Abstract
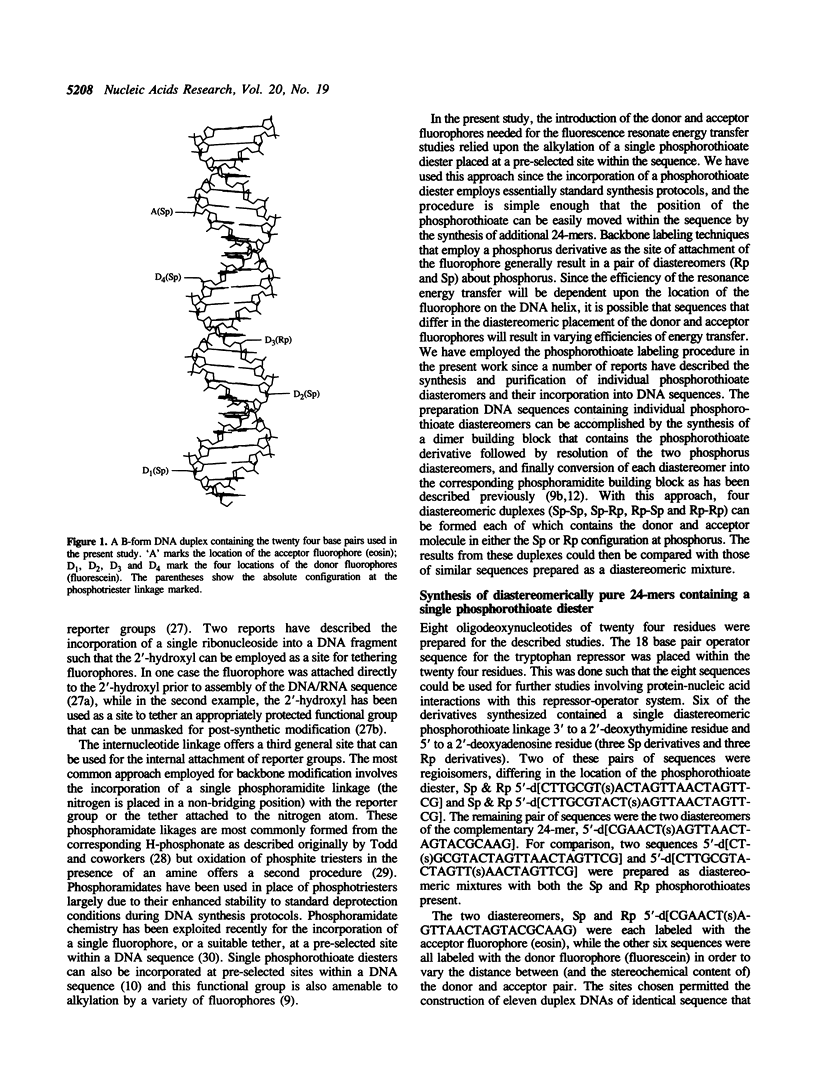
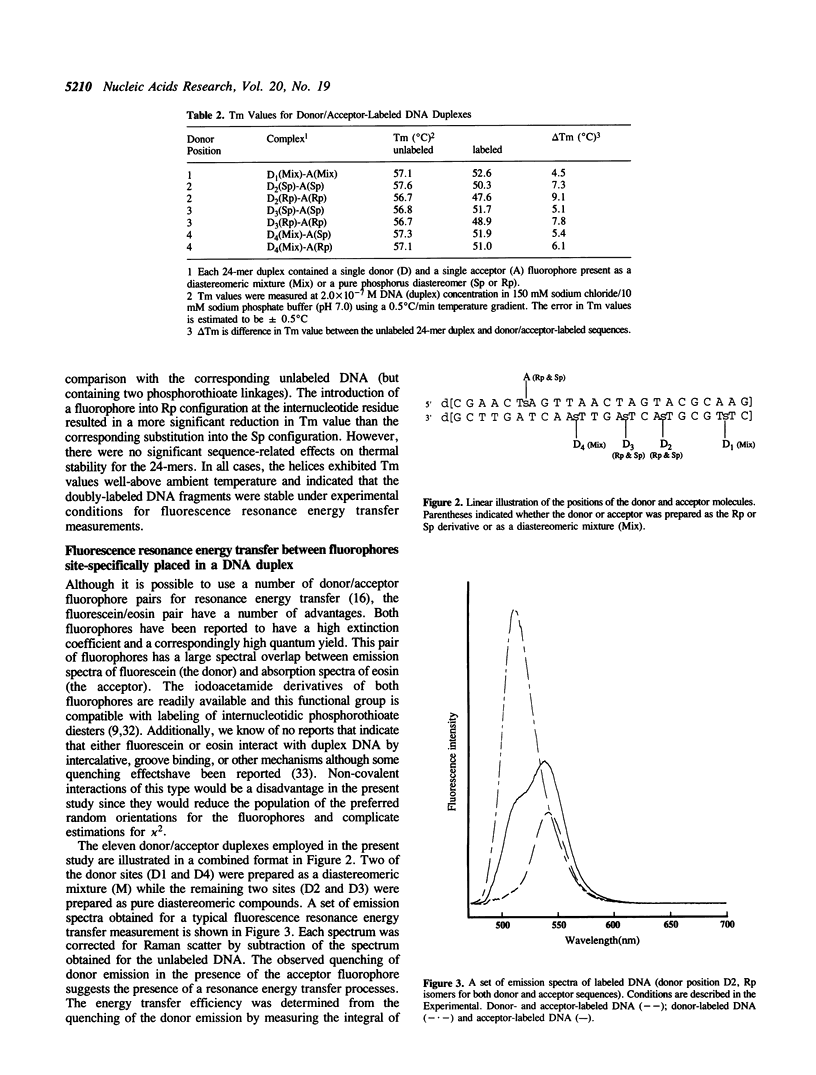
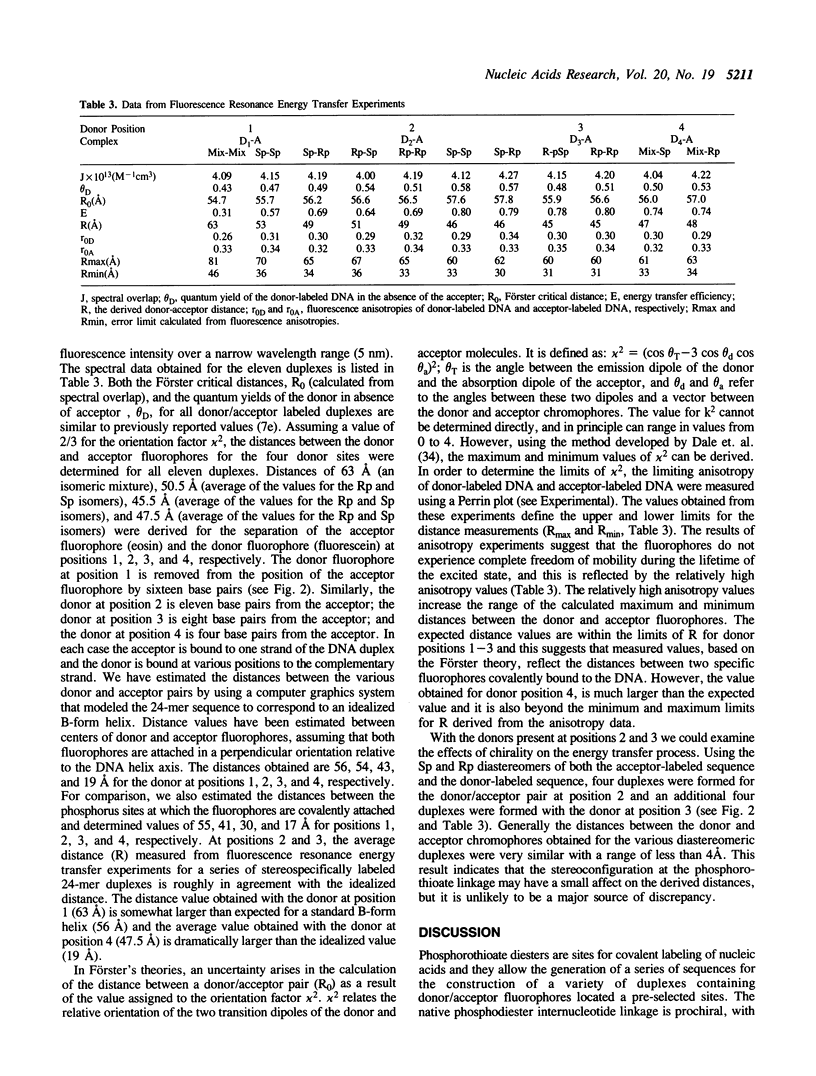
A series DNA helices of twenty-four base pairs has been prepared for the study of fluorescence resonance energy transfer. Each of the DNA helices contains two phosphorothioate diesters (one in each strand) at pre-selected sites for introduction of the desired donor and acceptor fluorophores. The phosphorothioate-containing oligodeoxynucleotides have been prepared as pure Rp or Sp derivatives or as deastereomeric mixtures. Fluorescein and eosin are employed as the respective donor and acceptor fluorophores. A series of donor-acceptor pairs was generated by labeling of the appropriate phosphorothioate diester with the desired fluorophore and annealing the two complementary DNA strands (one containing the acceptor and one containing the donor fluorophore) to form the double-stranded helix. The 24-mer helices containing two covalently attached fluorophores exhibited some thermal destabilization and the extent of this destabilization was dependent upon the stereochemical orientation of the fluorophore. The Sp derivatives direct the fluorophore out, away from the the DNA helix, while the Rp derivatives direct the fluorophore toward the major groove. As expected, the Sp labeled duplexes were more stable than the corresponding Rp labeled sequences. However, all of the duplex structures formed were stable under the conditions used to measure energy transfer. Energy transfer could be observed with these complexes from the quenching of the donor fluorescence in the presence of the acceptor fluorophore. Using Förster's theories, distances separating the fluorophores could be calculated that were generally in reasonable agreement with the distances expected in an idealized B-form DNA helix. However anomalous results were obtained for one donor/acceptor pair where the expected distance was less than 20 A. Fluorescence anisotropy values determined in solutions of varying viscosity were quite high suggesting that the fluorophores did not experience complete freedom of movement when attached to the DNA helix.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Christodoulou C., Gait M. J. Efficient methods for attaching non-radioactive labels to the 5' ends of synthetic oligodeoxyribonucleotides. Nucleic Acids Res. 1986 Aug 11;14(15):6227–6245. doi: 10.1093/nar/14.15.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broido M. S., James T. L., Zon G., Keepers J. W. Investigation of the solution structure of a DNA octamer [d(GGAATTCC)]2 using two-dimensional nuclear Overhauser enhancement spectroscopy. Eur J Biochem. 1985 Jul 1;150(1):117–128. doi: 10.1111/j.1432-1033.1985.tb08996.x. [DOI] [PubMed] [Google Scholar]
- Cardullo R. A., Agrawal S., Flores C., Zamecnik P. C., Wolf D. E. Detection of nucleic acid hybridization by nonradiative fluorescence resonance energy transfer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8790–8794. doi: 10.1073/pnas.85.23.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly B. A., Potter B. V., Eckstein F., Pingoud A., Grotjahn L. Synthesis and characterization of an octanucleotide containing the EcoRI recognition sequence with a phosphorothioate group at the cleavage site. Biochemistry. 1984 Jul 17;23(15):3443–3453. doi: 10.1021/bi00310a010. [DOI] [PubMed] [Google Scholar]
- Connolly B. A., Rider P. Chemical synthesis of oligonucleotides containing a free sulphydryl group and subsequent attachment of thiol specific probes. Nucleic Acids Res. 1985 Jun 25;13(12):4485–4502. doi: 10.1093/nar/13.12.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly B. A. The synthesis of oligonucleotides containing a primary amino group at the 5'-terminus. Nucleic Acids Res. 1987 Apr 10;15(7):3131–3139. doi: 10.1093/nar/15.7.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosstick R., Eckstein F. Synthesis of d(GC) and d(CG) octamers containing alternating phosphorothioate linkages: effect of the phosphorothioate group on the B-Z transition. Biochemistry. 1985 Jul 2;24(14):3630–3638. doi: 10.1021/bi00335a035. [DOI] [PubMed] [Google Scholar]
- Czworkowski J., Odom O. W., Hardesty B. Fluorescence study of the topology of messenger RNA bound to the 30S ribosomal subunit of Escherichia coli. Biochemistry. 1991 May 14;30(19):4821–4830. doi: 10.1021/bi00233a026. [DOI] [PubMed] [Google Scholar]
- Dale R. E., Eisinger J., Blumberg W. E. The orientational freedom of molecular probes. The orientation factor in intramolecular energy transfer. Biophys J. 1979 May;26(2):161–193. doi: 10.1016/S0006-3495(79)85243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairclough R. H., Cantor C. R. The use of singlet-singlet energy transfer to study macromolecular assemblies. Methods Enzymol. 1978;48:347–379. doi: 10.1016/s0076-6879(78)48019-x. [DOI] [PubMed] [Google Scholar]
- Gettins P., Beechem J. M., Crews B. C., Cunningham L. W. Separation and localization of the four cysteine-949 residues in human alpha 2-macroglobulin using fluorescence energy transfer. Biochemistry. 1990 Aug 21;29(33):7747–7753. doi: 10.1021/bi00485a025. [DOI] [PubMed] [Google Scholar]
- Giedroc D. P., Khan R., Barnhart K. Site-specific 1,N6-ethenoadenylated single-stranded oligonucleotides as structural probes for the T4 gene 32 protein-ssDNA complex. Biochemistry. 1991 Aug 20;30(33):8230–8242. doi: 10.1021/bi00247a020. [DOI] [PubMed] [Google Scholar]
- Goodchild J. Conjugates of oligonucleotides and modified oligonucleotides: a review of their synthesis and properties. Bioconjug Chem. 1990 May-Jun;1(3):165–187. doi: 10.1021/bc00003a001. [DOI] [PubMed] [Google Scholar]
- Hodges R. R., Conway N. E., McLaughlin L. W. "Post-assay" covalent labeling of phosphorothioate-containing nucleic acids with multiple fluorescent markers. Biochemistry. 1989 Jan 10;28(1):261–267. doi: 10.1021/bi00427a036. [DOI] [PubMed] [Google Scholar]
- Jäger A., Levy M. J., Hecht S. M. Oligonucleotide N-alkylphosphoramidates: synthesis and binding to polynucleotides. Biochemistry. 1988 Sep 20;27(19):7237–7246. doi: 10.1021/bi00419a010. [DOI] [PubMed] [Google Scholar]
- Kalman B., Sandström A., Johansson L. B., Lindskog S. Electronic energy transfer and fluorescence quenching in the active sites of mercuric reductase. Biochemistry. 1991 Jan 8;30(1):111–117. doi: 10.1021/bi00215a017. [DOI] [PubMed] [Google Scholar]
- Kochoyan M., Keutmann H. T., Weiss M. A. Alternating zinc fingers in the human male-associated protein ZFY: HX3H and HX4H motifs encode a local structural switch. Biochemistry. 1991 Oct 1;30(39):9396–9402. doi: 10.1021/bi00103a002. [DOI] [PubMed] [Google Scholar]
- LaPlanche L. A., James T. L., Powell C., Wilson W. D., Uznanski B., Stec W. J., Summers M. F., Zon G. Phosphorothioate-modified oligodeoxyribonucleotides. III. NMR and UV spectroscopic studies of the Rp-Rp, Sp-Sp, and Rp-Sp duplexes, [d(GGSAATTCC)]2, derived from diastereomeric O-ethyl phosphorothioates. Nucleic Acids Res. 1986 Nov 25;14(22):9081–9093. doi: 10.1093/nar/14.22.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letsinger R. L., Bach S. A., Eadie J. S. Effects of pendant groups at phosphorus on binding properties of d-ApA analogues. Nucleic Acids Res. 1986 Apr 25;14(8):3487–3499. doi: 10.1093/nar/14.8.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letsinger R. L., Zhang G. R., Sun D. K., Ikeuchi T., Sarin P. S. Cholesteryl-conjugated oligonucleotides: synthesis, properties, and activity as inhibitors of replication of human immunodeficiency virus in cell culture. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6553–6556. doi: 10.1073/pnas.86.17.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morden K. M., Gunn B. M., Maskos K. NMR studies of a deoxyribodecanucleotide containing an extrahelical thymidine surrounded by an oligo(dA).oligo(dT) tract. Biochemistry. 1990 Sep 18;29(37):8835–8845. doi: 10.1021/bi00489a047. [DOI] [PubMed] [Google Scholar]
- Murakami A., Nakaura M., Nakatsuji Y., Nagahara S., Tran-Cong Q., Makino K. Fluorescent-labeled oligonucleotide probes: detection of hybrid formation in solution by fluorescence polarization spectroscopy. Nucleic Acids Res. 1991 Aug 11;19(15):4097–4102. doi: 10.1093/nar/19.15.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie A. I., Clegg R. M., von Kitzing E., Duckett D. R., Diekmann S., Lilley D. M. Fluorescence energy transfer shows that the four-way DNA junction is a right-handed cross of antiparallel molecules. Nature. 1989 Oct 26;341(6244):763–766. doi: 10.1038/341763a0. [DOI] [PubMed] [Google Scholar]
- Nikonowicz E. P., Gorenstein D. G. Two-dimensional 1H and 31P NMR spectra and restrained molecular dynamics structure of a mismatched GA decamer oligodeoxyribonucleotide duplex. Biochemistry. 1990 Sep 18;29(37):8845–8858. doi: 10.1021/bi00489a048. [DOI] [PubMed] [Google Scholar]
- Odom O. W., Jr, Robbins D. J., Lynch J., Dottavio-Martin D., Kramer G., Hardesty B. Distances between 3' ends of ribosomal ribonucleic acids reassembled into Escherichia coli ribosomes. Biochemistry. 1980 Dec 23;19(26):5947–5954. doi: 10.1021/bi00567a001. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Nordheim A., Rich A. Right-handed and left-handed DNA: studies of B- and Z-DNA by using proton nuclear Overhauser effect and P NMR. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1413–1417. doi: 10.1073/pnas.79.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieles U., Sproat B. S., Neuner P., Cramer F. Preparation of a novel psoralen containing deoxyadenosine building block for the facile solid phase synthesis of psoralen-modified oligonucleotides for a sequence specific crosslink to a given target sequence. Nucleic Acids Res. 1989 Nov 25;17(22):8967–8978. doi: 10.1093/nar/17.22.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter B. V., Connolly B. A., Eckstein F. Synthesis and configurational analysis of a dinucleoside phosphate isotopically chiral at phosphorus. Stereochemical course of Penicillium citrum nuclease P1 reaction. Biochemistry. 1983 Mar 15;22(6):1369–1377. doi: 10.1021/bi00275a008. [DOI] [PubMed] [Google Scholar]
- Potter B. V., Eckstein F., Uznański B. A stereospecifically 18O-labelled deoxydinucleoside phosphate block for incorporation into an oligonucleotide. Nucleic Acids Res. 1983 Oct 25;11(20):7087–7103. doi: 10.1093/nar/11.20.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins D., Odom O. W., Jr, Lynch J., Kramer G., Hardesty B., Liou R., Ofengand J. Position of transfer ribonucleic acid on Escherichia coli ribosomes. Distance from the 3' end of 16S ribonucleic acid to three points on phenylalanine-accepting transfer ribonucleic acid in the donor site of 70S ribosomes. Biochemistry. 1981 Sep 1;20(18):5301–5309. doi: 10.1021/bi00521a033. [DOI] [PubMed] [Google Scholar]
- Roget A., Bazin H., Teoule R. Synthesis and use of labelled nucleoside phosphoramidite building blocks bearing a reporter group: biotinyl, dinitrophenyl, pyrenyl and dansyl. Nucleic Acids Res. 1989 Oct 11;17(19):7643–7651. doi: 10.1093/nar/17.19.7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., Fung S., Hunkapiller M. W., Hunkapiller T. J., Hood L. E. The synthesis of oligonucleotides containing an aliphatic amino group at the 5' terminus: synthesis of fluorescent DNA primers for use in DNA sequence analysis. Nucleic Acids Res. 1985 Apr 11;13(7):2399–2412. doi: 10.1093/nar/13.7.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproat B. S., Beijer B., Rider P., Neuner P. The synthesis of protected 5'-mercapto-2',5'-dideoxyribonucleoside-3'-O-phosphoramidites; uses of 5'-mercapto-oligodeoxyribonucleotides. Nucleic Acids Res. 1987 Jun 25;15(12):4837–4848. doi: 10.1093/nar/15.12.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproat B. S., Beijer B., Rider P. The synthesis of protected 5'-amino-2',5'-dideoxyribonucleoside-3'-O-phosphoramidites; applications of 5'-amino-oligodeoxyribonucleotides. Nucleic Acids Res. 1987 Aug 11;15(15):6181–6196. doi: 10.1093/nar/15.15.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. A., Subasinghe C., Shinozuka K., Cohen J. S. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988 Apr 25;16(8):3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L., Haugland R. P. Energy transfer: a spectroscopic ruler. Proc Natl Acad Sci U S A. 1967 Aug;58(2):719–726. doi: 10.1073/pnas.58.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. S., Garrett D. S., Brockie I. R., Svoboda D. L., Telser J. 1H NMR assignment and melting temperature study of cis-syn and trans-syn thymine dimer containing duplexes of d(CGTATTATGC).d(GCATAATACG). Biochemistry. 1990 Sep 18;29(37):8858–8866. doi: 10.1021/bi00489a049. [DOI] [PubMed] [Google Scholar]
- Torigoe H., Shimada I., Saito A., Sato M., Arata Y. Sequential 1H NMR assignments and secondary structure of the B domain of staphylococcal protein A: structural changes between the free B domain in solution and the Fc-bound B domain in crystal. Biochemistry. 1990 Sep 18;29(37):8787–8793. doi: 10.1021/bi00489a040. [DOI] [PubMed] [Google Scholar]
- Williamson M. P., Havel T. F., Wüthrich K. Solution conformation of proteinase inhibitor IIA from bull seminal plasma by 1H nuclear magnetic resonance and distance geometry. J Mol Biol. 1985 Mar 20;182(2):295–315. doi: 10.1016/0022-2836(85)90347-x. [DOI] [PubMed] [Google Scholar]
- Yamana K., Letsinger R. L. Synthesis and properties of oligonucleotides bearing a pendant pyrene group. Nucleic Acids Symp Ser. 1985;(16):169–172. [PubMed] [Google Scholar]


