Abstract
BACKGROUND/OBJECTIVES:
In studies performed in mice, rose hip powder has been shown to both prevent and reverse high-fat diet-induced obesity and glucose intolerance as well as reduce plasma levels of cholesterol. The aim of this study was to investigate whether daily intake of rose hip powder over 6 weeks exerts beneficial metabolic effects in obese individuals.
SUBJECTS/METHODS:
A total of 31 obese individuals with normal or impaired glucose tolerance were enrolled in a randomized, double-blind, cross-over study in which metabolic effects of daily intake of a rose hip powder drink over 6 weeks was compared with a control drink. Body weight, glucose tolerance, blood pressure, blood lipids and markers of inflammation were assessed in the subjects.
RESULTS:
In comparison with the control drink, 6 weeks of daily consumption of the rose hip drink resulted in a significant reduction of systolic blood pressure (−3.4% P=0.021), total plasma cholesterol (−4.9% P=0.0018), low-density lipoprotein (LDL) cholesterol (−6.0% P=0.012) and LDL/HDL ratio (−6.5% P=0.041). The Reynolds risk assessment score for cardiovascular disease was decreased in the rose hip group compared with the control group (−17% P=0.007). Body weight, diastolic blood pressure, glucose tolerance, and plasma levels of high-density lipoprotein (HDL) cholesterol, triglycerides, incretins and markers of inflammation did not differ between the two groups.
CONCLUSIONS:
Daily consumption of 40 g of rose hip powder for 6 weeks can significantly reduce cardiovascular risk in obese people through lowering of systolic blood pressure and plasma cholesterol levels.
Keywords: cholesterol, blood pressure, glucose tolerance, body weight, inflammation
Introduction
The incidence and prevalence rates of type 2 diabetes mellitus (T2DM) is increasing dramatically all over the world.1 Current estimates predict that 439 million people worldwide will be affected by 2030,1 and this figure does not take into account the large number of people in prediabetic states, that is, glucose intolerance accompanied or not by obesity. Obesity is a very strong risk factor for the development of T2DM and approximately 90% of people diagnosed with T2DM are either overweight or obese. The mechanisms whereby obesity leads to the development of T2DM are poorly understood, but dysfunction of the expanding adipose tissue, leading to ectopic lipid deposition, and low-grade inflammation are believed to be key events linking obesity to T2DM.2, 3 An inflammatory reaction may also be triggered via changes in the gut microbiota resulting in endotoxemia.4 Besides hyperglycemia, T2DM is characterized by elevated levels of circulating lipids and hyperlipidemia is causally linked to the cardiovascular complications of T2DM.5 Owing to the pathophysiological role of lipids in the development of both T2DM and its complications, diabetes care is strongly focused on the strict regulation of the plasma levels of both glucose and lipids.
It has been estimated that the development of diabetes to a large extent is preventable by adopting a healthy diet and increasing physical activity (International Diabetes Federation, http://www.idf.org/prevention). Thus, there is an urgent need for the identification and development of foods with documented effects on the prevention of development of obesity, T2DM, and associated diseases and complications. Rose hip is the pseudofruit of the rose plant.6 The fruit is rich in antioxidants, such as ascorbic acid, phenolic compounds and carotenoids. It has been used in traditional medicine for almost a century for its high content of ascorbic acid. During the last decade, the anti-inflammatory properties of rose hip have been documented in several studies and it has been used successfully to ameliorate symptoms in patients suffering from osteoarthritis, rheumatoid arthritis and lower-back pain.7, 8, 9, 10, 11 Recently, two independent studies, performed in two different strains of mice, demonstrated that rose hip exerts anti-obese and anti-diabetic effects. Ninomiya et al.12 reported that the administration of an acetone extract of fruits and seeds from dog rose (Rosa canina) prevented body-weight gain in mice fed with a normal chow diet. At least some of the effects could be ascribed to trans-tiliroside, a constituent of the extract, as administration of this reduced body-weight gain and improved glucose tolerance. In the other study, performed in our laboratory, the high-fat fed C57BL/6J mouse model, simulating human obesity and insulin resistance, was used to demonstrate that the administration of rose hip powder was able to both prevent and reverse diet-induced obesity and glucose intolerance.13 In both studies, it was ruled out that decreased food intake accounted for the effects. Furthermore, in both studies rose hip administration was also shown to exert lipid-lowering effects.
The aim of the present study was to investigate whether the beneficial metabolic effects observed in response to rose hip intake in mice could be reproduced in a human study. To this end, we performed a randomized, double-blind, cross-over study in which either a rose hip drink or a control drink was administered for 6 weeks to obese individuals with or without glucose intolerance. Primary endpoints were body weight, glucose tolerance, and fasting levels of glucose and insulin. Secondary endpoints were plasma total cholesterol, plasma low density lipoprotein (LDL) cholesterol, blood pressure and markers of inflammation.
Subjects and methods
Participants
The study was carried out in the period April 2009 to February 2010 at the Department of Clinical Sciences, Lund University Hospital. Study participants were recruited from the registry of obese patients at the Endocrinology Clinic. The patients of this registry had previously participated in a time-limited weight reduction program at the clinic. Despite poor results in terms of weight reduction, they were not interested in gastric bypass surgery, but had expressed their interest in forthcoming weight reduction programs and food intervention studies. Inclusion criteria were body mass index>30, willingness to participate in the study, comply with the daily intake of the drinks and the recommended energy intake during the course of the study. Exclusion criteria were diabetes, previous or ongoing insulin treatment, abnormal thyroid, liver or kidney status, known gastro-intestinal disorder, pregnancy and suspected allergy to ingredients of the control and test drinks. A total of 34 subjects, randomly selected from the registry, were assessed for eligibility (Supplementary Figure 1) and 31 of these (23 women and 9 men; age range 33–75, mean age 56, mean body mass index 35) signed the written consent and entered as participants in the study. Seven of the participants had impaired glucose tolerance, based on the results of the oral glucose tolerance test, nine were on hypertensive therapy and eight were on lipid-lowering medication with statins. There were no reported changes in medication during the study. Baseline characteristics of the participants at the start of the study were similar between individuals receiving control first and rose hip second, and individuals receiving rose hip first and control second, except for a significantly higher body weight in the group that was randomized to start with the rose hip drink (Table 1).
Table 1. Characteristics of study participants at time of inclusion.
| All participants | Individuals receiving control first and rose hip second | Individuals receiving rose hip first and control second | |
|---|---|---|---|
| Age (years) | 57±2 (37–75)a | 59±3 (42–75) | 55±3 (37–71) |
| Female (n=22) | 59±2 (42–75) | 61±3 (42–75) | 57±3 (44–69) |
| Male (n=9) | 52±4 (37–71) | 53±5 (48–67) | 52±7 (37–71) |
| Body weight (kg) | 102.9±2.8 (80.4–135.8) | 95.4±3.8 (80.4–129.5) | 110.9±3.3 (83.6–135.8) |
| BMI (kg/m2) | 35.3±0.6 (29.5–44) | 35.5±0.6 (29.5–38.9) | 37.2±0.9 (31.5–44) |
| Systolic BP (mm Hg) | 129±3 (105–170) | 130±3 (105–152) | 128±4 (105–170) |
| Diastolic BP (mm Hg) | 77±2 (60–110) | 74±1 (60–84) | 80±3 (60–110) |
| FP-glucose (mmol/l) | 5.6±0.1 (4.5–7.3) | 5.4±0.1 (4.5–6.8) | 5.9±0.2 (4.5–7.3) |
| FP-insulin (mIU/l) | 11.4±0.7 (6–22) | 10.6±0.8 (6–17) | 12.3±1.2 (6–22) |
| FP-cholesterol (mmol/l) | 5.4±0.2 (3.8–8.9) | 5.6±0.4 (3.8–8.9) | 5.1±0.2 (4.0–6.6) |
| FP-LDL cholesterol (mmol/l) | 3.2±0.2 (1.6–6.4) | 3.5±0.3 (2.1–6.4) | 2.9±0.2 (1.6–4.1) |
| FP-HDL cholesterol (mmol/l) | 1.4±0.07 (0.6–2.4) | 1.4±0.09 (0.6–2.2) | 1.4±0.1 (0.7–2.4) |
| FP-TG (mmol/l) | 1.6±0.1 (0.7–3.3) | 1.6±0.2 (0.7–3.1) | 1.6±0.2 (0.8–3.3) |
| P-ASAT (μkat/l) | 0.46±0.05 (0.22–1.90) | 0.48±0.09 (0.22–1.90) | 0.43±0.02 (0.29–0.61) |
| P-ALAT (μkat/l) | 0.49±0.07 (0.19–2.30) | 0.52±0.12 (0.19–2.30) | 0.47±0.05 (0.25–0.84) |
| P-ALP (μkat/l) | 1.16±0.05 (0.25–0.84) | 1.24±0.06 (0.76–1.70) | 1.08±0.07 (0.52–1.60) |
| P-GT (μkat/l) | 0.56±0.07 (0.22–1.70) | 0.49±0.08 (0.22–1.70) | 0.62±0.10 (0.25–1.50) |
| P-bilirubin (μmol/l) | 9±1 (3–21) | 10±1 (4–21) | 7±1 (3–16) |
Abbreviations: BMI, body mass index; BP, blood pressure; FP, fasting plasma; HDL, high density lipoprotein; LDL, low density lipoprotein; P-ALAT, plasma-alanine aminotransferase; P-ALP, plasma-alkaline phosphatase; P-ASAT, plasma-aspartate aminotransferase; P-GT, plasma-glutamyltransferase; TG, triglycerides.
Mean±s.e.m (min.–max.), n=30–31.
Study protocol
The study design was that of a randomized, double-blind, cross-over study with a 6-week treatment period and a 2-week wash-out period (Supplementary Figure 1). The participants were randomly assigned to start with either the control or the test drink. In addition to the consumption of the control or test drink (5 dl per day), the participants were instructed to restrict their caloric intake to 75% of resting metabolic rate × 1.3, where resting metabolic rate was calculated according to the equation provided by World Health Organization. Examples of daily menus, adhering to the national guidelines of macro- and micronutrient intake, were provided. The participants visited the clinic every 2 weeks to obtain test drinks for the following 2 weeks, measure body weight, give blood samples and report adverse effects. Intake was confirmed by questions to the participants at each visit at the clinic. At the start and end of each period a meal-based glucose tolerance test was performed. Participants visited the clinic on a total of nine occasions. Medical supervision and biological sampling took place at Lund University Hospital, Lund, under the responsibility of M.L-O. The study was approved by the Ethics Committee, Lund, Sweden (Dnr 2009/11) and was in accordance with the Helsinki Declaration of 1975, as revised in 1983.
Control and test drink
The fruit from rose hip (Rosa canina) was imported from Chile. In a two-step procedure, seeds were removed and the remainder of the fruit was ground and mixed with apple juice, citric acid solution and sugar in a mixing tank with stirring. The mixture was preheated with a heat exchanger to 55 °C and degassed before heating to 92 °C. After 95 s the mixture was cooled to 20 °C and stored in an aseptic tank until packaged in 500 ml aseptic Tetra Brik packages (Tetra Pak, Lund, Sweden). The control, consisting of apple juice, white grape juice, citric acid solution and sugar, was produced with the same procedure. Test drinks were analyzed at Eurofins (Lidköping, Sweden) for content of raw protein, raw fat, water, sucrose, fructose, glucose, total fiber, insoluble fiber and soluble fiber. The recipe and results of analyses of the drinks are shown in Table 2. The drinks were designed to have equal amounts of simple sugars and the analysis of the final products showed a difference of <10%.
Table 2. Recipe and analysis of test drinks.
| Control | Rose hip | |
|---|---|---|
| Ingredients (g per 1000 g) | ||
| Apple juice concentrate | 88 | 88 |
| Grape juice concentrate | 19.4 | — |
| Water | 858.8 | 815.1 |
| Citric acid solution | 1.5 | 1.5 |
| Sugar solution (65%) | 32.3 | 15.4 |
| Rose hip meal | — | 80 |
| Energy (kJ per 1000 g) | 1318 | 1326 |
| Analyses (g per 1000 g) | ||
| Raw protein | 0.9 | 3.0 |
| Raw fat | 1.3 | 3.2 |
| Glucose | 17 | 17 |
| Fructose | 32 | 33 |
| Sucrose | 26 | 19 |
| Fiber, total | ND | 31 |
| Fiber, insoluble | ND | ND |
| Fiber, soluble | ND | 31 |
Abbreviation: ND, non-detectable.
Meal-based tolerance test
Glucose tolerance was assessed by measuring plasma glucose at 0, 30, 60, 90 and 120 min, and plasma insulin at 0 and 30 min following the ingestion of a carbohydrate-rich breakfast. Participants came to the clinic at 0800 hours in fasting condition and had a breakfast corresponding to 521 kcal and 100 g carbohydrates, of which 38 g were disaccharides (26 sucrose) and 20.5 g monosaccharides. Blood samples were drawn in two separate tubes, one with Trasylol (aprotinin, Bayer AG, Leverkusen, Germany) and one with DPP-4 inhibitor, and centrifuged (4 °C) immediately after sampling. Plasma was frozen on dry ice and stored at −80 °C until analysis.
Body weight and blood pressure
Body weight was measured using an electronic body weight scale (Tanita, Tanita Europe BV, Amsterdam, The Netherlands), which was calibrated regularly. Blood pressure was measured using a mercury sphygmomanometer in the sitting position after 5 min resting. The mean of three consecutive measurements was recorded.
Laboratory analyses
Except for GLP-I, GIP, adiponectin and PAI-I, all blood and plasma analyses were conducted at the accredited clinical chemistry laboratory at Lund University Hospital. C-reactive protein (CRP) was measured using a high-sensitivity assay. PAI-I was analyzed using a high-sensitivity assay available at the accredited blood coagulation laboratory at Lund University Hospital, Malmö. GLP-I and GIP was analyzed using Luminex Technology (LX200, Luminex Corporation, Austin, TX, USA). Adiponectin was analyzed with ELISA (Millipore Corporation, Billerica, MA, USA).
Statistical analyses
All data are shown as means (95% confidence intervals) with s.e.m. To determine the differences in the measured variables following intake of the control and rose hip drink, paired t-tests were used when the data was Gaussian distributed according to D'agostino and Pearson omnibus normality test. When not normally distributed, Wilcoxon matched-pairs signed rank test was used. Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA) and the statistical software package SPSS for Windows (SPSS Inc., Chicago, IL, USA). P<0.05 was considered significant.
Results
Dietary intake and adverse effects
Both drinks were tolerated well by the participants, but mild gastrointestinal problems were reported in both the groups, more frequently in the rose hip group (11 compared with 2 in the control group). Loose stools and flatulence was the most commonly reported adverse effect (eight in the rose hip group, one in the control group), but obstipation was also reported (three in the rose hip group, one in the control group).
Body weight and glucose tolerance
The sequence of the drink did not influence the outcome, so data for each drink were pooled and referred to as control group and rose hip group, respectively. Daily intake of the rose hip drink was found to have no effect on body weight and body mass index (Table 3). Furthermore, no effects were observed on HbA1c or fasting levels of plasma glucose and insulin (Table 3), indicating that rose hip intake had no effects on glucose tolerance or insulin sensitivity. Possible effects on glucose tolerance were further assessed in meal-based glucose tolerance tests. These showed no significant differences between the groups with regard to plasma glucose levels (Supplementary Figure 2) or plasma insulin levels (Supplementary Table 1). Also, there were no differences with regard to the incretin response, assessed by the measurement of plasma levels of GLP-I and GIP at 0 and 30 min (Supplementary Table 1).
Table 3. End-point values after the 6-week intervention period with either the control drink (Control) or the drink containing rose hip (Rose hip).
| Control | Rose hip | P | |
|---|---|---|---|
| Body weight (kg) | 101.9±2.91 | 102.7±3.1 | NS |
| BMI (kg/m2) | 36.6±1.0 | 35.9±0.9 | NS |
| FP-glucose (mmol/l) | 5.6±0.2 | 5.7±0.2 | NS |
| FP-insulin (mIU/l) | 12.5±0.9 | 12.5±0.9 | NS |
| HbA1c (mmol/mol) | 35±0.4 | 35±0.6 | NS |
| TG (mmol/l) | 1.5±0.1 | 1.5±0.1 | NS |
| P-ASAT (μkat/l) | 0.48±0.06 | 0.43±0.03 | NS |
| P-ALAT (μkat/l) | 0.49±0.07 | 0.54±0.06 | NS |
| P-GT (μkat/l) | 0.49±0.07 | 0.48±0.05 | NS |
| P-ALP (μkat/l) | 1.1±0.05 | 1.2±0.05 | NS |
| P-bilirubin (μmol/l) | 7.8±0.5 | 7.9±0.5 | NS |
| CRP (mg/l) | 3.7±3.01 | 3.8±3.6 | NS |
| Adiponectin (mg/l) | 16.4±1.7 | 14.9±1.5 | NS |
| PAI-1 (IU/ml) | 27.6±3.2 | 29.9±3.4 | NS |
Abbreviations: BMI, body mass index; CRP, C-reactive protein; FP, fasting plasma; P-ALAT, plasma-alanine aminotransferase; P-ALP, plasma-alkaline phosphatase; P-ASAT, plasma-aspartate aminotransferase; P-GT, plasma-glutamyltransferase; TG, triglycerides.
Mean±s.e.m, n=25–28.
Plasma lipids and blood pressure
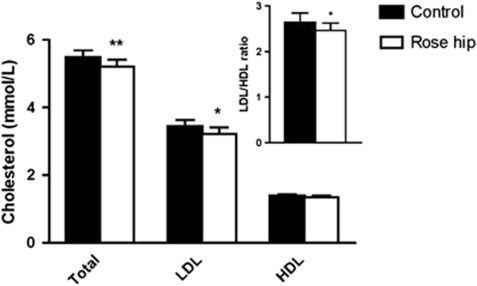
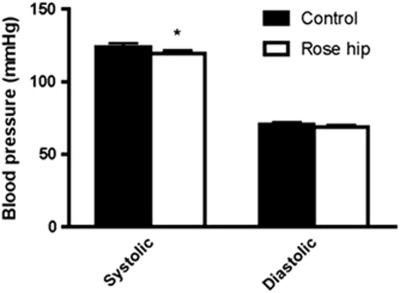
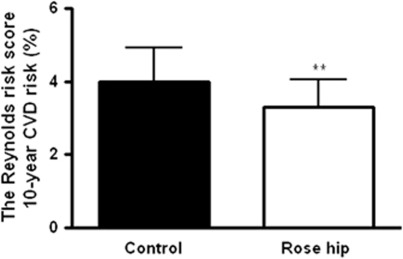
Intake of the rose hip drink was found to result in a small but significant reduction in plasma levels of total cholesterol (−4.9% P=0.0018), as well as LDL cholesterol (−6.0% P=0.012), compared the intake of the control drink (Figure 1). Plasma high-density lipoprotein (HDL) cholesterol, on the other hand, was not altered (Figure 1). The changes in cholesterol parameters were reflected in a decrease in the LDL/HDL ratio (−6.5% P=0.041) in the rose hip group (Figure 1). Exclusion of the eight participants who were on statin treatment in the analyses resulted in more pronounced cholesterol-lowering effects of rose hip, that is, a reduction of total cholesterol by 5.5% (P=0.0039; n=19) and a reduction of LDL cholesterol by 8.6% (P=0.0057; n=19). In contrast to cholesterol, no differences in plasma triglyceride levels were found between the rose hip and the control group (Table 3). Intake of rose hip was found to significantly lower systolic blood pressure, with no effect on diastolic blood pressure (Figure 2). Systolic blood pressure was significantly lower than that in the control group even when the eight participants who were on antihypertensive treatment were excluded from the analyses (−3.7% P=0.04, n=20). Applying the algorithm of the Reynolds risk assessment score14, 15 to the data demonstrated a significant reduction (17%, P=0.0075, n=25) in 10-year risk of cardiovascular disease (Figure 3).
Figure 1.
Plasma total cholesterol, LDL cholesterol, HDL cholesterol following 6 weeks of intake of the control drink or the rose hip drink. The LDL/HDL ratio is shown in the inset. Mean±s.e.m, n=27–28. *P<0.05; **P<0.01.
Figure 2.
Systolic and diastolic blood pressure following 6 weeks of intake of the control drink or the rose hip drink. Mean±s.e.m, n=28. *P<0.05.
Figure 3.
Reynolds risk assessment score following 6 weeks of intake of the control drink or the rose hip drink. Mean±s.e.m, n=25. **P<0.01.
Inflammatory markers
In order to assess the possible effects of rose hip intake on low-grade inflammation, we measured the plasma levels of CRP and PAI-I, two markers of inflammation, and adiponectin, an adipokine with anti-inflammatory properties. There were no significant differences between the groups for any of these markers (Table 3).
Discussion
To the best of our knowledge, this is the first study in humans investigating the metabolic effects of dietary administration of rose hip. Daily consumption of rose hip was found to significantly decrease plasma cholesterol and systolic blood pressure in obese, non-diabetic individuals, whereas no effects on body weight, glucose tolerance and markers of inflammation were observed. The reduction in plasma cholesterol and systolic blood pressure was estimated to lower the risk of developing cardiovascular disease by 17%, using the algorithm for Reynolds risk assessment score.
The magnitude of the lowering of plasma levels of LDL cholesterol in the rose hip group, that is 6.0% compared with the control, was within the range from 6 to 15% that has been reported in the few long-term studies performed on cholesterol-lowering effects of single foods16, 17, 18, 19 and accords very well with the 5% reduction considered realistic in real-world situations when dietary saturated fat levels are low.20 The observed decrease in LDL cholesterol in the rose hip group is below the minimum of 8–9% required to see a reduction in total mortality, although it is estimated to reduce the incidence of coronary heart disease by 14.5%.21 With the exception of two individuals who had slightly elevated cholesterol levels, the participants of the present study had normal plasma cholesterol levels. Thus, the full potency of rose hip to lower plasma cholesterol may not have been revealed and a follow-up study in hyperlipidemic individuals seems warranted. Cholesterol-lowering properties of rose hip have previously been demonstrated in a study performed in high-fat diet fed C57BL/6J mice.13 In that study, the reduction in total plasma cholesterol was shown to involve a reduction in both LDL cholesterol and HDL cholesterol with a larger effect on LDL cholesterol, resulting in a decreased LDL/HDL ratio. In the present study, a very similar profile of the cholesterol-lowering effects was observed, with a significant reduction in LDL cholesterol and LDL/HDL ratio, and a smaller, in this case non-significant, lowering of HDL cholesterol. In a previous human study where a rose hip drink was administered daily to hypercholesterolemic subjects for 6 weeks as a control to intake of the same drink supplemented with Lactobacillus plantarum 299v, no cholesterol-lowering effects were observed by rose hip alone.22 The reason for the discrepancy compared with the present study is not known, but it should be pointed out that besides differences in the characteristics of subjects, the dose of rose hip was only about 25% of that employed in the present study. In another study where 5 g of a standardized rose hip powder (Hyben Vital) was administered daily for 3 months to patients with osteoarthritis, total cholesterol was reduced by 8.5%, indicating that lower doses of rose hip may also promote lowering of plasma cholesterol if administered for longer periods.23 It should be pointed out, however, that besides differences in dose and length of administration, also the preparation of rose hip differs between studies, making comparisons difficult. For instance, the powder used by Rein et al.23 contains both the seeds and shells of the rose hip plant, whereas only the shells were used in our study. The fact that the cholesterol-lowering effects were exerted by both of these preparations may indicate that the shells rather than the seeds account for the cholesterol-lowering properties of rose hip.
The mechanism whereby rose hip is capable of reducing plasma cholesterol is unknown. In the study performed in C57BL/6J mice, the reduction in plasma cholesterol was accompanied by reduced levels of hepatic cholesterol, but the expression of SREBP-2 and HMG-CoA reductase was unaltered, indicating that cholesterol biosynthesis was unaffected.13 It is possible that the high fiber content of rose hip impairs the enterohepatic circulation of bile acids by preventing their re-absorption, thereby promoting increased synthesis of bile acids from cholesterol, that is, a mechanism of action similar to that of the cholesterol-lowering drug cholestyramin.
The observed decrease in systolic blood pressure (4 mm Hg) was close to the median reduction observed in 27 pharmacological trials estimated to result in decreases of the incidence of coronary heart disease and stroke by >15% and 25%, respectively.24 In a previous study, no decrease in blood pressure in smokers was observed by daily intake of rose hip for 6 weeks, whereas intake of the same rose hip drink supplemented by Lactobacillus plantarum resulted in a significant reduction of systolic blood pressure.25 The dose of rose hip employed was about 25% of the dose in the present study, which may explain why no effects were observed. The mechanism whereby rose hip lowers systolic blood pressure is unknown. As for the cholesterol-lowering effects, it could be speculated that the high fiber content of rose hip at least partially accounts for the effect. Consumption of the rose hip drink corresponded to a daily intake of 31 g of fiber. Two meta-analyses have concluded that an increase in fiber intake of 10–15 g per day for 8 weeks was associated with a decrease in systolic blood pressure of 1–2 mm Hg.26, 27 Thus, the reduction of 4 mm Hg in the present study is likely to be a result of the high fiber intake, although it may very well be that other bioactive components than fiber contribute to the blood pressure-lowering effects of rose hip.
Daily intake of rose hip had no effects on body weight or glucose tolerance. This is in contrast to two previous studies performed in mice, where dietary intake of rose hip potently inhibited body-weight gain and also significantly improved glucose tolerance.12, 13 The reason for this discrepancy is not known, but two obvious differences relate to the dose administered and the metabolic status of the study population. Although the daily intake of rose hip powder in the current study was high compared with previous studies in humans (40 vs 5 g8, 11, 23, 28), it was much lower than that in the study in C57BL/6J mice, where rose hip accounted for 30% of the daily food intake. The fact that the participants of the current study, in contrast to the mouse studies, were on an energy-restricted diet throughout the study could have obscured effects on body weight and future studies in a large number of overweight, or even lean, individuals on a non-restricted diet would be of interest. Also, the present study, designed to represent a first proof-of-concept study of possible metabolic effects of rose hip in humans, was performed in obese individuals with no diabetes and with only a few glucose-intolerant participants. This is in contrast to the high-fat fed C57BL/6J mouse model, where obesity is accompanied by glucose intolerance.
Several previous studies have demonstrated that the dietary administration of rose hip to individuals with osteoarthritis and rheumatoid arthritis exerts anti-inflammatory effects.7, 8, 11, 29 Obesity and its associated diseases are considered to be states of low-grade inflammation. However, we were unable to detect any significant differences with regard to two markers of systemic inflammation, that is, CRP and PAI-I, or with regard to adiponectin, an adipokine with anti-inflammatory as well as insulin-sensitizing effects. Possibly the most obvious explanation for the lack of effect on these markers is that the low inflammatory tonus of our participants, shown by levels of CRP and PAI-I within the normal range, makes it difficult to demonstrate anti-inflammatory actions, at least as effects on these markers of inflammation.
Although the mechanisms whereby rose hip lowers systolic blood pressure and plasma cholesterol levels remain unresolved, the findings of this study may have important health implications. The current study could be the starting point for exploring rose hip as a constituent of food portfolios aimed at reducing cholesterol and blood pressure, and thereby decrease the risk of coronary heart disease and mortality. Efficient food portfolios are urgently needed and they represent an attractive alternative to statin treatment for people that, because of muscle pain and increases in liver and muscle enzymes, do not tolerate statins, as well as for people at risk of developing diabetes, as statins recently were shown to increase the risk of diabetes.30
Food portfolios designed to lower plasma cholesterol typically contain soy, nuts or almonds, viscous fiber and plant sterols.20, 31 It would be of interest to explore the potential additive and synergistic effects of rose hip in such portfolios. Follow-up studies of dietary treatment with rose hip should not only be performed in hyperlipidemic and hypertensive individuals in order to study its potency in lowering cholesterol and blood pressure in more detail, but also in diabetic individuals in order to further explore its possible antidiabetic effects.
Acknowledgments
We thank Margit Bergström, Bertil Nilsson, Birgitta Danielsson and Sara Larsson for the excellent technical assistance. The authors' responsibilities were as follows: UA, MLO, AH and CH designed the study; UA and KB conducted research; AH was responsible for the production of control and test drinks; UA, KB and MLO analyzed data; CH wrote the paper; UA and CH had primary responsibility for final content of the paper. All authors read and approved the final manuscript. This study was supported by Lund University Antidiabetic Food Center, a VINNOVA VINN Excellence Center, the FuncFood PhD program at Lund University, the Swedish Research Council (grant 11284 to C.H.), the Swedish Diabetes Association and A. Påhlsson Foundation.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on European Journal of Clinical Nutrition website (http://www.nature.com/ejcn)
Contributors: UA, MLO, AH and CH designed the study; UA and KB conducted research; AH was responsible for the production of control and test drinks; UA, KB and MLO analyzed data; CH wrote the paper; UA and CH had primary responsibility for final content of the paper. All authors read and approved the final manuscript.
Supplementary Material
References
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:209–214. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Goldfine AB. Getting away from glucose: fanning the flames of obesity-induced inflammation. Nat Med. 2009;15:373–374. doi: 10.1038/nm0409-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Delzenne NM. Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr Opin Pharmacol. 2009;9:737–743. doi: 10.1016/j.coph.2009.06.016. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53:1270–1287. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrubasik C, Roufogalis BD, Muller-Ladner U, Chrubasik S. A systematic review on the Rosa canina effect and efficacy profiles. Phytother Res. 2008;22:725–733. doi: 10.1002/ptr.2400. [DOI] [PubMed] [Google Scholar]
- Kharazmi A, Winther K. Rose hip inhibits chemotaxis and chemiluminescence of human peripheral blood neutrophils in vitro and reduces certain inflammatory parameters in vivo. Inflammopharmacology. 1999;7:377–386. doi: 10.1007/s10787-999-0031-y. [DOI] [PubMed] [Google Scholar]
- Winther K, Apel K, Thamsborg G. A powder made from seeds and shells of a rose-hip subspecies (Rosa canina) reduces symptoms of knee and hip osteoarthritis: a randomized, double-blind, placebo-controlled clinical trial. Scand J Rheumatol. 2005;34:302–308. doi: 10.1080/03009740510018624. [DOI] [PubMed] [Google Scholar]
- Winther K, Rein E, Kharazmi A. The anti-inflammatory properties of rose-hip. Inflammopharmacology. 1999;7:63–68. doi: 10.1007/s10787-999-0026-8. [DOI] [PubMed] [Google Scholar]
- Jager AK, Eldeen IM, van Staden J. COX-1 and -2 activity of rose hip. Phytother Res. 2007;21:1251–1252. doi: 10.1002/ptr.2236. [DOI] [PubMed] [Google Scholar]
- Willich SN, Rossnagel K, Roll S, Wagner A, Mune O, Erlendson J, et al. Rose hip herbal remedy in patients with rheumatoid arthritis - a randomised controlled trial. Phytomedicine. 2010;17:87–93. doi: 10.1016/j.phymed.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Ninomiya K, Matsuda H, Kubo M, Morikawa T, Nishida N, Yoshikawa M. Potent anti-obese principle from Rosa canina: structural requirements and mode of action of trans-tiliroside. Bioorg Med Chem Lett. 2007;17:3059–3064. doi: 10.1016/j.bmcl.2007.03.051. [DOI] [PubMed] [Google Scholar]
- Andersson U, Henriksson E, Strom K, Alenfall J, Goransson O, Holm C. Rose hip exerts antidiabetic effects via a mechanism involving downregulation of the hepatic lipogenic program. Am J Physiol Endocrinol Metab. 2011;300:E111–E121. doi: 10.1152/ajpendo.00268.2010. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR.C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men Circulation 20081182243–2251.4p following 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake DB, Miller VT, LaRosa JC, Kinosian B, Jacobson T, Brown V, et al. Long-term treatment of hypercholesterolemia with dietary fiber. Am J Med. 1994;97:504–508. doi: 10.1016/0002-9343(94)90344-1. [DOI] [PubMed] [Google Scholar]
- Hendriks HF, Brink EJ, Meijer GW, Princen HM, Ntanios FY. Safety of long-term consumption of plant sterol esters-enriched spread. Eur J Clin Nutr. 2003;57:681–692. doi: 10.1038/sj.ejcn.1601598. [DOI] [PubMed] [Google Scholar]
- Miettinen TA, Puska P, Gylling H, Vanhanen H, Vartiainen E. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesterolemic population. N Engl J Med. 1995;333:1308–1312. doi: 10.1056/NEJM199511163332002. [DOI] [PubMed] [Google Scholar]
- Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc. 2003;78:965–978. doi: 10.4065/78.8.965. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Kendall CW, Marchie A, Faulkner DA, Wong JM, de Souza R, et al. Effects of a dietary portfolio of cholesterol-lowering foods vs lovastatin on serum lipids and C-reactive protein. JAMA. 2003;290:502–510. doi: 10.1001/jama.290.4.502. [DOI] [PubMed] [Google Scholar]
- Holme I. An analysis of randomized trials evaluating the effect of cholesterol reduction on total mortality and coronary heart disease incidence. Circulation. 1990;82:1916–1924. doi: 10.1161/01.cir.82.6.1916. [DOI] [PubMed] [Google Scholar]
- Bukowska H, Pieczul-Mroz J, Jastrzebska M, Chelstowski K, Naruszewicz M. Decrease in fibrinogen and LDL-cholesterol levels upon supplementation of diet with Lactobacillus plantarum in subjects with moderately elevated cholesterol. Atherosclerosis. 1998;137:437–438. doi: 10.1016/s0021-9150(97)00283-9. [DOI] [PubMed] [Google Scholar]
- Rein E, Kharazmi A, Winther K. A herbal remedy, Hyben Vital (stand. powder of a subspecies of Rosa canina fruits), reduces pain and improves general wellbeing in patients with osteoarthritis--a double-blind, placebo-controlled, randomised trial. Phytomedicine. 2004;11:383–391. doi: 10.1016/j.phymed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruszewicz M, Johansson ML, Zapolska-Downar D, Bukowska H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am J Clin Nutr. 2002;76:1249–1255. doi: 10.1093/ajcn/76.6.1249. [DOI] [PubMed] [Google Scholar]
- Whelton SP, Hyre AD, Pedersen B, Yi Y, Whelton PK, He J. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J Hypertens. 2005;23:475–481. doi: 10.1097/01.hjh.0000160199.51158.cf. [DOI] [PubMed] [Google Scholar]
- Streppel MT, Arends LR, van ‘t Veer P, Grobbee DE, Geleijnse JM. Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch Intern Med. 2005;165:150–156. doi: 10.1001/archinte.165.2.150. [DOI] [PubMed] [Google Scholar]
- Warholm O, Skaar S, Hedman E, Moelmer H, Eik L. The effect of a standardized herbal remedy made from a subtype of rosa canina in patients with osteoarthritis: a double-blind, randomized, placebo-controlled clinical trial. Curr Ther Res. 2003;64:21–31. doi: 10.1016/S0011-393X(03)00004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrubasik JE, Roufogalis BD, Chrubasik S. Evidence of effectiveness of herbal antiinflammatory drugs in the treatment of painful osteoarthritis and chronic low back pain. Phytother Res. 2007;21:675–683. doi: 10.1002/ptr.2142. [DOI] [PubMed] [Google Scholar]
- Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Kendall CW, Faulkner DA, Nguyen T, Kemp T, Marchie A, et al. Assessment of the longer-term effects of a dietary portfolio of cholesterol-lowering foods in hypercholesterolemia. Am J Clin Nutr. 2006;83:582–591. doi: 10.1093/ajcn.83.3.582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.