Abstract
Induced pluripotent stem cells (iPSCs) hold great hopes for therapeutic application in various diseases. While ongoing research is dedicated to achieving clinical translation of iPSCs, further understanding of the mechanisms that underlie complex pathogenic conditions is required. Compared to other classical models for studying diseases, iPSCs provide considerable advantages. A newly emerging application of iPSCs is in vitro disease modeling, which can significantly improve the never-ending search for new pharmacological cures. Here, we will discuss current efforts to create iPSC-dependent, patient-specific disease models. Furthermore, we will review the use of iPSCs for development and testing of new therapeutic agents, and the implications for high-throughput drug screening.
Keywords: Induced pluripotent stem cells, Disease modeling, Cardiovascular disease, Drug screening, High-throughput screening
Introduction
Reprogramming of adult somatic cells into induced pluripotent stem cells (iPSCs) is a powerful approach that holds great promise for regenerative medicine in the future. The first successful reprogramming of somatic cells into an induced pluripotent state was reported in 2006 by Yamanaka and colleagues [1]. This pioneering work defined a combination of four transcription factors, Oct4, Sox2, Klf4 and c-Myc, as both necessary and sufficient to return terminally differentiated cells to a pluripotent state, making these iPSCs capable of generating tissues of all three germ layers as well as whole organisms [1], similar to the capacity of human embryonic stem cells (hESCs) [2–4]. These findings were confirmed by many other researchers using various donor cell types, such as skin cells [5–7], neuronal cells [8–10], hematopoietic cells [11–13], adipose stromal cells [14], and others. Since then, iPSCs have evolved into an exciting, productive, and fast-moving research field. Considerable advances have also been made regarding various integration-free iPSC reprogramming techniques, including episomal plasmids [15–17], minicircles [18, 19], recombinant protein [20, 21], synthetic mRNA [22], microRNA [23, 24], and others [25, 26].
Because iPSCs are derived directly from the somatic tissues of patients, this technology overcomes major ethical concerns that have plagued hESCs, such as the destruction of human embryos and oocytes, thereby opening a vital portal for broader research on human pluripotent cells [27–30]. Furthermore, iPSCs provide an unlimited source of proliferating cells. This latter desirable feature of iPSCs overcomes the constraints of confined donor cell availability, as well as limited proliferation capacity and loss of functionality, both of which have been observed in ex vivo-expanded cells [31]. Hence, iPSCs not only provide promising therapeutic approaches for the future but may also pave the way for personalized medicine. However, recent studies have also reported substantial differences in genetic or epigenetic profiles of iPSCs versus hESCs [32–36], which must further investigated before therapeutic application of iPSCs in humans. Moreover, the possibility of an immunological response and rejection of iPSCs by a recipient patient cannot be completely precluded [37–39].
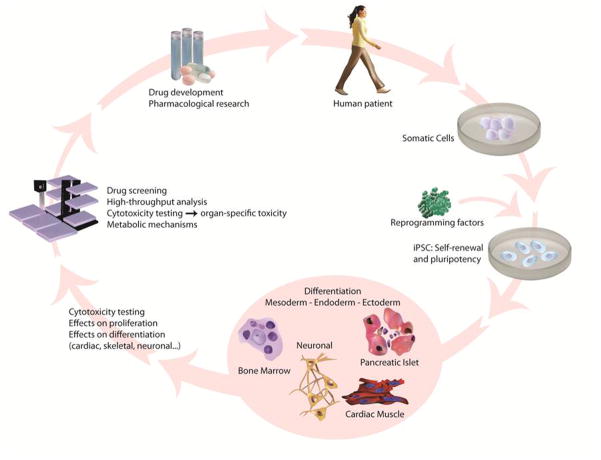
A different field of application establishes potential roles for iPSCs in modeling diseases and drug screening (Figure 1). Previously available human drug screening models relied on the patient’s samples or immortalized, tumor-derived cell lines. While a patient’s cells directly model the effects of a drug on humans, their availability and capacity for expansion are limited and finite compared to in vitro derived cell lines. The latter, however, may contain genetic and metabolic abnormalities due to their derivation, and thus would not represent a realistic or ideal drug model for human patients. These drawbacks restrict the capacity of these models to faithfully simulate human disease. By comparison, iPSCs can sidestep these limitations and thus provide a powerful and versatile tool for disease therapy as well as basic research.
Figure 1. Application of iPSCs for regenerative medicine, disease modeling, and drug screening.
After reprogramming of somatic, patient-derived cells to an induced pluripotent state, iPSCs can be differentiated into cell types of all three germ layers, allowing regeneration of tissue and posing great hopes for clinical translation of iPSCs and treatment of many diseases. Also, iPSC-derived tissue-specific cells may be employed for modeling diseases to understand the complex mechanisms underlying various diseases, and for assessing cytotoxicity of small chemicals in drug development. This illustrates how iPSCs provide an improved model system for disease modeling, high-throughput drug screening, and toxicity testing.
Disease modeling using iPSCs: a cardiac perspective
In recent years, researchers have begun to explore the iPSC technology’s full potential for creating disease models from patients with complex genetic defects [40–43]. Clinically relevant mutations can be derived from cells of patients with a particular genetic disease of choice. To date, various tissue-specific iPSC derivatives have been generated (Table 1), including hematopoietic [44–49], hepatic [50–52], endothelial [53], neurological [8–10, 54–56], and cardiovascular diseases [43, 57–61]. The number of diseases successfully modeled via iPSCs is also increasing constantly [9, 57–59, 62, 63], reflecting their growing utility and versatility as platforms for studying disease development and models in vitro, as well as investigating pathophysiology and testing therapeutic agents (Tables 1 and 2).
Table 1.
Overview on diseases modeled with patient-specific iPSCs.
| Disease background | Genetic disease | Donor cell type | Cell type generated | Drug testing | Reference |
|---|---|---|---|---|---|
| Hematologic | Fanconi anaemia | Fibroblasts | Hematocytes | ND | 43 |
| Fragile X Syndrome | Fibroblasts | ND | ND | 47 | |
| Thalassemia | Fibroblasts | Hematopoietic | ND | 45 | |
| Down syndrome (Trisomy 21) | Fibroblasts | ND | ND | 44 | |
| Type 1 diabetes | Fibroblasts | Insulin- and glucagon-producing cells | ND | 44 | |
| Swachman-Bodian-Diamond syndrome (SBDS) | Bone marrow mesenchymal cells | ND | ND | 44 | |
| Hurler syndrome (Mucopolysaccharidosis type I, MPS IH) | Fibroblasts, bone marrow mesenchymal cells | Hematopoietic | ND | 48 | |
| Cardiac/Cardiovascular | LQT syndrome type I | Fibroblasts | Cardiomyocytes | Yes | 57 |
| LQT syndrome type II | Fibroblasts | Cardiomyocytes | Yes | 56 | |
| Timothy syndrome (LQT syndrome type VII) | Fibroblasts | Cardiomyocytes | Yes | 58 | |
| LEOPARD syndrome | Fibroblasts | Cardiomyocytes | ND | 62 | |
| Duchenne muscular dystrophy (DMD) | Fibroblasts | ND | ND | 44, 126 | |
| Becker muscular dystrophy (BMD) | Fibroblasts | ND | ND | 44, 101 | |
| Hutchinson Gilford Progeria (HGPS) | Fibroblasts | Smooth muscle cells, Mesenchymal stem cells | ND | 59 | |
| Neurological | Amyotrophic lateral sclerosis (ALS) | Fibroblasts | Motor neurons, glial cells | ND | 8 |
| Parkinson disease (PD) | Fibroblasts | Dopaminergic neurons | Yes | 65, 66 | |
| Spinal muscular atrophy (SMA) | Fibroblasts | Motor neurons | Yes | 9 | |
| Huntington disease (HD) | Fibroblasts | ND | ND | 44 | |
| Schizophrenia (SCZD) | Fibroblasts | Neurons | ND | 61, 125 | |
| Autism spectrum disorders (ASD) | Fibroblasts | Neurons | ND | 67 | |
| Prader-Willi Syndrome | Fibroblasts | Neurons | ND | 54 | |
| Angelman and Prader-Willi Syndrome | Fibroblasts | Neurons | ND | 55 | |
| Familial dysautonomia | Fibroblasts | Neural crest cells | Yes | 64 | |
| RETT syndrome | Fibroblasts | Neurons | Yes | 67 | |
| Friedreich’s ataxia | Fibroblasts | ND | ND | 63 | |
| Others | Lesch-Nyhan syndrome (carrier stage) | Fibroblasts | ND | ND | 44 |
| Adenosine deaminase deficiencyrelated severe combined immunodeficiency (ADA-SCID) | Fibroblasts | ND | ND | 44 | |
| Gaucher disease type III (GD) | Fibroblasts | ND | ND | 44 | |
| X-linked chronic granulomatous disease (X-CGD) | Bone marrow mesenchymal cells | Neutrophil | ND | 41 | |
| A1-antitrypsin deficiency | Fibroblasts | Hepatocytes | Yes | 69 |
Table 2.
Advantages and disadvantages of different modeling approaches.
| Disease model | Advantage | Disadvantage |
|---|---|---|
| Animal (mouse, rat) |
|
|
| Classical tissue culture |
|
|
| hESCs |
|
|
| iPSCs |
|
|
| Adult stem cells |
|
|
In the past, experimental mouse model systems have been used to understand the functional changes of genetic mutations identified in patients with inherited disease. However, mouse models do not always demonstrate the same phenotypes as those observed in humans. For example, mice showed a much higher heart beating rate of 600 beats per minute (bpm) and much shorter action potentials than humans because the type and/or distribution of cardiac ion channels are different between mice and human. In vitro analysis of human cardiomyocytes is therefore important to understand the mechanism of human genetic arrhythmias, and iPSCs may be able to fill in this knowledge gap regarding genetic alterations in the ‘native’ cellular context.
Neuronal disease models using iPSCs were introduced as early as 2008 [8]. Dimos et al. reported reprogramming of an amyotrophic lateral sclerosis patient’s fibroblasts into iPSCs and their differentiation into functional motor neurons. Since then, various studies have successfully modeled neuronal disease [8–10, 45, 64–67], as reviewed elsewhere [68]. Very recent efforts include modeling of lysosomal storage diseases (LSDs), a most frequent cause of neurodegeneration originating from deficient recycling (and hence accumulation) of molecular catabolites [69]. Lemmonier et al. focused on mucopolysaccharidosis IIIB (MPSIIIB), a LSD resulting from α-N-acetylglucosaminidase deficiency. This lysosomal hydrolytic enzyme mediates heparan sulfate proteoglycan (HSPG) degradation and is involved in a critical step in protein turnover. Analysis of the disease via patient-derived iPSCs revealed that undifferentiated iPSCs rapidly displayed the disease phenotype-characteristic proliferation defects reflecting deficient FGF-2 signaling in the absence of lysosomal glucosaminidase and accumulation of the ganglioside GM3 in storage vesicles.
A different example providing insight into the field of iPSC-dependent disease modeling is hepatic differentiation. Significant advances have been made for in vitro differentiation of iPSCs into hepatocytes [50, 52, 70], and the unlimited proliferation potential of iPSC-derived hepatic cells holds great promise for regenerative tissue therapy, but challenges remain as it requires functional engraftment of hepatic cells into the liver. While the in vivo functionality of iPSC-derived hepatic cells has not been established in detail [31, 71], the properties of iPSC-derived hepatic cells that reflect disease features have been confirmed [50, 52, 70].
Cardiovascular disease modeling
Cardiomyopathies are defined as myocardial diseases, which can be due to myocardial infarction, genetic mutation, valvular regurgitation, storage disorder, endocrine disease, and toxicity from chemotherapy or alcohol. This complex disease requires an elaborate model to study the underlying functional mechanism. Recently, iPSCs have been utilized for in vitro disease modeling of cardiac arrhythmias [57–59]. A prominent example of cardiac arrhythmia is the long QT syndrome (LQTS). This rare inborn heart condition has an estimated prevalence of about 1:7000 persons (inherited LQTS), causing ~2000–3000 sudden deaths in children and young adults each year in the US alone [72–74]. QT describes a specific interval on an electrocardiogram (ECG), the time from the electrical stimulation (depolarization) of the heart’s pumping ventricles to the end of the recharging of the electrical system (repolarization). The total duration is measured in seconds or milliseconds (ms) and closely approximates the time from the beginning of the ventricles’ contraction until the end of relaxation. The normal QTc interval varies from 350–450 ms. About 95% of people show values between 338–440 ms, which is the range generally considered as the ‘normal’ range [75, 76].
In LQTS, delayed repolarization of the heart following a heartbeat increases the risk of episodes of Torsade de Pointes (TdP), a form of irregular heartbeat that originates from the ventricles [77–80]. These episodes may lead to palpitation, fainting, and sudden death due to ventricular fibrillation [81, 82]. It became evident that iPSC lines derived from patients with LQT1, LQT2, and LQT7 (also called Timothy Syndrome) can be differentiated into cardiomyocytes, showing the disease’s characteristic electrophysiological signature [57–59] and establishing a convenient and powerful system for studying mechanisms of pathogenesis and therapeutic compound testing. Moretti et al. generated for the first time iPSCs derived from LQT1 patients who are affected by an identified autosomal dominant missense mutation (R190Q) in the long-QT syndrome type 1 (LQT1) gene, which encodes the repolarizing potassium channel that mediates the delayed rectifier IKS current. Patient-derived iPSCs maintained the disease genotype of LQT1 and were successfully differentiated into functional cardiomyocytes. In “ventricular” and “atrial” cells derived from patients with LQT1, the duration of the action potential was markedly prolonged as compared to cells from control subjects. Interestingly, the R190Q–KCNQ1 mutation in the pathogenesis of LQT1 turned out to be associated with a dominant negative trafficking defect, leading to a 70~80% reduction in IKS current and altered channel activation as well as deactivation properties. Furthermore, the phenotype of iPSC-derived cardiomyocytes (iPSC-CMs) derived from patients with LQT1 had an increased susceptibility to catecholamine-induced tachyarrhythmia, which was diminished by beta-blockade treatment.
Following the same approach for LQT2, which is caused by a mutation in the KCNH1 gene and encodes the repolarizing potassium channel mediating the delayed rectifier IKr current, two studies generated iPSCs derived from LQT2 patients carrying the missense mutations A614V and G1681A, respectively [57, 83]. Detailed multielectrode array (MEA) and whole-cell patch clamp studies established a significant reduction of the cardiac potassium current IKr, which in turn can significantly prolong the action potential and cause early-after depolarization (EAD) [84, 85]. Intriguingly, several existing as well as novel pharmacological agents were tested on this newly established LQT human iPSC-derived cardiac tissue model, including potassium-channel blockers (E-4031), calcium-channel blockers (nifedipine), sodium-channel blockers (ranolazine), KATP-channel openers (pinacidil and nicorandil), stressor (isoprenaline), and β-blockers (propranolol and nadolol).
Collectively, these findings provide a powerful proof-of-principle demonstration that iPSCs can reliably reproduce abnormal cellular phenotypes and behaviors in vitro, thereby providing crucial mechanistic insights into the disease process [57–59]. Furthermore, these studies suggest that iPSCs may serve as a valuable platform for functional analysis of small molecules (Figure 2). Nevertheless, further optimization of the iPSC technology is required to facilitate its application in drug screening and pharmacological large-scale drug screening. Ongoing research is committed to improving both derivation efficiency and quality of iPSCs and their differentiated target cell progeny. These improvements will be highly beneficial not only for disease modeling research but also potential clinical applications, and for high-content, industrial-scale drug screening approaches.
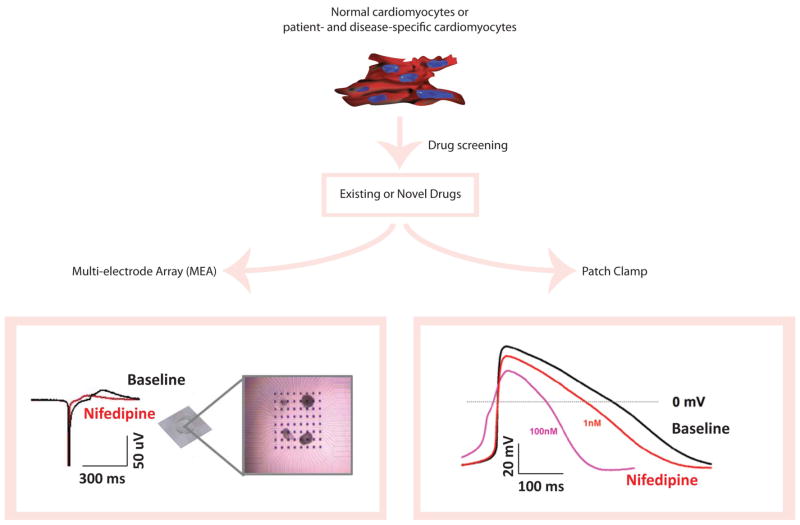
Figure 2. Schematic representation of cardiac drug screening and toxicity testing with human iPSC technology.
Cardiomyocytes are differentiated from normal or disease-specific, patient-derived iPSCs. Subsequently, these iPSC-derived cardiomyocytes (iPSC-CMs) undergo drug screening and toxicity testing with existing or novel drugs. The baseline properties of iPSC-CMs and their response to drugs are determined by electrophysiological assays such as extracellular multi-electrode array (MEA) and patch clamp recordings, using beating embryonic bodies (EBs) in MEA experiments (MED64 MEA amplifier, Alpha Med Scientific, Japan) and isolated single cardiomyocytes in patch clamp recordings (EPC-10 patch clamp amplifier, HEKA, Germany), respectively. Here, nifedipine (100nM) is tested in iPSC-CMs, which showed a significant shortening of field potential duration (FPD) on MEA compared to baseline. Similarly, nifedipine is tested with patch clamp recordings in a dose-dependent manner and exhibited an effect consistent with MEA data.
Employing iPSCs for drug screening
Pharmacological studies have been performed for many years and are an important part of the nonclinical drug evaluation prior to first-in-human clinical trials [86–89]. Well-defined models enable researchers to study specific pathogenic mechanisms and to identify relevant targets, by using chemical compound library screens while screening for new drug candidates. Candidate targets identified by such means will be subjected to secondary validation screens addressing their pharmacokinetic and safety properties [75]. Using in vitro models that resemble conditions in human patients increases the efficiency and accuracy of drug screening tremendously, as the targets can then in principle be scaled up for high-content production. The tremendous opportunities that iPSCs present for creating cardiac disease models highlight their exceptional potential for high predictive value drug screening (Figures 1 and 2), making iPSCs and iPSC-CMs an increasingly popular and powerful new tool for drug testing [75, 90].
Classical drug screening – The example of hERG
From 1990 to 2001, several non-cardiovascular drugs were withdrawn from clinical use because they prolonged the QT interval [91]. Blockage of the ion channel coded by the human ether-a-go-go-related gene (hERG) has been correlated to a prolongation of the QT interval in the ECG, which in turn is correlated to a potential risk of a life-threatening polymorphic ventricular tachycardia (Torsades de Pointes, TdP) [84, 92–96]. Given its possible role in life-threatening cardiac arrhythmias, ‘hERG’ is viewed with caution in the drug discovery community [95–97]. It is now widely accepted that data for the compound effects on hERG channel activity are generally part of the safety pharmacology risk assessment in regulatory submissions [86–89, 95, 96]. The current gold standard assay for hERG liability is the measurement of patch clamp currents in the absence and presence of a drug [98, 99]. In general, safety evaluation of biological agents is further complicated by the fact that they are designed to act on a human target, making it difficult to translate the results of experiments using non-human animal models to potential human toxicity. One well-known example is verapamil. In Chinese Hamster Ovary (CHO) cells overexpressing hERG, verapamil blocks the Ikr (delayed rectifying potassium channel), thereby predicting an association with prolonged QT interval [100]. In reality, although outward ion flux through Ikr channels is blocked in functional cardiomyocytes, verapamil also blocks inward flux through L-type calcium channels, and the overall effect on QT interval is cancelled out [100].
Drug screening employing iPSCs
Recent achievements in differentiation of hESC and iPSC into functional cardiomyocytes have provided the basis for this technology to develop into a platform for drug screening and toxicity testing (Figure 2). Available studies characterize hESC-CMs and iPSC-CMs as largely similar in their differentiation efficiency, gene expression profiles, and electrophysiological and pharmacological functionality [61, 101–104]. With regard to cardiac disease drug discovery, several independent research groups recently addressed the question whether the currently established models are capable of accurately predicting the safety of a candidate drug prior to its application to humans [105–107]. hESCs were differentiated into cardiomyocytes using staged protocols for stepwise cardiac progenitor cell induction. Subsequently, hESC-CMs were analyzed by electrophysiological means to explore their response towards a selection of well-characterized drugs [105–107]. Braam and colleagues made an important contribution showing that field potential duration (FPD) prolongation can be employed as readout to evaluate drug safety results in greater statistical significance and larger confidence level of data, as compared to standard hERG in vitro assays [105, 108, 109]. These studies showed that hESC-CMs have the potential of providing a reasonably economical human model for predictive cardiac pharmacological safety, shedding new light on the definition of a safety margin, which may be utilized to improve preclinical decision-making and to reduce drug development costs [105–107]. Further developments of iPSCs promise the generation of pluripotent cells from any individual and may ultimately provide differentiated cell types of any genetic background. Combined with automated culturing techniques and high-throughput screening approaches, the developing iPSC technology may enable pharmaceutical in vitro testing for both toxicity and efficacy over any individual genotype, making it possible to translate the potential of personalized medicine into the clinic (Tables 1 and 2).
It has been acknowledged that differentiation of iPSCs produces partially immature cells [110]. Many iPSC-derived cell types, such as iPSC-CMs, may still show gene expression profiles similar to human fetal cardiomyocytes, a phenomenon that has been extensively documented for hESC-CMs [75, 104, 111] and recently has been compared for hESC and hiPSC-derived progeny derivatives [112]. The electrophysiological properties of partially mature iPSC-CMs vary from those of human adult cardiomyocytes, particularly in the QT interval [113]. These limitations of current iPSC-CM derivation methods could not only prove problematic for potential therapeutic approaches in humans, but also compromise the iPSC-CM system for application in drug development. For example, early developmental stage and therefore restricted functionality of iPSC-CMs may lead to misinterpretation of a candidate drug’s deleterious features [107]. To overcome this constrains, it will be crucial to develop in the future maturation protocols for iPSC-CMs. Nevertheless, while “prematurity” of iPSC-CMs may have yet unknown implications for the clinical translation of iPSC-CMs, they still represent a considerable improvement for predicting and analyzing early pharmacological development and industrial-scale drug screening [90, 114].
High-throughput screening using iPSCs
Understanding disease mechanisms is crucial for development of effective and sensitive drugs with minimal side effects. In the past years, large-scale chemical screens and rational design have been employed to create and identify small molecules that modulate disease-specific molecular processes. Components revealed by such screens are validated in animal models and eventually in clinical trials, paving the way for new therapeutic approaches and cures [115–117]. However, due to the complexity of disease mechanisms in whole organisms, which is poorly reflected by cellular in vitro models, only a few out of the many initially promising small chemicals identified in large-scale chemical in vitro screens qualify for application in humans [115, 118]. A major advantage of iPSC-dependent disease modeling lies in its capacity to provide both a more direct resemblance of pathogenic conditions in vitro as well as an acceleration of the screening procedure (Table 2). Validation of putative drugs identified via iPSC-dependent chemical screening will not diminish the weight and relevance provided by downstream characterization and validation in animal disease models. However, the use of iPSC-dependent chemical screening may significantly improve the success rate and thereby accelerate the overall drug development process.
At the present, iPSC-dependent high-throughput drug screening is still in its infancy. To comprehensively benefit from the advantages that iPSCs provide as a large-scale drug screening platform, several parameters need to be optimized and explored in more detail. Examples are the potential influence of the donor cell type, as well as virus-free and integration-free reprogramming approaches, while maintaining the requirement of safe gene delivery and introducing minimal genetic aberrations [18, 20, 22–24, 119–123]. Further hurdles may depend on the particular features of iPSC growth and culture conditions, which can complicate their implementation in industrial scale screens [75]. Nevertheless, high-throughput screens in human pluripotent cells performed thus far demonstrate the effectiveness of these pioneer systems [122, 124–127].
Conclusion
In summary, a growing number of researchers have employed iPSCs for modeling a variety of diseases, including hematopoietic [44–46], hepatic [70], neurological [8, 128, 129], endothelial [53], and cardiovascular [45, 60, 61]. Tissue-specific differentiation protocols have been developed to derive these specialized cell types from human pluripotent cells. The challenge to re-create the disease phenotype in vitro as accurately as possible has given rise to striking advancements. For instance, three different cardiac arrhythmias—LQT1, LQT2 and LQT7—have been recapitulated by employing patient-specific iPSCs [43, 57–59]. Future studies will have to focus on how accurately iPSC models reflect a disease phenotype, as drugs for any given disease can be designed only as well as the methodological systems employed to create them. Advances in iPSC-dependent technologies cannot completely replace animal models for studying disease mechanisms and for pre-clinical evaluation of drugs. However, the emerging iPSC technology presents considerable advantages over classical in vitro models, optimizing the integrated approaches by using efficient, high-performance tools for clinical translational research and drug development.
Acknowledgments
We would like to acknowledge funding support from NIH DP2OD004437, HL099117, AG036142, and Burroughs Welcome Foundation Career Award Medical Scientists (JCW) and the German Research Foundation (ADE). Due to space limitations, we are unable to include all of the important papers relevant to induced pluripotent stem cell derivation and application; we apologize to those investigators whom we omitted here.
Footnotes
Disclosure
None
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Wernig M, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318–24. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 3.Maherali N, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1(1):55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 7.Park IH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 8.Dimos JT, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321(5893):1218–21. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 9.Ebert AD, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457(7227):277–80. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchetto MC, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143(4):527–39. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kambal A, et al. Generation of HIV-1 resistant and functional macrophages from hematopoietic stem cell-derived induced pluripotent stem cells. Mol Ther. 2011;19(3):584–93. doi: 10.1038/mt.2010.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown ME, et al. Derivation of induced pluripotent stem cells from human peripheral blood T lymphocytes. PLoS One. 2010;5(6):e11373. doi: 10.1371/journal.pone.0011373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye Z, et al. Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood. 2009;114(27):5473–80. doi: 10.1182/blood-2009-04-217406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun N, et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci U S A. 2009;106(37):15720–5. doi: 10.1073/pnas.0908450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu J, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stadtfeld M, et al. Induced pluripotent stem cells generated without viral integration. Science. 2008;322(5903):945–9. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woltjen K, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458(7239):766–70. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia F, et al. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. 2010;7(3):197–9. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narsinh KH, et al. Generation of adult human induced pluripotent stem cells using nonviral minicircle DNA vectors. Nat Protoc. 2011;6(1):78–88. doi: 10.1038/nprot.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou H, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4(5):381–4. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim D, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472–6. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warren L, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–30. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anokye-Danso F, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8(4):376–88. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyoshi N, et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8(6):633–8. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Chen G, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8(5):424–9. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ban H, et al. Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1103509108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamanaka S. Patient-specific pluripotent stem cells become even more accessible. Cell Stem Cell. 2010;7(1):1–2. doi: 10.1016/j.stem.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Nsair A, MacLellan WR. Induced pluripotent stem cells for regenerative cardiovascular therapies and biomedical discovery. Adv Drug Deliv Rev. 2011;63(4–5):324–30. doi: 10.1016/j.addr.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshida Y, Yamanaka S. Recent stem cell advances: induced pluripotent stem cells for disease modeling and stem cell-based regeneration. Circulation. 2010;122(1):80–7. doi: 10.1161/CIRCULATIONAHA.109.881433. [DOI] [PubMed] [Google Scholar]
- 30.Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24(20):2239–63. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chun YS, Chaudhari P, Jang YY. Applications of patient-specific induced pluripotent stem cells; focused on disease modeling, drug screening and therapeutic potentials for liver disease. Int J Biol Sci. 2010;6(7):796–805. doi: 10.7150/ijbs.6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bock C, et al. Quantitative comparison of genome-wide DNA methylation mapping technologies. Nat Biotechnol. 2010;28(10):1106–14. doi: 10.1038/nbt.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hussein SM, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471(7336):58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- 34.Gore A, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471(7336):63–7. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lister R, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471(7336):68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosh Z, et al. Persistent donor cell gene expression among human induced pluripotent stem cells contributes to differences with human embryonic stem cells. PLoS One. 2010;5(2):e8975. doi: 10.1371/journal.pone.0008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao T, et al. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474(7350):212–5. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 38.Boyd AS, Fairchild PJ. Approaches for immunological tolerance induction to stem cell-derived cell replacement therapies. Expert Rev Clin Immunol. 2010;6(3):435–48. doi: 10.1586/eci.10.20. [DOI] [PubMed] [Google Scholar]
- 39.Pearl JI, et al. Short-term immunosuppression promotes engraftment of embryonic and induced pluripotent stem cells. Cell Stem Cell. 2011;8(3):309–17. doi: 10.1016/j.stem.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saha K, Jaenisch R. Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell. 2009;5(6):584–95. doi: 10.1016/j.stem.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1(1):39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Zou J, et al. Oxidase-deficient neutrophils from X-linked chronic granulomatous disease iPS cells: functional correction by zinc finger nuclease-mediated safe harbor targeting. Blood. 2011;117(21):5561–72. doi: 10.1182/blood-2010-12-328161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narsinh K, Narsinh KH, Wu JC. Derivation of human induced pluripotent stem cells for cardiovascular disease modeling. Circ Res. 2011;108(9):1146–56. doi: 10.1161/CIRCRESAHA.111.240374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raya A, et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature. 2009;460(7251):53–9. doi: 10.1038/nature08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park IH, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134(5):877–86. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye L, et al. Induced pluripotent stem cells offer new approach to therapy in thalassemia and sickle cell anemia and option in prenatal diagnosis in genetic diseases. Proc Natl Acad Sci U S A. 2009;106(24):9826–30. doi: 10.1073/pnas.0904689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adler S, et al. First steps in establishing a developmental toxicity test method based on human embryonic stem cells. Toxicol In Vitro. 2008;22(1):200–11. doi: 10.1016/j.tiv.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 48.Urbach A, et al. Differential modeling of fragile X syndrome by human embryonic stem cells and induced pluripotent stem cells. Cell Stem Cell. 2010;6(5):407–11. doi: 10.1016/j.stem.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tolar J, et al. Hematopoietic differentiation of induced pluripotent stem cells from patients with mucopolysaccharidosis type I (Hurler syndrome) Blood. 2011;117(3):839–47. doi: 10.1182/blood-2010-05-287607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu H, et al. Generation of endoderm-derived human induced pluripotent stem cells from primary hepatocytes. Hepatology. 2010;51(5):1810–9. doi: 10.1002/hep.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song Z, et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19(11):1233–42. doi: 10.1038/cr.2009.107. [DOI] [PubMed] [Google Scholar]
- 52.Sullivan GJ, et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 2010;51(1):329–35. doi: 10.1002/hep.23335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Z, et al. Functional Characterization and Expression Profiling of Human Induced Pluripotent Stem Cell- and Embryonic Stem Cell-Derived Endothelial Cells. Stem Cells Dev. 2011 doi: 10.1089/scd.2010.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanna J, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318(5858):1920–3. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 55.Yang J, et al. Induced pluripotent stem cells can be used to model the genomic imprinting disorder Prader-Willi syndrome. J Biol Chem. 2010;285(51):40303–11. doi: 10.1074/jbc.M110.183392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chamberlain SJ, et al. Induced pluripotent stem cell models of the genomic imprinting disorders Angelman and Prader-Willi syndromes. Proc Natl Acad Sci U S A. 2010;107(41):17668–73. doi: 10.1073/pnas.1004487107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Itzhaki I, et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471(7337):225–9. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- 58.Moretti A, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363(15):1397–409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 59.Yazawa M, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230–4. doi: 10.1038/nature09855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, et al. A human iPSC model of Hutchinson Gilford Progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell. 2011;8(1):31–45. doi: 10.1016/j.stem.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Narsinh KH, et al. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J Clin Invest. 2011;121(3):1217–21. doi: 10.1172/JCI44635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brennand KJ, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473(7346):221–5. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carvajal-Vergara X, et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010;465(7299):808–12. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ku S, et al. Friedreich’s ataxia induced pluripotent stem cells model intergenerational GAATTC triplet repeat instability. Cell Stem Cell. 2010;7(5):631–7. doi: 10.1016/j.stem.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee G, et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009;461(7262):402–6. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soldner F, et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136(5):964–77. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen HN, et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011;8(3):267–80. doi: 10.1016/j.stem.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marchetto MC, Winner B, Gage FH. Pluripotent stem cells in neurodegenerative and neurodevelopmental diseases. Hum Mol Genet. 2010;19(R1):R71–6. doi: 10.1093/hmg/ddq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lemonnier T, et al. Modeling neuronal defects associated with a lysosomal disorder using patient-derived induced pluripotent stem cells. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr285. [DOI] [PubMed] [Google Scholar]
- 70.Rashid ST, et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest. 2010;120(9):3127–36. doi: 10.1172/JCI43122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanaka S, et al. Tumor progression in hepatocellular carcinoma may be mediated by p53 mutation. Cancer Res. 1993;53(12):2884–7. [PubMed] [Google Scholar]
- 72.Chiang CE. Congenital and acquired long QT syndrome. Current concepts and management. Cardiol Rev. 2004;12(4):222–34. doi: 10.1097/01.crd.0000123842.42287.cf. [DOI] [PubMed] [Google Scholar]
- 73.Moss AJ, Kass RS. Long QT syndrome: from channels to cardiac arrhythmias. J Clin Invest. 2005;115(8):2018–24. doi: 10.1172/JCI25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khositseth A, et al. Syncope in children and adolescents and the congenital long QT syndrome. Am J Cardiol. 2003;92(6):746–9. doi: 10.1016/s0002-9149(03)00846-4. [DOI] [PubMed] [Google Scholar]
- 75.Dick E, et al. Evaluating the utility of cardiomyocytes from human pluripotent stem cells for drug screening. Biochem Soc Trans. 2010;38(4):1037–45. doi: 10.1042/BST0381037. [DOI] [PubMed] [Google Scholar]
- 76.Johnson JN, Ackerman MJ. QTc: how long is too long? Br J Sports Med. 2009;43(9):657–62. doi: 10.1136/bjsm.2008.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schulze-Bahr E, et al. The LQT syndromes--current status of molecular mechanisms. Z Kardiol. 1999;88(4):245–54. doi: 10.1007/s003920050283. [DOI] [PubMed] [Google Scholar]
- 78.Lu JT, Kass RS. Recent progress in congenital long QT syndrome. Curr Opin Cardiol. 2010 doi: 10.1097/HCO.0b013e32833846b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Charpentier F, Demolombe S, Escande D. Cardiac channelopathies: from men to mice. Ann Med. 2004;36(Suppl 1):28–34. doi: 10.1080/17431380410032508. [DOI] [PubMed] [Google Scholar]
- 80.Moss AJ. The QT interval and torsade de pointes. Drug Saf. 1999;21(Suppl 1):5–10. doi: 10.2165/00002018-199921001-00002. discussion 81–7. [DOI] [PubMed] [Google Scholar]
- 81.Del Rosario ME, Weachter R, Flaker GC. Drug-induced QT prolongation and sudden death. Mo Med. 2010;107(1):53–8. [PMC free article] [PubMed] [Google Scholar]
- 82.Tamargo J. Drug-induced torsade de pointes: from molecular biology to bedside. Jpn J Pharmacol. 2000;83(1):1–19. doi: 10.1254/jjp.83.1. [DOI] [PubMed] [Google Scholar]
- 83.Matsa E, et al. Drug evaluation in cardiomyocytes derived from human induced pluripotent stem cells carrying a long QT syndrome type 2 mutation. Eur Heart J. 2011;32(8):952–62. doi: 10.1093/eurheartj/ehr073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Curran ME, et al. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80(5):795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 85.Waldo AL, et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet. 1996;348(9019):7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- 86.Friedrichs GS, Patmore L, Bass A. Non-clinical evaluation of ventricular repolarization (ICH S7B): results of an interim survey of international pharmaceutical companies. J Pharmacol Toxicol Methods. 2005;52(1):6–11. doi: 10.1016/j.vascn.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 87.Pugsley MK. Methodology used in safety pharmacology: appraisal of the state-of-the-art, the regulatory issues and new directions. J Pharmacol Toxicol Methods. 2005;52(1):1–5. doi: 10.1016/j.vascn.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 88.Lindgren S, et al. Benchmarking safety pharmacology regulatory packages and best practice. J Pharmacol Toxicol Methods. 2008;58(2):99–109. doi: 10.1016/j.vascn.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 89.Giorgi MA, et al. QT interval prolongation: preclinical and clinical testing arrhythmogenesis in drugs and regulatory implications. Curr Drug Saf. 2010;5(1):54–7. doi: 10.2174/157488610789869148. [DOI] [PubMed] [Google Scholar]
- 90.Liang H, et al. Human and murine embryonic stem cell-derived cardiomyocytes serve together as a valuable model for drug safety screening. Cell Physiol Biochem. 2010;25(4–5):459–66. doi: 10.1159/000303051. [DOI] [PubMed] [Google Scholar]
- 91.Fermini B, Fossa AA. The impact of drug-induced QT interval prolongation on drug discovery and development. Nat Rev Drug Discov. 2003;2(6):439–47. doi: 10.1038/nrd1108. [DOI] [PubMed] [Google Scholar]
- 92.Sanguinetti MC, et al. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81(2):299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 93.Zhou Z, et al. HERG channel dysfunction in human long QT syndrome. Intracellular transport and functional defects. J Biol Chem. 1998;273(33):21061–6. doi: 10.1074/jbc.273.33.21061. [DOI] [PubMed] [Google Scholar]
- 94.Keating MT. The long QT syndrome. A review of recent molecular genetic and physiologic discoveries. Medicine (Baltimore) 1996;75(1):1–5. doi: 10.1097/00005792-199601000-00001. [DOI] [PubMed] [Google Scholar]
- 95.Taglialatela M, et al. Human ether-a-gogo related gene (HERG) K+ channels as pharmacological targets: present and future implications. Biochem Pharmacol. 1998;55(11):1741–6. doi: 10.1016/s0006-2952(98)00002-1. [DOI] [PubMed] [Google Scholar]
- 96.Thomas D, Karle CA, Kiehn J. The cardiac hERG/IKr potassium channel as pharmacological target: structure, function, regulation, and clinical applications. Curr Pharm Des. 2006;12(18):2271–83. doi: 10.2174/138161206777585102. [DOI] [PubMed] [Google Scholar]
- 97.Priest BT, I, Bell M, Garcia ML. Role of hERG potassium channel assays in drug development. Channels (Austin) 2008;2(2):87–93. doi: 10.4161/chan.2.2.6004. [DOI] [PubMed] [Google Scholar]
- 98.De Ponti F, et al. Safety of non-antiarrhythmic drugs that prolong the QT interval or induce torsade de pointes: an overview. Drug Saf. 2002;25(4):263–86. doi: 10.2165/00002018-200225040-00004. [DOI] [PubMed] [Google Scholar]
- 99.Hancox JC, et al. The hERG potassium channel and hERG screening for drug-induced torsades de pointes. Pharmacol Ther. 2008;119(2):118–32. doi: 10.1016/j.pharmthera.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 100.Redfern WS, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs. evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58(1):32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- 101.Itskovitz-Eldor J, et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6(2):88–95. [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang J, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104(4):e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yokoo N, et al. The effects of cardioactive drugs on cardiomyocytes derived from human induced pluripotent stem cells. Biochem Biophys Res Commun. 2009;387(3):482–8. doi: 10.1016/j.bbrc.2009.07.052. [DOI] [PubMed] [Google Scholar]
- 104.Cao F, et al. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS One. 2008;3(10):e3474. doi: 10.1371/journal.pone.0003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Braam SR, et al. Prediction of drug-induced cardiotoxicity using human embryonic stem cell-derived cardiomyocytes. Stem Cell Res. 2010;4(2):107–16. doi: 10.1016/j.scr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 106.Jonsson MK, et al. Quantified proarrhythmic potential of selected human embryonic stem cell-derived cardiomyocytes. Stem Cell Res. 2010;4(3):189–200. doi: 10.1016/j.scr.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 107.Otsuji TG, et al. Progressive maturation in contracting cardiomyocytes derived from human embryonic stem cells: Qualitative effects on electrophysiological responses to drugs. Stem Cell Res. 2010;4(3):201–13. doi: 10.1016/j.scr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 108.Braam SR, Mummery CL. Human stem cell models for predictive cardiac safety pharmacology. Stem Cell Res. 2010;4(3):155–6. doi: 10.1016/j.scr.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 109.Braam SR, Passier R, Mummery CL. Cardiomyocytes from human pluripotent stem cells in regenerative medicine and drug discovery. Trends Pharmacol Sci. 2009;30(10):536–45. doi: 10.1016/j.tips.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 110.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453(7193):322–9. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 111.Braam SR, et al. Improved genetic manipulation of human embryonic stem cells. Nat Methods. 2008;5(5):389–92. doi: 10.1038/nmeth.1200. [DOI] [PubMed] [Google Scholar]
- 112.Patterson M, et al. Defining the nature of human pluripotent stem cell progeny. Cell Res. 2011 doi: 10.1038/cr.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mandenius CF, et al. Cardiotoxicity testing using pluripotent stem cell-derived human cardiomyocytes and state-of-the-art bioanalytics: a review. J Appl Toxicol. 2011;31(3):191–205. doi: 10.1002/jat.1663. [DOI] [PubMed] [Google Scholar]
- 114.Laposa R. Stem cells for Drug Screening. J Cardiovasc Pharmacol. 2011 doi: 10.1097/FJC.0b013e31821823f5. [DOI] [PubMed] [Google Scholar]
- 115.Sartipy P, et al. The application of human embryonic stem cell technologies to drug discovery. Drug Discov Today. 2007;12(17–18):688–99. doi: 10.1016/j.drudis.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 116.Kramer JA, Sagartz JE, Morris DL. The application of discovery toxicology and pathology towards the design of safer pharmaceutical lead candidates. Nat Rev Drug Discov. 2007;6(8):636–49. doi: 10.1038/nrd2378. [DOI] [PubMed] [Google Scholar]
- 117.Bremer S, et al. The development of new concepts for assessing reproductive toxicity applicable to large scale toxicological programmes. Curr Pharm Des. 2007;13(2):3047–58. doi: 10.2174/138161207782110462. [DOI] [PubMed] [Google Scholar]
- 118.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3(8):711–5. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 119.Baharvand H, et al. Human-induced pluripotent stem cells: derivation, propagation, and freezing in serum- and feeder layer-free culture conditions. Methods Mol Biol. 2010;584:425–43. doi: 10.1007/978-1-60761-369-5_23. [DOI] [PubMed] [Google Scholar]
- 120.Huangfu D, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26(7):795–7. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu X, et al. Small molecules that induce cardiomyogenesis in embryonic stem cells. J Am Chem Soc. 2004;126(6):1590–1. doi: 10.1021/ja038950i. [DOI] [PubMed] [Google Scholar]
- 122.Li W, Ding S. Small molecules that modulate embryonic stem cell fate and somatic cell reprogramming. Trends Pharmacol Sci. 2010;31(1):36–45. doi: 10.1016/j.tips.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 123.Zhu S, et al. A small molecule primes embryonic stem cells for differentiation. Cell Stem Cell. 2009;4(5):416–26. doi: 10.1016/j.stem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 124.Barbaric I, et al. Novel regulators of stem cell fates identified by a multivariate phenotype screen of small compounds on human embryonic stem cell colonies. Stem Cell Res. 2010;5(2):104–19. doi: 10.1016/j.scr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 125.Barbaric I, Gokhale PJ, Andrews PW. High-content screening of small compounds on human embryonic stem cells. Biochem Soc Trans. 2010;38(4):1046–50. doi: 10.1042/BST0381046. [DOI] [PubMed] [Google Scholar]
- 126.Outten JT, et al. A high-throughput multiplexed screening assay for optimizing serum-free differentiation protocols of human embryonic stem cells. Stem Cell Res. 2011;6(2):129–42. doi: 10.1016/j.scr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 127.Casalino L, et al. An Automated High Throughput Screening-Compatible Assay to Identify Regulators of Stem Cell Neural Differentiation. Mol Biotechnol. 2011 doi: 10.1007/s12033-011-9413-7. [DOI] [PubMed] [Google Scholar]
- 128.Pedrosa E, et al. Development of Patient-Specific Neurons in Schizophrenia Using Induced Pluripotent Stem Cells. J Neurogenet. 2011 doi: 10.3109/01677063.2011.597908. [DOI] [PubMed] [Google Scholar]
- 129.Kazuki Y, et al. Complete genetic correction of ips cells from Duchenne muscular dystrophy. Mol Ther. 2010;18(2):386–93. doi: 10.1038/mt.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]