Personalized medicine applied to cancer treatment exploits established clinical-pathological features of the disease combined with state-of-the-art molecular profiling in order to create diagnostic, prognostic and therapeutic strategies tailored to specific patient/disease requirements. On the path toward personalized medicine of cancer patients, genomic aberrations driving tumor behavior represent an invaluable tool to improve diagnosis, patient stratification and, most importantly, therapeutic efficacy by targeted treatments.
Several lymphoid malignancies harbor characteristic genetic abnormalities. These are relevant in determining their biological features, can be useful in differential diagnosis, and may provide therapeutic targets. Genetic hallmarks in the setting of mature B-cell tumors include chromosomal translocations, such as the t(11;14) translocation in mantle cell lymphoma, t(14;18) translocation in follicular lymphoma, and MYC rearrangements in Burkitt’s lymphoma, but also point mutations, such as the p.V600E mutation of BRAF that has been recently identified in 100% of hairy cell leukemia patients.1,2 Exome and transcriptome sequencing have revealed additional new genetic lesions in several B-cell malignancies, such as chronic lymphocytic leukemia, diffuse large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, multiple myeloma, and the role of these lesions in diagnosis, prognosis and therapy is currently under scrutiny.
Despite these advancements, little is known about the genetics of other lymphoma types. Among B-cell tumors, no disease-specific genetic lesions have yet been identified in splenic marginal zone lymphoma (SMZL).
SMZL is an indolent B-cell neoplasm involving spleen, bone marrow and, in a small number of cases, peripheral blood. The clinical course of SMZL is heterogeneous, although the majority of cases show an indolent course with a median survival of approximately ten years. Approximately 30% of cases present a worse outcome than this3 and a further 10% of cases undergo transformation to diffuse large B-cell lymphoma (DLBCL).3,4
The pathogenesis of SMZL is still not known. Clinical and epidemiological data point to an association with hepatitis C virus (HCV). However, the infection rate in SMZL patients does not exceed around 15–20%, even in geographical areas where the virus is highly prevalent.3 The contribution of antigen stimulation to SMZL pathogenesis is suggested by the highly restricted immunoglobulin gene repertoire, including selective usage of the immunoglobulin heavy variable (IGHV) 1–2*04 allele in around 20–30% of SMZL and by the stereotyped B-cell receptor in approximately 10% of cases.5 However, the relevant antigen has not been identified. Cytogenetic abnormalities associated with SMZL include 7q31-q32 deletions in around 30% of cases and 3q gains in around 20%, although the genes targeted by these lesions are not known.
Not much is known about somatic gene lesions associated with SMZL and this makes diagnosis and classification based on genetics difficult to realize, even though it is one of the mainstay criteria adopted by the World Health Organization Classification of Tumours of Haematopietic and Lymphoid Tissues for the diagnosis of B-cell lymphoma.6 In addition, the lack of knowledge about SMZL biology has prevented the design of target therapy approaches directed at the genetic lesions involved in SMZL growth.
No randomized trials have addressed the issue of SMZL management, and there is no consensus on optimal treatment for naive and relapsed patients. Therefore, the therapeutic armamentarium for SMZL covers a wide range of approaches and these have mainly been derived from approaches developed for the treatment of other types of B-cell lymphoma. Therapeutic options for SMZL include splenectomy,7,8 chemotherapy,9,10 rituximab alone11,12 or rituximab + chemotherapy.11–13 In addition, antiviral treatment should be considered in patients with SMZL and concurrent HCV-related chronic hepatitis who do not immediately need conventional chemo/immunotherapy against the lymphoma clone.14–16
NF-κB signaling is transiently engaged when normal B lymphocytes respond to antigens, promoting cell survival and differentiation. In a variety of lymphoid cancers, NF-κB is constitutively active owing to diverse abnormalities that ultimately subvert the normal function of NF-κB in tumor cells. These include somatic mutations, genomic amplifications and deletions, and chromosomal translocations.
There is indirect evidence pointing to the nuclear factor-κB (NF-κB) pathway as an attractive candidate also in SMZL pathogenesis. Abnormal marginal zone B-cell expansions are often observed in animal models with constitutive NF-κB activation.17–19 Also, up-regulated expression of NF-κB target genes in SMZL suggests the occurrence of NF-κB activation20 that, in other B-cell malignancies, is sustained by cancer-specific genetic lesions.21
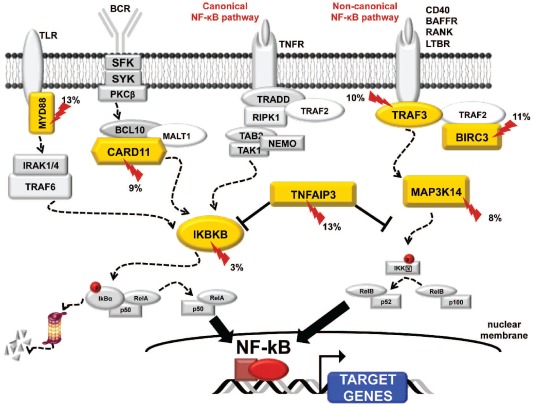
Consistent with this, a recent study22 demonstrated that the NF-κB pathway is constitutively active in most (~60%) SMZL and that constitutive NF-κB activation is driven by genetic lesions targeting key regulators of both canonical (TNFAIP3 and IKBKB) and non-canonical (BIRC3, TRAF3, MAP3K14) NF-κB signaling, accounting for more than 30% of cases overall. Mutations clustered in genes of the TRAF3/MAP3K14-TRAF2/BIRC3 regulatory complex of non-canonical NF-κB signaling that was targeted in 25% SMZL cases. In this context, BIRC3 was repeatedly inactivated because of somatic mutations that disrupted the same RING domain that in extranodal marginal zone lymphoma is removed by the t(11;18) translocation. This suggests BIRC3 disruption is a common mechanism across marginal zone B-cell lymphomagenesis.
In the April issue of Haematologica, Yan et al.23 provide further evidence of the pathogenetic role of NF-κB dysregulation in SMZL. Yan et al.13 identified inactivating mutations of the TNFAIP3 (13%), a negative regulator of the canonical NF-κB signaling, as well as activating lesions of MYD88 (13%) and CARD11 (9%). Among their many functions, these act as positive regulators of NF-κB in signaling from B-cell and Toll-like receptors. Overall, mutations of negative and positive NF-κB regulators were distributed for the most part in a mutually exclusive fashion and accounted for approximately 30% of SMZL cases. This suggests activation of NF-κB is a major contributor to the pathogenesis of this disease.
Genetic lesions of NF-κB provide a new molecular basis for the pathogenesis of SMZL (Figure 1), and offer a suitable target for NF-κB tailored therapeutic strategies in this lymphoma.
Figure 1.
Genes of the canonical and non-canonical NF-kB pathways targeted by mutations in splenic marginal zone lymphoma.
Bortezomib is a proteasome inhibitor with proapoptotic effects mediated by inhibition of the NF-κB pathway. Bortezomib is active in marginal zone lymphomas of extranodal type,24–28 a disease that shares several characteristics with SMZL, including the same putative cell of origin, constitutive NF-κB activation as driving mechanism, and the NF-κB pathway gene BIRC3 as molecular target of genetic lesions. Although no trials have specifically examined the activity and safety of bortezomib in SMZL, clinical research has presented some evidence to suggest a more general activity of this compound against marginal zone lymphomas. For example, Conconi et al.27 reported 31 patients with relapsed/refractory MALT lymphoma treated with bortezomib 1.3 mg/m2 i.v. on Days 1, 4, 8, and 11 for up to six 21-day cycles. Among the 29 patients assessable for response, the overall response rate (ORR) was 48%, including 9 patients who achieved a complete response. The most relevant adverse events were fatigue, thrombocytopenia, neutropenia and peripheral neuropathy. Bortezomib plus rituximab combination regimens are also effective in relapsed or refractory marginal zone lymphoma, as documented by a randomized trial in which patients received bortezomib 1.3 mg/m2 twice weekly (arm A) or weekly bortezomib 1.6 mg/m2 (arm B) plus rituximab 375 mg/m2 weekly for four weeks (both arms).25 ORR was 49% in arm A and 43% in arm B (including 10% complete remissions).
Further evidence to support the activity of bortezomib in marginal zone lymphomas is provided by observation of a rapid response when these lymphomas are challenged with proteasomal inhibitors.29 Among 69 indolent B-cell lymphoma patients (follicular lymphoma 60%, marginal zone lymphoma 22%), the median time to treatment response to bortezomib in marginal zone lymphomas was four weeks compared to 12 weeks in follicular lymphoma.
NF-κB dysregulation in SMZL represents a new biological basis for novel therapeutic strategies for SMZL patients. Proteasome inhibitors, that are already approved for other malignancies, or more specific anti-NF-κB compounds under development should be tested for their efficacy in clinical trials specifically dedicated to SMZL.
References
- 1.Tiacci E, Schiavoni G, Forconi F, Santi A, Trentin L, Ambrosetti A, et al. Simple genetic diagnosis of hairy cell leukemia by sensitive detection of the BRAF-V600E mutation. Blood. 2012;119(1):192–5. doi: 10.1182/blood-2011-08-371179. [DOI] [PubMed] [Google Scholar]
- 2.Tiacci E, Trifonov V, Schiavoni G, Holmes A, Kern W, Martelli MP, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305–15. doi: 10.1056/NEJMoa1014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arcaini L, Lazzarino M, Colombo N, Burcheri S, Boveri E, Paulli M, et al. Splenic marginal zone lymphoma: a prognostic model for clinical use. Blood. 2006;107:4643–9. doi: 10.1182/blood-2005-11-4659. [DOI] [PubMed] [Google Scholar]
- 4.Dungarwalla M, Appiah-Cubi S, Kulkarni S, Saso R, Wotherspoon A, Osuji N, et al. High-grade transformation in splenic marginal zone lymphoma with circulating villous lymphocytes: the site of transformation influences response to therapy and prognosis. Br J Haematol. 2008;143:71–4. doi: 10.1111/j.1365-2141.2008.07301.x. [DOI] [PubMed] [Google Scholar]
- 5.Zibellini S, Capello D, Forconi F, Marcatili P, Rossi D, Rattotti S, et al. Stereotyped patterns of B-cell receptor in splenic marginal zone lymphoma. Haematologica. 2010;95:1792–6. doi: 10.3324/haematol.2010.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. [Google Scholar]
- 7.Chacon JI, Mollejo M, Munoz E, Algara P, Mateo M, Lopez L, et al. Splenic marginal zone lymphoma: clinical characteristics and prognostic factors in a series of 60 patients. Blood. 2002;100:1648–54. [PubMed] [Google Scholar]
- 8.Thieblemont C, Felman P, Callet-Bauchu E, Traverse-Glehen A, Salles G, Berger F, et al. Splenic marginal-zone lymphoma: a distinct clinical and pathological entity. Lancet Oncol. 2003;4:95–103. doi: 10.1016/s1470-2045(03)00981-1. [DOI] [PubMed] [Google Scholar]
- 9.Lefrere F, Hermine O, Belanger C, Francois S, Tilly H, Lebas de La Cour JC, et al. Fludarabine: an effective treatment in patients with splenic lymphoma with villous lymphocytes. Leukemia. 2000;14:573–5. doi: 10.1038/sj.leu.2401710. [DOI] [PubMed] [Google Scholar]
- 10.Iannitto E, Minardi V, Calvaruso G, Ammatuna E, Florena AM, Mule A, et al. Deoxycoformycin (pentostatin) in the treatment of splenic marginal zone lymphoma (SMZL) with or without villous lymphocytes. Eur J Haematol. 2005;75:130–5. doi: 10.1111/j.1600-0609.2005.00426.x. [DOI] [PubMed] [Google Scholar]
- 11.Cervetti G, Galimberti S, Sordi E, Buda G, Orciuolo E, Cecconi N, et al. Significant efficacy of 2-CdA with or without rituximab in the treatment of splenic marginal zone lymphoma (SMZL) Ann Oncol. 2010;21:851–4. doi: 10.1093/annonc/mdp395. [DOI] [PubMed] [Google Scholar]
- 12.Tsimberidou AM, Catovsky D, Schlette E, O’Brien S, Wierda WG, Kantarjian H, et al. Outcomes in patients with splenic marginal zone lymphoma and marginal zone lymphoma treated with rituximab with or without chemotherapy or chemotherapy alone. Cancer. 2006;107:125–35. doi: 10.1002/cncr.21931. [DOI] [PubMed] [Google Scholar]
- 13.Arcaini L, Orlandi E, Scotti M, Brusamolino E, Passamonti F, Burcheri S, et al. Combination of rituximab, cyclophosphamide, and vincristine induces complete hematologic remission of splenic marginal zone lymphoma. Clin Lymphoma. 2004;4:250–2. doi: 10.3816/clm.2004.n.005. [DOI] [PubMed] [Google Scholar]
- 14.Hermine O, Lefrere F, Bronowicki JP, Mariette X, Jondeau K, Eclache-Saudreau V, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med. 2002;347:89–94. doi: 10.1056/NEJMoa013376. [DOI] [PubMed] [Google Scholar]
- 15.Saadoun D, Suarez F, Lefrere F, Valensi F, Mariette X, Aouba A, et al. Splenic lymphoma with villous lymphocytes, associated with type II cryoglobulinemia and HCV infection: a new entity? Blood. 2005;105:74–6. doi: 10.1182/blood-2004-05-1711. [DOI] [PubMed] [Google Scholar]
- 16.Kelaidi C, Rollot F, Park S, Tulliez M, Christoforov B, Calmus Y, et al. Response to antiviral treatment in hepatitis C virus-associated marginal zone lymphomas. Leukemia. 2004;18:1711–6. doi: 10.1038/sj.leu.2403443. [DOI] [PubMed] [Google Scholar]
- 17.Xie P, Stunz LL, Larison KD, Yang B, Bishop GA. Tumor necrosis factor receptor-associated factor 3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity. 2007;27:253–67. doi: 10.1016/j.immuni.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki Y, Calado DP, Derudder E, Zhang B, Shimizu Y, Mackay F, et al. NIK overexpression amplifies, whereas ablation of its TRAF3-binding domain replaces BAFF:BAFF-R-mediated survival signals in B cells. Proc Natl Acad Sci USA. 2008;105:10883–8. doi: 10.1073/pnas.0805186105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conze DB, Zhao Y, Ashwell JD. Non-canonical NF-kappaB activation and abnormal B cell accumulation in mice expressing ubiquitin protein ligase-inactive c-IAP2. PLoS Biol. 2010;8:e1000518. doi: 10.1371/journal.pbio.1000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz-Ballesteros E, Mollejo M, Rodriguez A, Camacho FI, Algara P, Martinez N, et al. Splenic marginal zone lymphoma: proposal of new diagnostic and prognostic markers identified after tissue and cDNA microarray analysis. Blood. 2005;106:1831–8. doi: 10.1182/blood-2004-10-3898. [DOI] [PubMed] [Google Scholar]
- 21.Compagno M, Lim WK, Grunn A, Nandula SV, Brahmachary M, Shen Q, et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009;459:717–21. doi: 10.1038/nature07968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi D, Deaglio S, Dominguez-Sola D, Rasi S, Vaisitti T, Agostinelli C, et al. Alteration of BIRC3 and multiple other NF-kappaB pathway genes in splenic marginal zone lymphoma. Blood. 2011;118:4930–4. doi: 10.1182/blood-2011-06-359166. [DOI] [PubMed] [Google Scholar]
- 23.Yan Y, Huang Y, Watkins AJ, Kocialkowski S, Zeng N, Hamoudi RA, et al. BCR and TLR signalling pathways are recurrently targeted by genetic changes in splenic marginal zone lymphomas. Haematologica. 2012;97(4):595–8. doi: 10.3324/haematol.2011.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Connor OA, Wright J, Moskowitz C, Muzzy J, MacGregor-Cortelli B, Stubblefield M, et al. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin’s lymphoma and mantle cell lymphoma. J Clin Oncol. 2005;23:676–84. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 25.de Vos S, Goy A, Dakhil SR, Saleh MN, McLaughlin P, Belt R, et al. Multicenter randomized phase II study of weekly or twice-weekly bortezomib plus rituximab in patients with relapsed or refractory follicular or marginal-zone B-cell lymphoma. J Clin Oncol. 2009;27:5023–30. doi: 10.1200/JCO.2008.17.7980. [DOI] [PubMed] [Google Scholar]
- 26.Troch M, Jonak C, Mullauer L, Puspok A, Formanek M, Hauff W, et al. A phase II study of bortezomib in patients with MALT lymphoma. Haematologica. 2009;94:738–42. doi: 10.3324/haematol.2008.001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conconi A, Martinelli G, Lopez-Guillermo A, Zinzani PL, Ferreri AJ, Rigacci L, et al. Clinical activity of bortezomib in relapsed/refractory MALT lymphomas: results of a phase II study of the International Extranodal Lymphoma Study Group (IELSG) Ann Oncol. 2011;22:689–95. doi: 10.1093/annonc/mdq416. [DOI] [PubMed] [Google Scholar]
- 28.Di Bella N, Taetle R, Kolibaba K, Boyd T, Raju R, Barrera D, et al. Results of a phase 2 study of bortezomib in patients with relapsed or refractory indolent lymphoma. Blood. 2010;115:475–80. doi: 10.1182/blood-2009-08-233155. [DOI] [PubMed] [Google Scholar]
- 29.O’Connor OA, Portlock C, Moskowitz C, Hamlin P, Straus D, Gerecitano J, et al. Time to treatment response in patients with follicular lymphoma treated with bortezomib is longer compared with other histologic subtypes. Clin Cancer Res. 2010;16:719–26. doi: 10.1158/1078-0432.CCR-08-2647. [DOI] [PubMed] [Google Scholar]