Abstract
Background
The management of patients with relapsed or refractory Hodgkin’s lymphoma who achieve less than a partial response to first-line salvage chemotherapy is unclear. The objective of this study was to evaluate response and outcomes to second-line salvage and autologous stem cell transplantation in patients not achieving a complete or partial response to platinum-containing first-line salvage chemotherapy.
Design and Methods
Consecutively referred transplant-eligible patients with relapsed/refractory Hodgkin’s lymphoma after primary chemotherapy received gemcitabine, dexamethasone, and cisplatin as first salvage chemotherapy. Those achieving a complete or partial response, and those with a negative gallium scan and stable disease with bulk <5 cm proceeded to high-dose chemotherapy and autologous stem cell transplantation. Patients with progressive disease or stable disease with a positive gallium scan or bulk ≥5 cm were given second salvage chemotherapy with mini-BEAM (carmustine, etoposide, cytarabine, melphalan). Patients who responded (according to the same definition) proceeded to autologous stem cell transplantation.
Results
One hundred and thirty-one patients with relapsed/refractory Hodgkin’s lymphoma received first-line salvage gemcitabine, dexamethasone, and cisplatin; of these patients 99 had at least a partial response (overall response rate 76%). One hundred and twelve (85.5%) patients proceeded to autologous stem cell transplantation, while the remaining 19 (14.5%) patients received mini-BEAM. Among these 19 patients, six had at least a partial response (overall response rate 32%), and nine proceeded to autologous stem cell transplantation. The remaining ten patients received palliative care. Seven of the nine patients transplanted after mini-BEAM had a subsequent relapse. Patients receiving second salvage mini-BEAM had poor outcomes, with a 5-year progression-free survival rate of 11% and a 5-year overall survival rate of 20%.
Conclusions
Patients who require a second salvage regimen to achieve disease control prior to autologous stem cell transplantation have a relatively poor outcome and should be considered for alternative treatment strategies.
Keywords: autologous transplantation, GDP, Hodgkin’s lymphoma, miniBEAM, salvage chemotherapy
Introduction
The current standard of care for patients with Hodgkin’s lymphoma (HL) who relapse from or are refractory to primary chemotherapy is salvage (or second-line) chemotherapy, and in those who demonstrate chemotherapy-sensitive disease, autologous stem cell transplantation (ASCT).1–4 The commonly used salvage regimens are platinum-containing,5–8 although there is no gold standard regimen due to the lack of randomized comparative trials.
Although there are no controlled trials to support the use of one salvage regimen over another, the combination of gemcitabine, dexamethasone, and cisplatin (GDP) has become the preferred chemotherapy for relapsed and refractory HL at our center.9,10 We had previously employed mini-BEAM (carmustine, etoposide, cytarabine, melphalan) as our regimen of choice with acceptable response rates and post-ASCT outcomes.11 GDP has similar activity to other salvage regimens, with the advantages of outpatient administration, a favorable toxicity profile, and minimal impact on the ability to mobilize peripheral blood stem cells. The response rate to GDP is 60–70%, although only 17–28% patients achieve a complete response.9,10
The optimal management of patients with HL that does not respond to platinum-containing chemotherapy is unknown. Options include proceeding to single or tandem ASCT, alternative aggressive combination chemotherapy (second salvage) before ASCT, allogeneic stem cell transplantation, or non-curative approaches such as involved field radiotherapy, or experimental systemic therapy.
The mini-BEAM combination is a non-platinum-containing salvage regimen that produces a high response rate when used as first-line salvage for patients with relapsed/refractory HL (68–85%).11–14 However, mini-BEAM must be administered in an inpatient setting, causes significant myelosuppression with severe infections and need for blood product support, impairs peripheral stem cell mobilization, and increases the risk of secondary myeloid leukemia.11–14 After adopting GDP as our first salvage regimen in HL and since mini-BEAM is relatively non-cross resistant with GDP, it has been used at our center as a second salvage regimen in patients in whom GDP fails and who are otherwise fit and eligible for transplantation.
The objective of this study was to evaluate response and outcomes to second-line salvage and ASCT in patients not achieving a complete or partial response to GDP.
Design and Methods
Patients
This was a retrospective review of prospectively collected data stored in a computerized database and the medical records of all transplant-eligible patients referred to the Autologous Blood and Marrow Transplant Program at Princess Margaret Hospital with relapsed/refractory HL between January 2001 and December 2008. Additional data were collected from the patients’ electronic records, and supplemented with information from the referring or primary care physicians, as necessary. This study was approved by the University Health Network Research Ethics Board.
Patients 18–65 years of age with biopsy-proven HL who had recurrent/refractory disease to first-line chemotherapy, and who received second-line (i.e., first salvage) GDP chemotherapy with the intention of performing ASCT were included. A central pathology review was not routinely performed. Primary refractory HL was defined as progressive disease during primary chemotherapy or within 3 months of its completion.15,16
Patients underwent restaging investigations at the time of progression with computed tomography scans of the chest, abdomen, and pelvis and with magnetic resonance imaging when appropriate, as well as bone marrow aspirate and biopsy in all patients. Gallium scans were recommended, especially for patients with disease bulk ≥5 cm at relapse, but were not mandatory, and positron emission scans (PET) were not routinely performed. Repeat biopsy was not mandatory in all patients but was performed if the original biopsy was unclear, if the relapse was late (4–5 years after primary therapy) or if clinically there was suspicion of an alternative diagnosis.
Salvage chemotherapy and response assessment
GDP chemotherapy consisted of gemcitabine 1000 mg/m2 intravenously (IV) on days 1 and 8, dexamethasone 40 mg IV on day 1, 40 mg orally on days 2–4, and cisplatin 75 mg/m2 IV on day 1. GDP was administered every 21 days in an outpatient setting. Response was evaluated by physical examination and computed tomography scans in all patients after two cycles of GDP. Bone marrow biopsy was only repeated in patients with documented bone marrow involvement prior to salvage chemotherapy. Similarly, gallium scans were only repeated if performed and positive prior to salvage chemotherapy.
Responses were defined according to the 1999 International Working Group criteria.17 The overall response rate was the sum of patients achieving a complete remission, unconfirmed complete remission, and partial remission. These patients proceeded to stem cell mobilization and ASCT. In addition, patients with stable disease after GDP with gallium-negative residual mass(es) measuring <5 cm were considered to have a sufficiently adequate response to salvage chemotherapy to proceed directly to stem cell mobilization and ASCT.
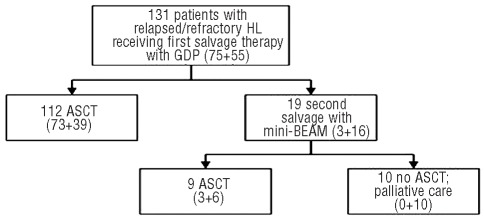
All other patients with stable disease (i.e., positive gallium scan or with disease ≥5 cm) and all patients with progressive disease after GDP with a good performance status received mini-BEAM as second salvage chemotherapy (Figure 1). Mini-BEAM consists of carmustine 60 mg/m2 IV on day 1, etoposide 75 mg/m2 IV on days 2–5, cytarabine 100 mg/m2 twice daily IV on days 2–5, and melphalan 30 mg/m2 IV on day 5, given as inpatient treatment every 21–28 days depending on hematologic recovery. Response was usually assessed after one cycle of mini-BEAM; patients rarely received two cycles. Patients achieving a partial remission or stable disease (gallium negative or bulk <5 cm) in response to mini-BEAM proceeded to stem cell collection and ASCT.
Figure 1.
Patients’ management following GDP. Numbers in parentheses refer to patients’ status after primary chemotherapy. (relapsed + refractory = total).
Stem cell collection, high-dose chemotherapy and autologous stem cell transplantation
Patients responding to first- or second-line salvage chemotherapy underwent peripheral stem cell mobilization with cyclophosphamide 2 g/m2 IV on day 1, etoposide 200 mg/m2 IV on days 1–3, and filgrastim 10 μg/kg/day subcutaneously starting on day 6 and continued until the completion of leukapheresis. If peripheral blood stem cell collection was inadequate (<2.0×106/kg CD34+ cells), peripheral stem cells were mobilized with pleraxifor or bone marrow was harvested.
The high-dose chemotherapy regimen contained etoposide 60 mg/kg IV on day −4 and melphalan 180 mg/m2 IV on day −3, followed by stem cell infusion on day 0, and inpatient supportive care. This regimen was adopted at our institution based on earlier data showing that response rates to this regimen compare favorably with those of three or four other high-dose drug combination regimens.18 Consolidative involved-field radiotherapy (30–35 Gy in 20 fractions) was given 6–12 weeks after ASCT to patients with bulky (≥5 cm) disease prior to GDP.19 Patients were monitored frequently after completion of all treatments, with the follow-up including imaging studies at 3 months and 1 year after transplantation, or sooner if clinically indicated.
Statistical methods
The primary end-point of this study was the overall response rate (complete remission + unconfirmed complete remission + partial remission) to mini-BEAM. Outcomes and characteristics at diagnosis and relapse were compared using Fisher’s exact test for discrete variables, and the Wilcoxon rank sum test for continuous variables.
Secondary end-points were progression-free and overall survival. The progression-free survival was calculated from the date of ASCT to progression or last follow-up. Overall survival was calculated from the date of ASCT to death or last follow-up. For patients not undergoing ASCT, progression-free and overall survival were calculated from the date the decision was made not to proceed with ASCT, which in most instances occurred during clinical and radiologic reassessment after mini-BEAM. Survival calculations were estimated using the Kaplan-Meier method20 and differences between groups were compared with the log-rank test.
Data were analyzed with SPSS Statistics 17.0 software (SPSS Inc, Chicago IL, USA).
Results
Baseline characteristics
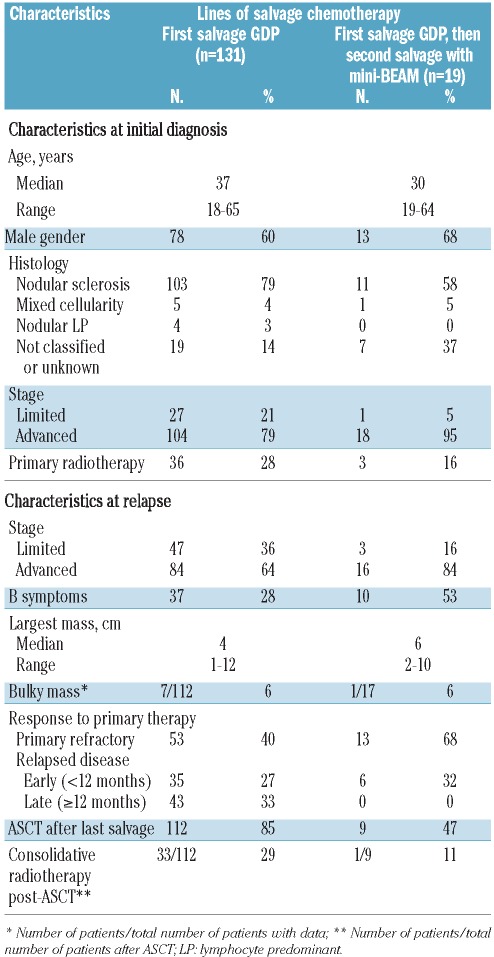
A total of 131 patients receiving first salvage chemotherapy with GDP were identified (Table 1). Their median age was 37 years (range, 18–65), 60% patients were male, and 79% had nodular sclerosis histology. At diagnosis, most patients had advanced stage HL, and 65% had achieved at least a partial remission in response to primary chemotherapy, which was ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) in the majority of patients. Forty percent of patients had primary refractory HL, and 60% had relapsed disease (27% relapsed within 12 months and 33% relapsed ≥12 months after completion of primary chemotherapy). Most relapses were also advanced stage; 28% patients had B symptoms and a minority had bulky disease.
Table 1.
Comparison of baseline characteristics and outcomes between different cohort subsets.
Responses to first-line salvage gemcitabine, dexamethasone and cisplatin
Figure 1 displays the responses to GDP, and the subsequent management of patients according to the aforementioned treatment algorithm. After two cycles of GDP, 19/131 (15%) patients achieved a complete remission and 80 (61%) achieved a partial remission (overall response rate, 76%), and all proceeded to ASCT. Twenty-two (17%) patients achieved stable disease, of whom 13 proceeded directly to ASCT on the basis of gallium scan negativity and disease bulk <5 cm, in accordance with the local treatment algorithm. The remaining nine patients whose disease remained stable despite treatment with GDP (4 gallium-positive, 4 bulk ≥5 cm, 1 both) as well as the ten patients with disease progression on GDP (all gallium-positive) received second salvage with mini-BEAM as their disease was felt to be resistant to platinum-based chemotherapy.
Responses to second-line salvage mini-BEAM therapy
A total of 19 patients received second salvage chemotherapy with mini-BEAM (Table 1). The median age of these patients was 30 years (range, 19–64), 68% were male, and 58% had nodular sclerosis histology. The majority of patients had advanced stage HL both at diagnosis and progression, with 53% patients experiencing B symptoms during the latter. Sixty-eight percent of patients had primary refractory HL, and 32% had relapsed disease (all relapsed within 12 months of primary chemotherapy). Patients receiving mini-BEAM were similar, with regards to age, gender, use of primary radiotherapy, and stage at diagnosis and progression, to those who had responded to GDP and proceeded directly to ASCT (n=112). However, compared to this latter subgroup, in the group receiving mini-BEAM there was a lower proportion of patients with nodular sclerosis histology (58% versus 82%, P=0.030), more B symptoms at progression (53% versus 24%, P=0.024), bulkier masses (median 6 cm versus 4 cm, P=0.049), and lower rates of chemosensitivity to primary therapy including primary refractory disease (68% versus 36%, P=0.011) and late relapses (0 versus 38%, P=0.006).
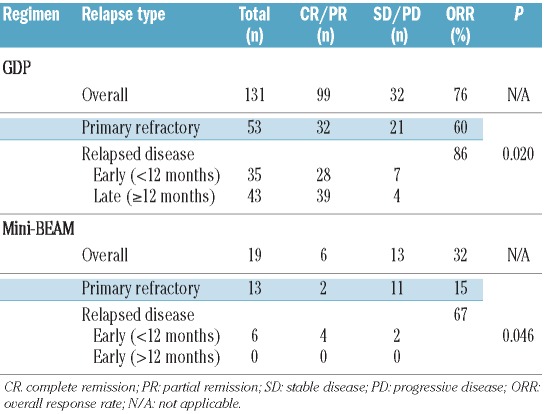
Patients received a median of one cycle of mini-BEAM (range, 1–3), and over 50% received only one cycle. Following mini-BEAM, six of 19 patients achieved a partial remission; no complete remissions were documented (overall response rate 32%). Five patients achieved stable disease, as determined by computed tomography imaging, of whom three proceeded directly to ASCT on the basis of negative functional imaging by gallium scans and disease bulk <5 cm, while the other two patients remained gallium avid and were felt to have chemo-refractory disease. The remaining eight of 19 patients who received mini-BEAM had progressive disease and did not proceed to ASCT. The majority (68%) of the patients treated with mini-BEAM had primary refractory disease, and their overall response rate to mini-BEAM was lower than that of patients with relapsed disease (15% versus 67%, P=0.046) (Figure 1 and Table 2).
Table 2.
Response rates to first and second salvage regimens. The term “primary refractory” and “relapsed disease” refer to the patients’ status after primary chemotherapy.
Nine of the 19 patients who received second salvage with mini-BEAM underwent ASCT, while the other ten patients did not proceed to ASCT. Compared to patients who underwent ASCT following mini-BEAM, these ten patients had a higher proportion of primary refractory HL (100% versus 33%, P=0.003) and a greater median mass size at progression (7 cm versus 4 cm, P=0.040). All other characteristics were similar between these two subgroups. Seven patients subsequently received palliative radiotherapy, and four patients received palliative systemic therapy. All patients have died, with a median overall survival of 3 months (range, 0 – 1.3 years), and 2-year progression-free survival of 0%.
Figure 2.
Progression-free survival (solid line) and overall survival (dotted line) of the 19 patients receiving mini-BEAM. Because ten patients developed progressive disease immediately after mini-BEAM, the progression-free survival curve does not begin at 1.0.
Outcomes following salvage chemotherapy
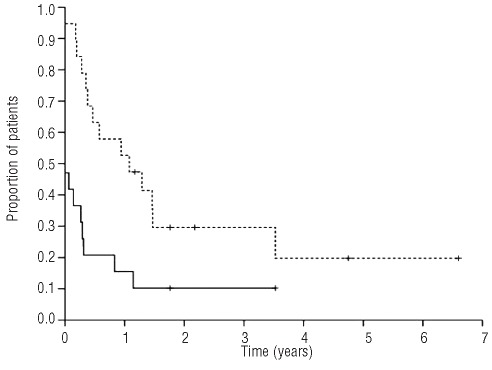
With a median follow-up of 3.3 years (range, 0.4–8.5), the 5-year progression-free and overall survival rates were 11% and 20%, respectively, for the 19 patients treated with mini-BEAM (Figure 3). In addition to the ten patients with progressive disease, seven of nine patients transplanted after mini-BEAM relapsed. Two of these seven patients (one with relapsed and the other with refractory HL) received salvage radiotherapy upon disease progression, and remain alive 6.6 and 4.8 years after ASCT. Finally, only two of 19 patients transplanted after mini-BEAM required no further treatment (both had refractory HL) and remain in remission with 1.8 and 3.5 years of follow-up. Consolidative radiotherapy was given to the former patient. The latter patient received no further treatment after ASCT, and died of an unrelated cause without known progression of HL.
Figure 3.
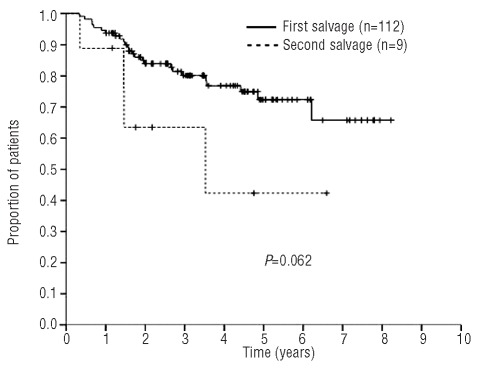
Overall survival of patients transplanted after first salvage (GDP) versus second salvage (mini-BEAM).
In patients transplanted after second salvage therapy the median progression-free survival was 4 months (range, 0 –3.5 years) and the median overall survival was 3.5 years (range, 4 months – 6.6 years) (Figure 3). These outcomes are significantly worse than those of patients transplanted after first salvage, for whom the median progression-free and overall survivals have not been reached. To date, one patient transplanted after GDP has developed myelodysplastic syndrome, and no cases of acute myeloid leukemia have been documented.
Discussion
We observed an overall response rate of 32% to mini-BEAM as a second salvage treatment in patients who did not respond adequately to GDP, a platinum-containing salvage regimen. As would be expected, this rate appears much lower than the overall response rate for mini-BEAM as first salvage therapy reported in the literature,11–14 and is likely a reflection of the high proportion of patients with advanced stage and disease refractory to anthracycline-based and subsequent platinum-based salvage therapy. These two characteristics have been previously described as negative prognostic factors for freedom from treatment failure and overall survival in prospective21,22 and retrospective23 studies of patients with relapsed/refractory HL receiving salvage chemotherapy and ASCT. In these studies, the prognostic impact of chemoresistance was shown for patients with an initial remission lasting less than 12 months, as well as those with primary refractory HL.
Even though some platinum-refractory patients responding to third-line mini-BEAM were able to proceed to ASCT, their outcomes were suboptimal. Patients receiving two or more lines of chemotherapy before ASCT are known to have higher rates of treatment failure, higher transplant-related mortality, and decreased survival.11,21,24–27 For example, by multivariate analyses, in the study by Sureda et al. (n=494), patients with relapsed/refractory HL who received two or more lines of chemotherapy prior to ASCT had a worse overall survival (HR 2.58, P<0.001),26 and in the study by Nademanee et al. (n=85), the number of chemotherapy regimens prior to ASCT was associated with worse overall survival (HR 3.9, P=0.003).25 In the present study, the utility of mini-BEAM prior to ASCT appears modest, with a disappointing median progression-free survival of 4 months and only two of nine (22%) patients remaining disease-free after transplantation. It is, therefore, debatable whether the low probability of long-term benefit from such treatment intensity in the form of ASCT outweighs its morbidity and risks.
Functional imaging by gallium and PET scanning after first-line salvage chemotherapy (and prior to ASCT) in patients with relapsed/refractory HL predicts both progression/event-free and overall survival.28–33 Although PET has largely replaced gallium scanning for response assessment in many centers, limited data suggest that both modalities are similarly effective at predicting outcome prior to ASCT.28,30,31 However, it is unknown whether functional imaging, in particular PET, could be used to identify those patients who may benefit from ASCT after more than one line of salvage therapy. In the present study, gallium status was only known for five of ten patients undergoing ASCT after mini-BEAM. Given the poor outcomes experienced by this small group of patients, it is possible that functional imaging has less prognostic significance in this setting because this is a more heavily pre-treated group of patients than many reported in the PET series.28–33 Larger prospective studies are needed to determine whether functional imaging is able to determine which groups of patients defined by PET-determined response may benefit from ASCT. While a treatment algorithm adapted according to PET-determined response could be utilized to improve outcomes for these poor prognosis patients, there are no published reports evaluating this strategy.
There is a paucity of published data describing the response rate and outcomes to second salvage chemotherapy given with the intent to proceed to ASCT. In an earlier cohort study of 37 patients with relapsed/refractory HL receiving first-line salvage therapy with DHAP, ten patients had a suboptimal response and received alternative salvage regimens (mini-BEAM, CEP, augmented CVP), of whom six achieved at least a partial remission and proceeded to ASCT.34 In a similar cohort, mini-BEAM allowed eight of 11 patients with an insufficient response to first salvage therapy with DHAP to proceed to ASCT, and four of them achieved a durable complete remission.35 In a small study by Ardeshna et al., six of 17 HL patients given second-line salvage chemotherapy had a satisfactory response and proceeded to stem cell transplantation (3 allotransplants, 3 ASCT), which resulted in complete remission and long-term remission in five patients.36 The results presented in these studies are of limited value, as they include small, mixed populations (non-Hodgkin’s lymphoma and HL) of highly-selected patients receiving heterogeneous treatments for which detailed outcome data are not provided. These studies provided the rationale for the present study, which is the largest published series evaluating response to second-line chemotherapy in this population of patients.
Our results are limited by the small number of patients studied due to the rarity of the condition under investigation, as well as to the favorable response rates with second-line regimens in HL. Given the lack of phase III data examining stem cell transplantation in patients in whom salvage chemotherapy has failed (mini-BEAM or dexa-BEAM in the published trials testing ASCT as the experimental arm), it is relevant to compare patients who do and do not undergo ASCT; if post-ASCT patients had favorable outcomes, this would validate the strategy of pursuing aggressive salvage chemotherapy in cases of platinum failure in an attempt to proceed to ASCT. However, because of the higher rate of treatment failure in this group, further modification to this strategy, such as alternative salvage therapy incorporating novel agents, maintenance therapy or tandem ASCT should be studied.
Strategies employing successive intensive systemic therapies should be abandoned and these patients should be offered alternative treatment modalities in lieu of conventional cytotoxic chemotherapy. The incorporation of novel agents as part of salvage or maintenance (such as brentuximab vedotin, panobinostat, and lenalidomide) should be considered. Alternatively, tandem ASCT may be considered in patients in whom salvage chemotherapy was failed. Four prospective studies have evaluated tandem ASCT in patients with primary refractory and high-risk relapsed HL selected for different poor prognostic factors, suggesting that this strategy compares favorably to single ASCT, with high response rates and the potential for durable remissions while maintaining an acceptable toxicity profile.22,37–39 Additionally, allogeneic stem cell transplantation following reduced intensity conditioning (RIC-allogeneic SCT) may also be useful in patients with relapsed/refractory HL in whom salvage therapy, including ASCT, has failed. The reported relatively low relapse rate, presumably as a result of a graft-versus-tumor effect, comes at the expense of high morbidity and mortality associated with this procedure.40–45 Furthermore, RIC-allogeneic SCT has been demonstrated to produce inferior outcomes in patients with chemorefractory disease and thus is not likely to be a successful strategy in this population.
In summary, patients with HL resistant to GDP are infrequently rescued by second-line salvage mini-BEAM therapy, even when they eventually proceed to ASCT. Data in this area will continue to be limited due to the small number of patients reported in series and the retrospective nature of the analyses. In these patients, prospective evaluation of alternative treatment modalities should be considered, including the incorporation of novel agents into the patients’ management, as well as dose-intensive strategies that could include tandem autografting or RIC-allogeneic SCT, and innovative strategies to reduce relapse after stem cell transplantation.
Footnotes
Presented in part during the 50th Annual Meeting of the American Society of Hematology, San Francisco CA, USA, December 6-9, 2008.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Lazarus HM, Rowlings PA, Zhang MJ, Vose JM, Armitage JO, Bierman PJ, et al. Autotransplants for Hodgkin’s disease in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol. 1999;17(2):534–45. doi: 10.1200/JCO.1999.17.2.534. [DOI] [PubMed] [Google Scholar]
- 2.Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341(8852):1051–4. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359(9323):2065–71. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 4.Yuen AR, Rosenberg SA, Hoppe RT, Halpern JD, Horning SJ. Comparison between conventional salvage therapy and high-dose therapy with autografting for recurrent or refractory Hodgkin’s disease. Blood. 1997;89(3):814–22. [PubMed] [Google Scholar]
- 5.Josting A, Rudolph C, Reiser M, Mapara M, Sieber M, Kirchner HH, et al. Time-intensified dexamethasone/cisplatin/cytarabine: an effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin’s disease. Ann Oncol. 2002;13(10):1628–35. doi: 10.1093/annonc/mdf221. [DOI] [PubMed] [Google Scholar]
- 6.Moskowitz CH, Nimer SD, Zelenetz AD, Trippett T, Hedrick EE, Filippa DA, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97(3):616–23. doi: 10.1182/blood.v97.3.616. [DOI] [PubMed] [Google Scholar]
- 7.Ribrag V, Nasr F, Bouhris JH, Bosq J, Brault P, Girinsky T, et al. VIP (etoposide, ifosfamide and cisplatinum) as a salvage intensification program in relapsed or refractory Hodgkin’s disease. Bone Marrow Transplant. 1998;21(10):969–74. doi: 10.1038/sj.bmt.1701202. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez J, Rodriguez MA, Fayad L, McLaughlin P, Swan F, Sarris A, et al. ASHAP: a regimen for cytoreduction of refractory or recurrent Hodgkin’s disease. Blood. 1999;93(11):3632–6. [PubMed] [Google Scholar]
- 9.Baetz T, Belch A, Couban S, Imrie K, Yau J, Myers R, et al. Gemcitabine, dexamethasone and cisplatin is an active and non-toxic chemotherapy regimen in relapsed or refractory Hodgkin’s disease: a phase II study by the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol. 2003;14(12):1762–7. doi: 10.1093/annonc/mdg496. [DOI] [PubMed] [Google Scholar]
- 10.Kuruvilla J, Nagy T, Pintilie M, Tsang R, Keating A, Crump M. Similar response rates and superior early progression-free survival with gemcitabine, dexamethasone, and cisplatin salvage therapy compared with carmustine, etoposide, cytarabine, and melphalan salvage therapy prior to autologous stem cell transplantation for recurrent or refractory Hodgkin lymphoma. Cancer. 2006;106(2):353–60. doi: 10.1002/cncr.21587. [DOI] [PubMed] [Google Scholar]
- 11.Colwill R, Crump M, Couture F, Danish R, Stewart AK, Sutton DM, et al. Mini-BEAM as salvage therapy for relapsed or refractory Hodgkin’s disease before intensive therapy and autologous bone marrow transplantation. J Clin Oncol. 1995;13(2):396–402. doi: 10.1200/JCO.1995.13.2.396. [DOI] [PubMed] [Google Scholar]
- 12.Chopra R, Linch DC, McMillan AK, Blair S, Patterson KG, Moir D, et al. Mini-BEAM followed by BEAM and ABMT for very poor risk Hodgkin’s disease. Br J Haematol. 1992;81(2):197–202. doi: 10.1111/j.1365-2141.1992.tb08207.x. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Jimenez MC, Canales MA, Ojeda E, de Bustos JG, Aguado MJ, Hernandez-Navarro F. Salvage chemotherapy with mini-BEAM for relapsed or refractory Hodgkin’s disease prior to autologous peripheral blood stem cell transplantation. Haematologica. 1999;84(11):1007–11. [PubMed] [Google Scholar]
- 14.Martin A, Fernandez-Jimenez MC, Caballero MD, Canales MA, Perez-Simon JA, Garcia de Bustos J, et al. Long-term follow-up in patients treated with Mini-BEAM as salvage therapy for relapsed or refractory Hodgkin’s disease. Br J Haematol. 2001;113(1):161–71. doi: 10.1046/j.1365-2141.2001.02714.x. [DOI] [PubMed] [Google Scholar]
- 15.Josting A, Reiser M, Rueffer U, Salzberger B, Diehl V, Engert A. Treatment of primary progressive Hodgkin’s and aggressive non-Hodgkin’s lymphoma: is there a chance for cure? J Clin Oncol. 2000;18(2):332–9. doi: 10.1200/JCO.2000.18.2.332. [DOI] [PubMed] [Google Scholar]
- 16.Josting A, Rueffer U, Franklin J, Sieber M, Diehl V, Engert A. Prognostic factors and treatment outcome in primary progressive Hodgkin lymphoma: a report from the German Hodgkin Lymphoma Study Group. Blood. 2000;96(4):1280–6. [PubMed] [Google Scholar]
- 17.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 18.Crump M, Smith AM, Brandwein J, Couture F, Sherret H, Sutton DM, et al. High-dose etoposide and melphalan, and autologous bone marrow transplantation for patients with advanced Hodgkin’s disease: importance of disease status at transplant. J Clin Oncol. 1993;11(4):704–11. doi: 10.1200/JCO.1993.11.4.704. [DOI] [PubMed] [Google Scholar]
- 19.Tsang RW, Gospodarowicz MK, Sutcliffe SB, Crump M, Keating A. Thoracic radiation therapy before autologous bone marrow transplantation in relapsed or refractory Hodgkin’s disease. PMH Lymphoma Group, and the Toronto Autologous BMT Group. Eur J Cancer. 1999;35(1):73–8. doi: 10.1016/s0959-8049(98)00304-9. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 21.Josting A, Muller H, Borchmann P, Baars JW, Metzner B, Dohner H, et al. Dose intensity of chemotherapy in patients with relapsed Hodgkin’s lymphoma. J Clin Oncol. 2010;28(34):5074–80. doi: 10.1200/JCO.2010.30.5771. [DOI] [PubMed] [Google Scholar]
- 22.Morschhauser F, Brice P, Ferme C, Divine M, Salles G, Bouabdallah R, et al. Risk-adapted salvage treatment with single or tandem autologous stem-cell transplantation for first relapse/refractory Hodgkin’s lymphoma: results of the prospective multicenter H96 trial by the GELA/SFGM study group. J Clin Oncol. 2008;26(36):5980–7. doi: 10.1200/JCO.2007.15.5887. [DOI] [PubMed] [Google Scholar]
- 23.Josting A, Franklin J, May M, Koch P, Beykirch MK, Heinz J, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin’s lymphoma registered in the database of the German Hodgkin’s lymphoma study group. J Clin Oncol. 2002;20(1):221–30. doi: 10.1200/JCO.2002.20.1.221. [DOI] [PubMed] [Google Scholar]
- 24.Bierman PJ, Anderson JR, Freeman MB, Vose JM, Kessinger A, Bishop MR, et al. High-dose chemotherapy followed by autologous hematopoietic rescue for Hodgkin’s disease patients following first relapse after chemotherapy. Ann Oncol. 1996;7(2):151–6. doi: 10.1093/oxfordjournals.annonc.a010542. [DOI] [PubMed] [Google Scholar]
- 25.Nademanee A, O’Donnell MR, Snyder DS, Schmidt GM, Parker PM, Stein AS, et al. High-dose chemotherapy with or without total body irradiation followed by autologous bone marrow and/or peripheral blood stem cell transplantation for patients with relapsed and refractory Hodgkin’s disease: results in 85 patients with analysis of prognostic factors. Blood. 1995;85(5):1381–90. [PubMed] [Google Scholar]
- 26.Sureda A, Arranz R, Iriondo A, Carreras E, Lahuerta JJ, Garcia-Conde J, et al. Autologous stem-cell transplantation for Hodgkin’s disease: results and prognostic factors in 494 patients from the Grupo Espanol de Linfomas/Transplante Autologo de Medula Osea Spanish Cooperative Group. J Clin Oncol. 2001;19(5):1395–404. doi: 10.1200/JCO.2001.19.5.1395. [DOI] [PubMed] [Google Scholar]
- 27.Sweetenham JW, Carella AM, Taghipour G, Cunningham D, Marcus R, Della Volpe A, et al. High-dose therapy and autologous stem-cell transplantation for adult patients with Hodgkin’s disease who do not enter remission after induction chemotherapy: results in 175 patients reported to the European Group for Blood and Marrow Transplantation. Lymphoma Working Party. J Clin Oncol. 1999;17(10):3101–9. doi: 10.1200/JCO.1999.17.10.3101. [DOI] [PubMed] [Google Scholar]
- 28.Jabbour E, Hosing C, Ayers G, Nunez R, Anderlini P, Pro B, et al. Pretransplant positive positron emission tomography/gallium scans predict poor outcome in patients with recurrent/refractory Hodgkin lymphoma. Cancer. 2007;109(12):2481–9. doi: 10.1002/cncr.22714. [DOI] [PubMed] [Google Scholar]
- 29.Mocikova H, Pytlik R, Markova J, Steinerova K, Kral Z, Belada D, et al. Pre-transplant positron emission tomography in patients with relapsed Hodgkin lymphoma. Leuk Lymphoma. 2011;52(9):1668–74. doi: 10.3109/10428194.2011.573889. [DOI] [PubMed] [Google Scholar]
- 30.Moskowitz AJ, Yahalom J, Kewalramani T, Maragulia JC, Vanak JM, Zelenetz AD, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood. 2010;116(23):4934–7. doi: 10.1182/blood-2010-05-282756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moskowitz CH, Yahalom J, Zelenetz AD, Zhang Z, Filippa D, Teruya-Feldstein J, et al. High-dose chemo-radiotherapy for relapsed or refractory Hodgkin lymphoma and the significance of pre-transplant functional imaging. Br J Haematol. 2010;148(6):890–7. doi: 10.1111/j.1365-2141.2009.08037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poulou LS, Thanos L, Ziakas PD. Unifying the predictive value of pretransplant FDG PET in patients with lymphoma: a review and meta-analysis of published trials. Eur J Nucl Med Mol Imaging. 2010;37(1):156–62. doi: 10.1007/s00259-009-1258-y. [DOI] [PubMed] [Google Scholar]
- 33.Smeltzer JP, Cashen AF, Zhang Q, Homb A, Dehdashti F, Abboud CN, et al. Prognostic Significance of FDG-PET in relapsed or refractory classical Hodgkin lymphoma treated with standard salvage chemotherapy and autologous stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(11):1646–52. doi: 10.1016/j.bbmt.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandwein JM, Callum J, Sutcliffe SB, Scott JG, Keating A. Evaluation of cytoreductive therapy prior to high dose treatment with autologous bone marrow transplantation in relapsed and refractory Hodgkin’s disease. Bone Marrow Transplant. 1990;5(2):99–103. [PubMed] [Google Scholar]
- 35.Stewart AK, Brandwein J, Sutcliffe S, Scott G, Keating A. Mini-beam as salvage chemotherapy for refractory Hodgkin’s disease and Non-Hodgkin lymphoma. Leuk Lymphoma. 1991;5(2–3):111–5. doi: 10.3109/10428199109068113. [DOI] [PubMed] [Google Scholar]
- 36.Ardeshna KM, Kakouros N, Qian W, Powell MG, Saini N, D’Sa S, et al. Conventional second-line salvage chemotherapy regimens are not warranted in patients with malignant lymphomas who have progressive disease after first-line salvage therapy regimens. Br J Haematol. 2005;130(3):363–72. doi: 10.1111/j.1365-2141.2005.05603.x. [DOI] [PubMed] [Google Scholar]
- 37.Brice P, Divine M, Simon D, Coiffier B, Leblond V, Simon M, et al. Feasibility of tandem autologous stem-cell transplantation (ASCT) in induction failure or very unfavorable (UF) relapse from Hodgkin’s disease (HD). SFGM/GELA Study Group. Ann Oncol. 1999;10(12):1485–8. doi: 10.1023/a:1008343823292. [DOI] [PubMed] [Google Scholar]
- 38.Castagna L, Magagnoli M, Balzarotti M, Sarina B, Siracusano L, Nozza A, et al. Tandem high-dose chemotherapy and autologous stem cell transplantation in refractory/relapsed Hodgkin’s lymphoma: a mono-center prospective study. Am J Hematol. 2007;82(2):122–7. doi: 10.1002/ajh.20790. [DOI] [PubMed] [Google Scholar]
- 39.Fung HC, Stiff P, Schriber J, Toor A, Smith E, Rodriguez T, et al. Tandem autologous stem cell transplantation for patients with primary refractory or poor risk recurrent Hodgkin lymphoma. Biol Blood Marrow Transplant. 2007;13(5):594–600. doi: 10.1016/j.bbmt.2007.01.072. [DOI] [PubMed] [Google Scholar]
- 40.Akpek G, Ambinder RF, Piantadosi S, Abrams RA, Brodsky RA, Vogelsang GB, et al. Long-term results of blood and marrow transplantation for Hodgkin’s lymphoma. J Clin Oncol. 2001;19(23):4314–21. doi: 10.1200/JCO.2001.19.23.4314. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez I, Sureda A, Caballero MD, Urbano-Ispizua A, Ribera JM, Canales M, et al. Nonmyeloablative stem cell transplantation is an effective therapy for refractory or relapsed hodgkin lymphoma: results of a spanish prospective cooperative protocol. Biol Blood Marrow Transplant. 2006;12(2):172–83. doi: 10.1016/j.bbmt.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Anderlini P, Saliba R, Acholonu S, Okoroji GJ, Donato M, Giralt S, et al. Reduced-intensity allogeneic stem cell transplantation in relapsed and refractory Hodgkin’s disease: low transplant-related mortality and impact of intensity of conditioning regimen. Bone Marrow Transplant. 2005;35(10):943–51. doi: 10.1038/sj.bmt.1704942. [DOI] [PubMed] [Google Scholar]
- 43.Gajewski JL, Phillips GL, Sobocinski KA, Armitage JO, Gale RP, Champlin RE, et al. Bone marrow transplants from HLA-identical siblings in advanced Hodgkin’s disease. J Clin Oncol. 1996;14(2):572–8. doi: 10.1200/JCO.1996.14.2.572. [DOI] [PubMed] [Google Scholar]
- 44.Milpied N, Fielding AK, Pearce RM, Ernst P, Goldstone AH. Allogeneic bone marrow transplant is not better than autologous transplant for patients with relapsed Hodgkin’s disease. European Group for Blood and Bone Marrow Transplantation. J Clin Oncol. 1996;14(4):1291–6. doi: 10.1200/JCO.1996.14.4.1291. [DOI] [PubMed] [Google Scholar]
- 45.Peggs KS, Hunter A, Chopra R, Parker A, Mahendra P, Milligan D, et al. Clinical evidence of a graft-versus-Hodgkin’s-lymphoma effect after reduced-intensity allogeneic transplantation. Lancet. 2005;365(9475):1934–41. doi: 10.1016/S0140-6736(05)66659-7. [DOI] [PubMed] [Google Scholar]