate peripheral B cells. Notably, IGCR1 and its CBEs are not required for overall VH to DJH recombination levels, but rather to decrease relative recombination of proximal VHs, particularly VH81X. Inability of the dominant VH81X to promote B cell development likely leads to developmental defects associated with IGCR1 mutations. Yet, the enigmatic VH81X is strongly conserved across mouse strains39 and, correspondingly, has been suggested to play important roles in early antibody repertoires40. Now, we find that IGCR1 plays a key role in regulating VH81X rearrangement. IGCR1 also is required to allow feedback regulation of proximal VH to DJH rearrangements, implicating IGCR1 as a critical element for the allelic exclusion of VH81X and other very proximal VHs. Our findings indicate IGCR1 allows feedback by suppressing early, unordered proximal VH rearrangement, providing the first evidence in support of long-standing hypothesis that ordered VH to DJH joining provides a means of mediating allelic exclusion5,6. However, we found no evidence for loss of feedback regulation of distal VHs, in accord with the proposal that locus contraction mediates their allelic exclusion24.
Our findings show that IGCR1-mediated promotion of the utilization of VHs up to several Mb distant does not involve alterations in distal VH transcription. In pro-B cells, IgH contraction promotes distal VH usage23,24. In the absence of certain transcription (e.g. Pax-5 or YY1) or chromatin modifying (e.g. Ezh2) factors, distal VH transcription is unimpaired but IgH contraction does not occur, diminishing distal VH rearrangement. In such factor-deficient pro-B cells, transcription and rearrangement of proximal VHs does not increase8,41-43, in marked contrast to the dramatic increases in IGCR1-/- pro-B cells. This phenotypic difference is consistent with IGCR1 normalizing VH repertoires via mechanisms other than IgH contraction. We suggest that IGCR1 promotes distal VH usage indirectly by preventing premature proximal VH rearrangement via insulating functions prior to contraction, thereby, preserving DJH substrates for distal VH rearrangement. The location of CBEs throughout the VH portion of IgH led to the notion that recruitment of VHs into DJH recombination centers44 subsequent to contraction is promoted via interaction of VH and IGCR1 CBEs45. Due to the dominance of proximal VH rearrangements on IGCR1-mutant alleles, assays for such putative IGCR1 functions require additional model systems.
IGCR1 CBEs suppress inappropriate transcription and rearrangement of proximal VHs 100kb or more upstream. These suppressive functions are consistent with enhancer insulating functions of CBEs in vitro29,35, which may relate to loop formation35. We propose that IGCR1 CBEs mediate loops with downstream 3′IgHCBEs that segregate the D/JH and VH portions of IgH into separate regulatory domains during the D to JH rearrangement stage of B cell development, blocking activity of iEμ or other elements beyond IGCR117,19 (Supp. Fig. 14a). Thus, inactivation of the IGCR1 CBEs allows transcriptional enhancing activity to extend to the proximal VHs promoting their premature rearrangement (Supp. Fig. 14b). Notably, such activity does not appear to extend beyond the most proximal VHs, which may result from formation of new CBE-mediated loops to upstream VH CBEs in the absence of IGCR1. In DJH-containing pro-B cells, IGCR1-insulating functions that prevent VH to DH rearrangements must be neutralized to allow VH to DJH joining (Supp. Fig. 14c). As CTCF binding to IgHCBEs does not vary with B cell stage32,33, other factors must modulate activity of bound CTCF within IGCR1 to allow for IgH-specific functions. Such factors might include CTCF modifications, interacting proteins such as cohesin32,33, or CBE sequence context35 and orientation46,47. In addition, other putative binding elements within IGCR1 may recruit proteins, such as YY1, that have been implicated in modulating CTCF function35.
Methods Summary
Mice
The targeting strategy and analysis of IGCR1-deleted and CBE-mutated ES cells is diagrammed in Supp. Figs. 2a and 3a (see Full Methods for details). The Institutional Animal Care and Use Committee of Children's Hospital (Boston, MA) approved all animal work.
V(D)J rearrangement assays
PCR assays for D to JH or VH to DJH rearrangements were performed as described30 (see Supp. Table 1 for primers). Generation of B cell hybridomas and VDJ recombination analyses was performed as described48.
RT-PCR and Northern blot
RT-PCR and Northern blotting assays for germline transcripts of IgH gene segments were performed as described30 (primers for RT-PCR and Northern blot probes are in Supp. Table 1).
Chromosomal conformation capture assay (3C)
3C assays were performed as described49.
ChIP-seq/ChIP-qPCR assays
Assays were done as described32.
Supplementary Material
Acknowledgments
We thank Yuko Fujiwara and Pei-Yi Huang for generating chimeric mice. This work was supported by NIH grants RO1 AI20047 (to FA), RO1 HL48702 and AI40227 (MS), CA054198-20 (to CM) and K08 AI070839 (to CCG). MB was supported by the Austrian GEN-AU initiative and Boehringer Ingelheim. CG is supported by an Irvington Institute Postdoctoral Fellowship from Cancer Research Institute. CV is supported by a Marie Curie Fellowship. CB is supported by an EMBO fellowship. FWA is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Author Contributions: C.G., H.S.Y., A.F., C.C.G. and F.W.A. conceived and designed most experiments, interpreted most results, and wrote most of the manuscript. Other authors designed and performed certain experiments, helped interpret some results, and provided important insights.
Author Information: Reprint and permissions information is available at www.nature.com/reprints.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of this article at www.nature.com/nature.
References
- 1.Schatz DG. Antigen receptor genes and the evolution of a recombinase. Semin Immunol. 2004;16:245–56. doi: 10.1016/j.smim.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, et al. The role of mechanistic factors in promoting chromosomal translocations found in lymphoid and other cancers. Adv Immunol. 2010;106:93–133. doi: 10.1016/S0065-2776(10)06004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yancopoulos GD, et al. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984;311:727–33. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]
- 4.Perlot T, Alt FW. Cis-regulatory elements and epigenetic changes control genomic rearrangements of the IgH locus. Adv Immunol. 2008;99:1–32. doi: 10.1016/S0065-2776(08)00601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alt FW, et al. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984;3:1209–19. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung D, Giallourakis C, Mostoslavsky R, Alt FW. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu Rev Immunol. 2006;24:541–70. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- 7.Bates JG, Cado D, Nolla H, Schlissel MS. Chromosomal position of a VH gene segment determines its activation and inactivation as a substrate for V(D)J recombination. J Exp Med. 2007;204:3247–56. doi: 10.1084/jem.20071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuxa M, et al. Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes Dev. 2004;18:411–22. doi: 10.1101/gad.291504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reth MG, Ammirati P, Jackson S, Alt FW. Regulated progression of a cultured pre-B-cell line to the B-cell stage. Nature. 1985;317:353–5. doi: 10.1038/317353a0. [DOI] [PubMed] [Google Scholar]
- 10.Melchers F, et al. Repertoire selection by pre-B-cell receptors and B-cell receptors, and genetic control of B-cell development from immature to mature B cells. Immunol Rev. 2000;175:33–46. [PubMed] [Google Scholar]
- 11.Malynn BA, Yancopoulos GD, Barth JE, Bona CA, Alt FW. Biased expression of JH-proximal VH genes occurs in the newly generated repertoire of neonatal and adult mice. J Exp Med. 1990;171:843–59. doi: 10.1084/jem.171.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decker DJ, Boyle NE, Klinman NR. Predominance of nonproductive rearrangements of VH81X gene segments evidences a dependence of B cell clonal maturation on the structure of nascent H chains. J Immunol. 1991;147:1406–11. [PubMed] [Google Scholar]
- 13.ten Boekel E, Melchers F, Rolink AG. Changes in the V(H) gene repertoire of developing precursor B lymphocytes in mouse bone marrow mediated by the pre-B cell receptor. Immunity. 1997;7:357–68. doi: 10.1016/s1074-7613(00)80357-x. [DOI] [PubMed] [Google Scholar]
- 14.Yancopoulos GD, Alt FW. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985;40:271–81. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- 15.Yancopoulos GD, Blackwell TK, Suh H, Hood L, Alt FW. Introduced T cell receptor variable region gene segments recombine in pre-B cells: evidence that B and T cells use a common recombinase. Cell. 1986;44:251–9. doi: 10.1016/0092-8674(86)90759-2. [DOI] [PubMed] [Google Scholar]
- 16.Corcoran AE. The epigenetic role of non-coding RNA transcription and nuclear organization in immunoglobulin repertoire generation. Semin Immunol. 2010 doi: 10.1016/j.smim.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Feeney A. Epigenetic regulation of V(D)J recombination. Semin Immunol. 2010 doi: 10.1016/j.smim.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subrahmanyam R, Sen R. RAGs' eye view of the immunoglobulin heavy chain gene locus. Semin Immunol. 2010 doi: 10.1016/j.smim.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Jhunjhunwala S, van Zelm MC, Peak MM, Murre C. Chromatin architecture and the generation of antigen receptor diversity. Cell. 2009;138:435–48. doi: 10.1016/j.cell.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abarrategui I, Krangel MS. Germline transcription: a key regulator of accessibility and recombination. Adv Exp Med Biol. 2009;650:93–102. doi: 10.1007/978-1-4419-0296-2_8. [DOI] [PubMed] [Google Scholar]
- 21.Bergman Y, Cedar H. Epigenetic control of recombination in the immune system. Semin Immunol. 2010;22:323–9. doi: 10.1016/j.smim.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sayegh CE, Jhunjhunwala S, Riblet R, Murre C. Visualization of looping involving the immunoglobulin heavy-chain locus in developing B cells. Genes Dev. 2005;19:322–7. doi: 10.1101/gad.1254305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jhunjhunwala S, et al. The 3D structure of the immunoglobulin heavy-chain locus: implications for long-range genomic interactions. Cell. 2008;133:265–79. doi: 10.1016/j.cell.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roldan E, et al. Locus ‘decontraction’ and centromeric recruitment contribute to allelic exclusion of the immunoglobulin heavy-chain gene. Nat Immunol. 2005;6:31–41. doi: 10.1038/ni1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinaud E, et al. The IgH locus 3′ regulatory region: pulling the strings from behind. Adv Immunol. 2011 doi: 10.1016/B978-0-12-387663-8.00002-8. In press. [DOI] [PubMed] [Google Scholar]
- 26.Sakai E, Bottaro A, Davidson L, Sleckman BP, Alt FW. Recombination and transcription of the endogenous Ig heavy chain locus is effected by the Ig heavy chain intronic enhancer core region in the absence of the matrix attachment regions. Proc Natl Acad Sci U S A. 1999;96:1526–31. doi: 10.1073/pnas.96.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perlot T, Alt FW, Bassing CH, Suh H, Pinaud E. Elucidation of IgH intronic enhancer functions via germ-line deletion. Proc Natl Acad Sci U S A. 2005;102:14362–7. doi: 10.1073/pnas.0507090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afshar R, Pierce S, Bolland DJ, Corcoran A, Oltz EM. Regulation of IgH gene assembly: role of the intronic enhancer and 5′DQ52 region in targeting DHJH recombination. J Immunol. 2006;176:2439–47. doi: 10.4049/jimmunol.176.4.2439. [DOI] [PubMed] [Google Scholar]
- 29.Featherstone K, Wood AL, Bowen AJ, Corcoran AE. The mouse immunoglobulin heavy chain V-D intergenic sequence contains insulators that may regulate ordered V(D)J recombination. J Biol Chem. 2010;285:9327–38. doi: 10.1074/jbc.M109.098251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giallourakis CC, et al. Elements between the IgH variable (V) and diversity (D) clusters influence antisense transcription and lineage-specific V(D)J recombination. Proc Natl Acad Sci U S A. 2010;107:22207–12. doi: 10.1073/pnas.1015954107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin YC, et al. A global network of transcription factors, involving E2A, EBF1 and Foxo1, that orchestrates B cell fate. Nat Immunol. 2010;11:635–43. doi: 10.1038/ni.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ebert A, et al. The distal v(h) gene cluster of the igh locus contains distinct regulatory elements with pax5 transcription factor-dependent activity in pro-B cells. Immunity. 2011;34:175–87. doi: 10.1016/j.immuni.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Degner SC, Wong TP, Jankevicius G, Feeney AJ. Cutting edge: developmental stage-specific recruitment of cohesin to CTCF sites throughout immunoglobulin loci during B lymphocyte development. J Immunol. 2009;182:44–8. doi: 10.4049/jimmunol.182.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams A, Flavell RA. The role of CTCF in regulating nuclear organization. J Exp Med. 2008;205:747–50. doi: 10.1084/jem.20080066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips JE, Corces VG. CTCF: master weaver of the genome. Cell. 2009;137:1194–211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bulger M, Groudine M. Enhancers: the abundance and function of regulatory sequences beyond promoters. Dev Biol. 2010;339:250–7. doi: 10.1016/j.ydbio.2009.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Degner SC, et al. CCCTC-binding factor (CTCF) and cohesin influence the genomic architecture of the Igh locus and antisense transcription in pro-B cells. Proc Natl Acad Sci U S A. 2011;108:9566–71. doi: 10.1073/pnas.1019391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wuerffel R, et al. S-S synapsis during class switch recombination is promoted by distantly located transcriptional elements and activation-induced deaminase. Immunity. 2007;27:711–22. doi: 10.1016/j.immuni.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirano SL, et al. Identity of IGHV-7183.1 (V81x) coding and recombination signal sequences among wild-derived mice. Immunogenetics. 2001;53:54–8. doi: 10.1007/s002510000287. [DOI] [PubMed] [Google Scholar]
- 40.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 41.Hesslein DG, et al. Pax5 is required for recombination of transcribed, acetylated, 5′ IgH V gene segments. Genes Dev. 2003;17:37–42. doi: 10.1101/gad.1031403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su IH, et al. Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat Immunol. 2003;4:124–31. doi: 10.1038/ni876. [DOI] [PubMed] [Google Scholar]
- 43.Liu H, et al. Yin Yang 1 is a critical regulator of B-cell development. Genes Dev. 2007;21:1179–89. doi: 10.1101/gad.1529307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji Y, et al. The in vivo pattern of binding of RAG1 and RAG2 to antigen receptor loci. Cell. 2010;141:419–31. doi: 10.1016/j.cell.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Degner-Leisso SC, Feeney AJ. Epigenetic and 3-dimensional regulation of V(D)J rearrangement of immunoglobulin genes. Semin Immunol. 2010 doi: 10.1016/j.smim.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacPherson MJ, Sadowski PD. The CTCF insulator protein forms an unusual DNA structure. BMC Mol Biol. 2010;11:101. doi: 10.1186/1471-2199-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kyrchanova O, Chetverina D, Maksimenko O, Kullyev A, Georgiev P. Orientation-dependent interaction between Drosophila insulators is a property of this class of regulatory elements. Nucleic Acids Res. 2008;36:7019–28. doi: 10.1093/nar/gkn781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dudley DD, et al. Impaired V(D)J recombination and lymphocyte development in core RAG1-expressing mice. J Exp Med. 2003;198:1439–50. doi: 10.1084/jem.20030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hagege H, et al. Quantitative analysis of chromosome conformation capture assays (3C-qPCR) Nat Protoc. 2007;2:1722–33. doi: 10.1038/nprot.2007.243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


