Abstract
Hyperosmolar stress acts in two ways on the implanting embryo and its major constituent, placental trophoblast stem cells (TSC). Stress causes homeostasis that slows development with lesser cell accumulation, increased cell cycle arrest, and apoptosis. Stress may also cause placental differentiation at implantation. To test for the homeostatic and differentiation-inducing consequences of stress, TSC were exposed to hyperosmolar stress for 24hr and tested using whole mouse genome arrays and Real-time quantitative (Q)PCR. At 0.5hr, all 31 highly changing mRNA (>1.5-fold compared with unstressed TSC) decreased, but by 24hr 158/288 genes were upregulated. Many genes upregulated at 24hr were near baseline levels in unstressed TSC, suggesting new transcription. Thus few genes change during the early stress response, but by 24hr TSC have adapted to start new transcription with large gene sets. Types of genes upregulated at 24hr included homeostatic genes regulating growth and DNA damage induced (GADD45β/γ), activator protein (AP)-1 (junB/junC/ATF3/4), heat shock proteins (HSP22/68), and cyclin-dependent kinase inhibitor [CDKI; p15, p21]. But, stress also induced transcription factors that mediate TSC differentiation to trophoblast giant cells (TGC)(Stra13, HES1, GATA-binding2), placental hormones [proliferin, placental lactogen (PL)1, prolactin-like peptide (PLP)M], and extracellular matrix genes (CCN1/2). Transcription factors for later placental cell lineages, spongiotrophoblast (MASH2, TPBPα) and syncytiotrophoblast (GCM1, TEF5) and placental hormones (PLPA, PLII), were not induced by 24hr stress. Thus stress induced the temporal and spatial placental differentiation normal after implantation. Although differentiation was induced, markers of TSC stemness such as inhibitor of differentiation (ID)2 remained at 100% of levels of unstressed TSC, suggesting that retained mRNA might mediate dedifferentiation were stress to subside.
Keywords: microarray, placental trophoblast stem cells, hyperosmolar stress, differentiation
Introduction
Peri-implantation stress leads to slower embryo development [1; 2], with accumulation of fewer stem cells through mechanisms of apoptosis and cell cycle arrest in embryos and TSC [3; 4]. In addition, models such as delayed implantation may also be an example of a balanced stress which can cause embryonic stasis and cell cycle arrest [5]. However, shear stress on the hatching blastocyst [6] or cytokine-to-receptor signaling from the uterus to placental cells [7] may initiate placental differentiation [8]. The predominant cell type in the implanting blastocyst is the TSC and a subpopulation of this cell type must differentiate soon after implantation to produce the first placental hormones that mediate the antiluteolytic response needed for survival of the conceptus [9]. Proliferation and differentiation defects in cytotrophoblasts early in the first trimester can lead to diseases of placental insufficiency such as preeclampsia [10].
Hyperosmolar stress can induce yeast undergoing somatic growth to differentiate and produce α-mating type factor that induces development through induction of conjugation structures and sporulation [8]. After hyperosmolar stress was used to clone stress enzymes in yeast, hyperosmolar stress and inflammatory factors and cytokines such as lipopolysaccharides and tumor necrosis factor, respectively, were used to clone stress enzymes in mammalian cells. Hyperosmolar stimulation and inflammation are inducers of stress signaling and can be used to probe prioritized developmental decision-making. Hyperosmolar stress can induce multiple homeostatic changes in the implanting embryo and its constituent TSC [3; 4], but it is not known if hyperosmolar stress modulates developmental programs.
Peri-implantation development is the most susceptible period of mammalian development to embryo loss [11] and embryos in this period are susceptible to sublethal in vitro and in vivo stress effects leading to post-natal consequences such as hypertension and learning anomalies [12; 13; 14]. During assisted reproductive technology (ART), embryo culture [1; 15] and handling techniques such as pipetting activate stress activated protein kinase (SAPK)[16] and if sustained leads to loss of cell accumulation at lower stress levels and apoptosis at higher levels. In addition, the stress of embryo culture during IVF can lead to changes in placental hormone production in the first trimester [17]. Understanding stress effects on normal and pathophysiologic peri-implantation development is needed to understand how stress may have short-term lethal and long-term sublethal placental consequences.
We report here that hyperosmolar stress induces a time-dependent program of developmental mRNA in TSC consistent with the hypothesis that stress can regulate normal or pathophysiologic development.
Materials and Methods
Reagents
Sorbitol, FGF4, and heparin were from Sigma Chemical Co. (St. Louis, MO). KSOM and KSOM+ amino acids (KSOM+AA) were from Specialty Media (Phillipsburg, NJ). DMEM/F-12, fetal bovine serum, and RPMI1640 were from Gibco (Grand Island, NY). The primary antibody for total JunB (SC73) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and the antibody for actin (CS4967) was purchased from Cell Signaling Technology (Beverly, MA). CCN2 antibodies were described previously [18].
Collection and culture conditions for mouse embryos, culture of TSC
Standard techniques were used for obtaining mouse embryos [19]. Female MF1 mice (4–5 weeks old, Harlan Sprague Dawley, Indianapolis, IN) were super-ovulated, and their embryos were obtained as described previously [1; 6; 15]. Animal use protocols were approved by the Wayne State University Animal Investigation Committee (AIC). In all studies, embryos were equilibrated for 2 hour in KSOMaa and stressed with the 400mM sorbitol for the time period indicated. KSOMaa had ranges of 250–270mOsmol that increased 2.82-fold to 674mOsmol with 400 mM sorbitol. TSC were from Dr. Rossant (Lunenfeld Research Institute, Ontario, Canada). TSC were cultured as described previously [20]. TSC media range from 265–300mOsmol. The change in osmolality caused by sorbitol in all media were measured as described previously [3; 4]. For TSC basal media produced 298mOsmol and the addition of 400 mM sorbitol produced 713mOsmol. This was a 2.4-fold increase.
Microarray experiments
Total RNA was isolated from TSC stressed with 400mM sorbitol for each time point during 24hr in 2–3 independent biological experiments. All amplifications started with 2ug total RNA. For each replicate, 10ug aRNA was fragmented and hybridized to Murine Genome 430.2 gene chips following Affymetrix instructions. Analysis of the raw data was performed with dCHIP, a model-based method for expression analysis. Repeat biological and technical experiments were performed at Stanford and Wayne State (WSU). In comparing the replicates between Stanford and WSU, there was an anticipated time-dependent correlation. Unstressed TSC have biological variability, putatively due to changing fractions of default differentiation, and had the lowest correlation. Correlation increased to the highest level at 24hr of hyperosmolar stress, when biological and molecular responses became the most focused to achieve cellular (homeostatic) and ‘organismal’ (developmental) survival.
Analysis of microarray data
The microarray data were analyzed using several software packages. D-Chip (2005 Version) (www.dchip.org) was used to normalized the data set [21]. The median intensity from all 11 un-normalized array chips varies between 91 to 122 with P call between 48.1% ~57.1%. The original signals were scaled towards the median intensity array (the default is the one with median overall intensity of 95) in the group at the probe intensity level. Normalization was done by determining the normalization curve with invariant probe sets. The resulting signals, or model-based expression indexes, were the weighted average of PM/MM differences (PM/MM model) of selected probes estimated using a multiplicative model [21]. In our normalization process, those low values were truncated to 100. GeneSpring 7.0 (Silicon Genetics, Redwood City, CA) was used to identify differentially expressed genes and hierarchical clustering. Samples were grouped according to different time points. ANOVA method was used to analysis the difference. In this ANOVA analysis, we used parametric test, variances were assumed equal, and p-value cutoff was 0.05. Since there are 45116 probes in Affymatrix Chips, Benjamini and Hochberg method was used for multiple testing corrections for false discovery rate (FDR). All the significant genes between two groups were filtered with expression level based on 1.2 and 1.5 fold changes.
Gene Ontologies (GO) classification
Differentially expressed genes among different embryo culture conditions, identified by microarray gene expression analysis, were classified into gene ontology groups using GO Tree software [22]. Gene Ontology Tree Machine (GOTM) interprets biological, molecular, and cellular functions of from lists of genes identified in response to TSC stress. GOTM performs a statistical analysis of the GO categories for the input gene list and outputs potential biological areas for further investigation. The differentially expressed genes are classified by their corresponding GO categories, and the observed number of genes in each of these GO categories is recorded. Genes represented on the Affymetrix 430 2.0 chip comprise the reference gene list. The expected number of genes in each GO category corresponds to the number of genes falling into that GO category in the reference gene list. A given GO category is considered enriched when the observed number of genes in that category is greater than the expected number. The GO classifications for stress vs. unstressed conditions were derived, taking into account only non-redundant and specific GO classes for each condition.
Indirect immunocytochemistry and Western blot analysis conditions
Indirect immunocytochemistry was performed as described previously [3; 4; 6]. Photomicrography was done with a Leica DM IRE2 automated epifluorescence microscope (Wetzlar, Germany) controlled by SimplePCI AI software (Compix Inc., Imaging Systems, Cranberry Township, PA). Photomicrographs were formatted using Adobe Photoshop 6.0 (San Jose, CA). FITC intensity measurement and comparison were done with SimplePCI DNN software. SDS-PAGE and Western blots were done as described previously [3; 4].
Real-Time QPCR
TSC were stimulated as for microarray experiments during three independent biological experiments. Primer sequences for were used from PrimerBank Harvard (Wang and Seed, 2003)(Supplemental Table 1). Real-time quantitative (Q)PCR was performed using a SmartCycler Thermal Cycler (Cepheid, Sunnyvale, CA, USA) and carried out with QuantiTect SYBR Green PCR Master Mix (Qiagen), which contained HotStarTaq DNA Polymerase, QuantiTect SyBR Green PCR Buffer, and SYBR Green I. The QPCR reaction mixture contained 1X QuantiTect SYBR Green PCR Master Mix, 0.3uM primer pairs, and 1ul cDNA in a total volume of of 25ul. Melting curves were done by incremental temperature increases of 1.0°C from 60°C to 90°C. These were repeated to ensure that primer-dimers and other nonspecific products had been eliminated. All QPCR measurements were performed in triplicate, with similar results.
Results
Immediate response to hyperosmolar stress at 0.5hr is to irreversibly downregulate a small set of highly changing mRNA transcripts
The kinetics of the TSC hyperosmolar stress response was analyzed by whole mouse genome Affymetrix arrays (430MOE v2). Two or three 400 mM sorbitol stimulation periods replicated with high correlation, 0.86–0.94 for time points from 0 to 24hr (Supplemental Table 2). Sorbitol at 400mM was chosen because it induces peak SAPK activation in TSC, and activates p38MAPK and AMPK in TSC and embryos at high stimulation indices [[3; 4], unpublished data]. Biological replicates within a single stimulation period had a 0.95 correlation from array series one at 0.5hr and a 0.99 correlation from array series two at 6hr.
Microarray data repeated in array series one and two were normalized for intensity using positive and negative control probes in the array. DCHIP software was used to calculate the significance of changes in intensity for paired time points by two-way ANOVA. The following analyses featured significantly changing genes using 1.5-fold change from unstimulated as a threshold.
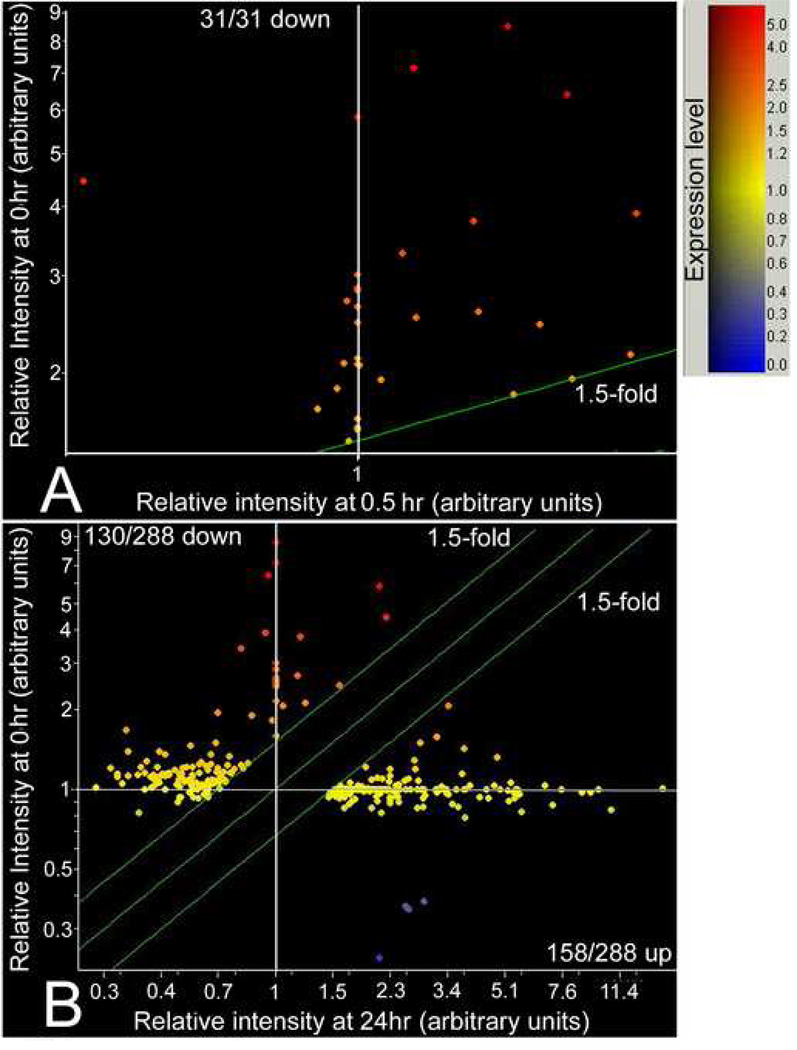
By 0.5hr, 100% of significantly changing genes (31 genes) decreased in magnitude (Figure 1A, Supplemental Table 3). The early stress response of TSC is only to downregulate highly-changing mRNA. This is a specific response as >1.5 fold downregulated genes are only 31/17,411 sequences expressed in the unstressed TSC (from a total of 45,101 probes). In contrast to 0.5hr, at 24hr 158/288 highly-changing sequences increased in magnitude (Figure 1B) and 130/288 decreased.
Figure 1.
Scatter plots of highly downregulated genes at 0.5hr and a mix of downregulated and upregulated genes at 24hr. Scatter plots of 45,101 genes on Affymetrix 430 MOE v2. Microarray comparing gene expression in unstressed and stress TSC. Microarray data were analyzed by ANOVA statistics. The combined results of two series of hybridizations identified 31 downregulated genes at 0.5hr (>1.5fold change comparing stressed with unstressed TSC, p<0.05) and 130 downregulated and 158 upregulated genes at 24hr of 400 mM sorbitol.
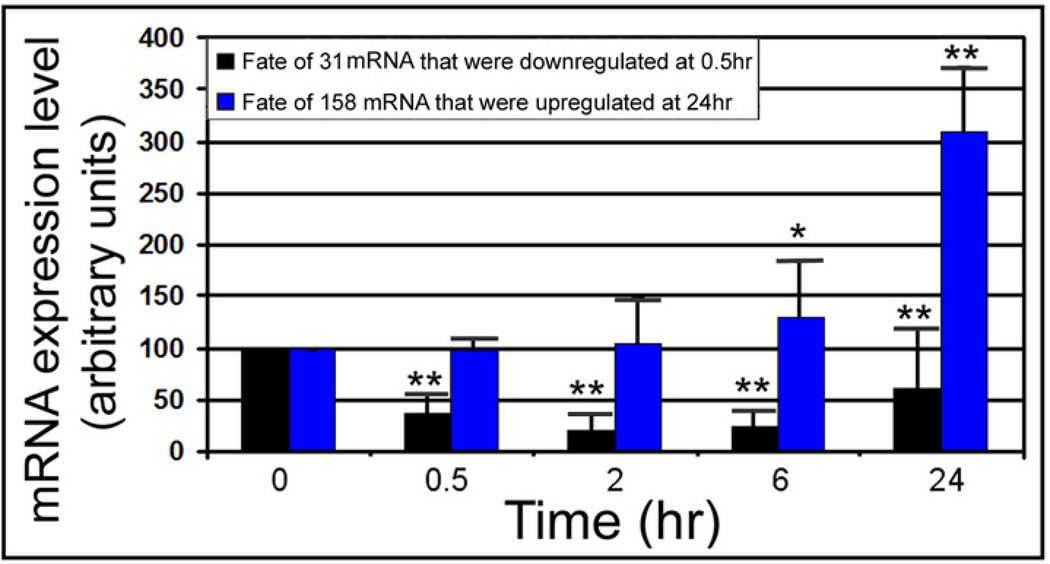
What was the fate at 24hr of the genes downregulated at 0.5hr? The 31 downregulated genes are significantly decreased (p<0.001) at 33.6%, 18.9%, 21.3%, and 52.8% of unstressed levels (mean intensity ratios of 128/381, 72/381, 81/381, and 201/381) at 0.5hr, 2hr, 6hr, and 24hr, respectively (Figure 2, Supplemental Table 3). Although there is a significant drift upwards from 18.9% to 52.8% between 2hr and 24hr (p<.05), the initial downregulation of the 31 genes remains significant through 24hr.
Figure 2.
Of highly changing genes, downregulated genes decrease rapidly and remain significantly lower than unstressed and upregulated genes increase slowly, becoming significantly higher by 24hr. Time courses of the 31 genes downregulated at 0.5hr and 158 genes upregulated at 24hr are displayed in a histogram series. ** Signifies highly significant change (p<0.001), and * signifies significance (p<0.05). Y error flags show standard errors of average intensity of the group.
In contrast upregulation occurs slowly with a small, insignificant increase through 6hr (123.5%, mean intensity ratio=270/219, p=0.25), and a high significant increase (303%, mean intensity ratio=663/219, p<0.001) occurring at 24hr. By 6hr, 84% of the 158 genes upregulated at 24hr (Supplemental Table 3) are still expressed at low levels.
The upregulated response at 24hr is homeostatic
What was the quality of the new mRNA synthesis occurring at 24hr? We analyzed the upregulated and downregulated genes at 24hr and the downregulated genes at 0.5hr for the types of functions they might perform. This was done for general cell biological, putatively homeostatic, functions using GOTM software.
The hyperosmolar stress response included many categories of function in the set of the 158 genes upregulated at 24hr. These included regulation of cell cycle, apoptosis, cell signaling pathways, and nuclear function including transcription regulation (Table 1 shows significant categories, Supplemental table 4 shows individual genes). The functional categories in the set of 130 genes downregulated at 24hr include decreased cell cycle commitment, mediators of mitosis, and several changes in macromolecular synthesis (Table 1, Supplemental Table 5). The functions of the set of 31 genes downregulated at 0.5hr included RNA polymerases, processing, and binding proteins and cellular metabolism (Table 1, Supplemental Table 6).
Table 1.
Ontology of upregulated and downregulated genes modulated by hyperosmolar stress
| A. Gene ontology: up-regulated 158 mRNA at 24hr compared to 0hr. |
| 1. Protein kinase cascade: p=0.0093 (3), 1B. Activation of MAPKK activity: p=0.0005 (2) |
| 2. Regulation of enzyme activity: p=0.0066 (2), 2B. Negative reg. of protein kinase activity: p=0.0012 (3) |
| 3. Regulation of cell cycle: p=0.0002 (5), 3B. Negative reg. of cell cycle progression: p=0.0007(5) |
| 4. Apoptosis: p=0.0036 (6), 4B. Negative regulation of apoptosis: p=0.0087 (4) |
| 5. Reg. of transcription: p=0.0006 (23), 5B. Positive reg. of transcription, DNA-dependent: p=0.0095 (4) |
| 6. Cell differentiation: p=0.0064 (10) |
| 7. Response to heat: p=5.1735E-05 (4) |
| 8. Organ development: p=0.0099 (8). 8B. Blood vessel development: p=0.0009 (4) |
| 9. Protein dimerization activity: p=0.0019 (4) |
| 10. Nucleic acid binding: p=0.0039 (29), 10B. Single-stranded DNA binding: p=0.0050 (2) |
| 11. Transcription reg. activity: p=0.0002 (4), 11B. Transcription factor activity: p=0.0009 (12) |
| 11C. Transcriptional repressor activity: p=0.0004 (5) |
| 12. Inosine kinase activity: p=0.0001 (2) |
| 13. Polyphosphate-glucose phosphotranferase activity: p=0 (2) |
| 14. Cyclin-dependent protein kinase inhibitor activity: p=0.0018 (3) |
| 15. Nucleus: p=0.0029 (38), 15B. Transcription elongation factor complex: p=2.6779E-21 (6) |
| 16. Nucleolus organizer complex: p=0.00001 (2) |
| B. Gene ontology: down-regulated 130 mRNA at 24hr compared to 0hr |
| 1. Germ cell development: p=0.0064 (2) |
| 2. Chromosome segregation: p=0.0010 (3) |
| 3. Cell cycle: p=6.9944E-05 (7), 3B. M phase of mitotic cell cycle: p=0.0005 (5) |
| 4. Cell organization and biogenesis: p=0.0076 (10), 4B. Mitotic chromosome condensation: p=0.0016 (2), |
| 4C. Telomere maintenance: p=0.0070 (2), 4D. mRNA export from nucleus: p=0.0077 (2) |
| 5. Cell division: p=9.3031E-06 (8) |
| 6. Nucleic base, nucleoside, nucleotide metabolism: p=0.0018 (20), 6B. mRNA processing: p=0.0005 (6) |
| 7. Macromolecule metabolism: p=0.0093 (24), 7B. DNA replication: p=0.0036 (4) |
| 8. Response to DNA damage stimulus: p=0.0033 (5) |
| 9. Phospholipid binding: p=0.0080 (3) |
| 10. Nucleic acid bind p=4.5E-06 (5), 10B. DNA bind p=0.0094 (18), 10C. RNA bind p=8.37E-06 (12) |
| 11. Nucleotide binding: p=0.0002 (7). 11B. ATP binding: p=0.0043 (15) |
| 12. Transferase activity, phosphorus-containing groups: p=0.0024 (8), 12B. Polyphosphate-glucose phosphotransferase activity: p=0.0001 (7). |
| 13. Chromosome: p=1.2486E-07 (11) |
| 14. Cytosol: p=0.0016(7) |
| 15. Nucleus: p=8.1541E-07 (39) |
| 16. MHC protein complex: p=0.0019 (2). |
| 17. DNA topoisomerase IV complex: p=0 (2) |
| 18. Transcription factor TFIID complex: p=0.0049 (2) |
| C. Gene ontology: down-regulated 31 mRNA at 0.5hr compared to 0hr |
| 1. Nucleo base, nucleoside, nucleotide metabolism: p=0.0054 (3), 1B. RNA processing: p=0.0010 (3) |
| 2. RNA binding: p=0.0002 (4) |
| 3. Intracellular components: p=0.0052 (7), 3B. RNA polymerase complex: p=3.9866E-05 (2) |
Subsets of genes typical of hyperosmolar stress responses were upregulated in homeostatic gene groups such as adaptor protein (AP)1, growth arrest and DNA damage (GADD)45 and DNA damage inducible transcripts (DDIT), heat shock protein (HSP), and cyclin-dependent kinase inhibitors (CDKI)(Table 2). The function of these genes is to resist stress by stopping the cell cycle and mediating survival.
Table 2.
Transcriptional changes induced by hyperosmolar stress in selected homeostatic genes
|
Functional Group |
Ratio of expression; 24hr stress/T0 (unstressed) | ||||
|---|---|---|---|---|---|
| Genes | 0hr (Arbitrary units) |
24hr | T24/T0 | P | Accession number |
|
CDKI p15 p21 |
118 268 |
283 1443 |
2.4 5.4 |
0.004 0.03 |
AF059567 AK007630 |
|
GADD45 and DDIT GADD45β GADD45γ DDIT3(CHOP10) DDIT4 |
341 133 100 484 |
1614 1687 529 1133 |
4.7 12.7 5.3 2.3 |
0.002 0.007 0.0004 0.022 |
AI323528 AK007410 NM_007837 AK017926 |
|
HSP HSP22/Hspb8 HSP68/Hspa1a HSP70.1/Hspa1b HSP70.2/Hspa2 |
325 479 100 100 |
1079 2142 518 208 |
3.3 4.5 5.2 2.1 |
0.033 5.81E-05 0.01 0.0003 |
AF250139 AW763765 M12573 BC004714 |
|
AP1 JunC ATF3 ATF4 |
210 159 822 |
1243 736 1561 |
5.9 4.6 1.9 |
0.04 0.019 0.023 |
NM_010591 BC019946 M94087 |
| Msx2/Hox8 280 | 949 3.4 | 0.03 | NM_010261 | ||
The upregulated response at 24hr mediates TS cell differentiation
A homeostatic response had been anticipated (Table 2), but an unexpectedly large fraction of the genes upregulated at 24hr were specific to TS cell differentiation (Table 3). Since some other markers for primary and secondary TGC were not significantly changed despite replicate high stimulation indices, we also tested 16 of them by QPCR, rather than repeating a third array time course (Table 3). Transcription factors and endocrine hormones associated with differentiation to primary TGC, such as Stra13, HES1, and PL1 were induced by hyperosmolar stress, as were some secondary TGC genes such as GATA2, proliferin, and PLP-M. Extracellular matrix molecules important in placental differentiation after implantation, CCN1/CCN2, were also induced by hyperosmolar stress. Other secondary TGC markers such as PLPA and PLII were not expressed. The QPCR results confirmed the results from arrays and all 16 genes studied had the same changes with both techniques, although there was some variation in magnitude using the two techniques.
Table 3.
Transcriptional changes induced by hyperosmolar stress after 24hr. Assayed by microarray and Real-Time quantitative PCR (QPCR).
|
Functional Group |
Ratio of expression; 24hr stress/T0 (unstressed) | ||||
|---|---|---|---|---|---|
| Genes | Microarray |
Real-time QPCR |
|||
| 0hr | 24hr | T24/T0% | T24/T0 | Accession | |
| (P value) | (P value) | Number (QPCR) | |||
| (Arbitrary units) | |||||
| Developmental | |||||
| TS cell | |||||
| Id2 | 2604 | 2401 | 0.9(1) | 1.0(0.7) | NM_010496 |
| Cdx2 | 630 | 359 | 0.6 (1) | 0.1 (0.02) | NM_007673 |
| TGC primary | |||||
| STRA13 | 346 | 1698 | 4.9 (0.002) | 3.0 (0.001) | NM_011498 |
| HES1 | 158 | 395 | 2.5 (0.01) | 2.2 (0.02) | BC018375 |
| PL1 | 100 | 160 | 1.6 (0.008) | 3.2 (0.003) | BB414739 |
| TGC secondary | |||||
| PLF | 108 | 930 | 8.6 (0.0005) | 4.6 (0.0002) | X75557 |
| PLP-M | 100 | 401 | 4.1 (0.004) | 10.0(0.003) | NM_019991 |
| GATA2 | 655 | 1574 | 2.4 (0.02) | 3.7 (0.008) | AK004675 |
| ECM | |||||
| CTGF/CCN2 | 228 | 1912 | 8.4 6.04E-05 | 7.3 (0.005) | NM_010516 |
| CYR61/CCN1 | 795 | 2753 | 3.5 (0.005) | 3.3 (0.004) | NM_010217 |
| Homeostatic | |||||
| GADD45Y | 133 | 1687 | 12.7(0.0001) | 5.9 (0.0003) | AK007410 |
| HSP68 | 479 | 2142 | 4.5 (0.004) | 5.7 (0.0005) | M12573 |
| AP1 family | |||||
| ATF3 | 210 | 1243 | 5.9 (0.02) | 3.9 (0.007) | BC019946 |
| JunC | 159 | 736 | 4.6 (0.006) | 4.4 (0.02) | NM_010591 |
| JunB | 155 | 483 | 3.1 (0.0004) | 2.7 (0.03) | NM_008416 |
| Fos-like 2 | 100 | 536 | 5.4 (0.007) | 3.1 (0.01) | BM245170 |
Interestingly, although many TGC markers were induced, many genes specific to TSC were still expressed at nearly the same level and did not change significantly through 24hr of hyperosmolar stress (Tables 4). Extra embryonic development (Eed)/polycombs, Inhibitor of DNA binding (Id)1/2, AP2γ, and cyclin D3 are expressed after 24hr of stress at 81–117% of unstressed cells. Since these genes did not change between 0 and 24hr, the differences were not significant (p>0.05). Typically markers of spongiotrophoblasts and syncytiotrophoblasts arise later during induction of differentiation of TSC in vitro and in vivo, and markers for these cell types were not induced significantly. Specifically, for spongiotrophoblasts, MASH2 and TPBPa/4311 weren’t induced and for syncytiotrophoblasts, GCM1 and TEF5 weren’t induced.
Table 4.
Lack of significant transcriptional changes induced by sorbitol are detected for some markers of pluripotent TSC, primary and secondary TGC, spongiotrophoblasts, and syncytiotrophoblasts.”
|
Functional Group |
Ratio of expression; 24hr stress/T0 (unstressed) | ||||
|---|---|---|---|---|---|
| Genes | 0hr (Arbitrary units) |
24hr | T24/T0 (%) |
P value |
Accession number |
| TSC | |||||
| Cdx2 | 630 | 359 | 0.6 | 1 | NM_007673 |
| Eomesodermin | 1218 | 649 | 0.5 | 1 | BB128925 |
| ErrBeta | 478 | 226 | 0.5 | 0.22 | BB386239 |
| Cyclin A2 | 1200 | 714 | 0.6 | 1 | X75483 |
| Cyclin B1 | 2159 | 1351 | 0.6 | 0.68 | AU015121 |
| Eed | 905 | 965 | 1.1 | 1 | U97675 |
| Id1 | 244 | 285 | 1.2 | 1 | U43884 |
| Id2 | 2604 | 2401 | 0.9 | 1 | NM_010496 |
| AP2y | 1676 | 1576 | 0.9 | 1 | BB550860 |
| Cyclin D3 | 749 | 607 | 0.8 | 0.08 | NM_007632 |
| TGC primary | |||||
| HAND1 | 1607 | 1581 | 1.0 | 1 | U43714 |
| PL1 | 100 | 160 | 1.6 | 1 | BB414739 |
| TGC secondary | |||||
| PL-II | 100 | 100 | 1.0 | 1 | NM_008865 |
| PLP-A | 100 | 100 | 1.0 | 1 | |
| Spongiotropoblast | |||||
| MASH2 | 100 | 100 | 1.0 | 1 | NM_008554 |
| TPBPa/4311 | 100 | 100 | 1.0 | 1 | NM_009411 |
| Flt1 | 117 | 307 | 2.6 | 0.16 | D88690 |
| Placenta specific 8 | 131 | 473 | 3.6 | 0.15 | AF263458 |
| Svncvtiotrophoblast | |||||
| GCM1 | 100 | 100 | 1.0 | 1 | NM_008103 |
| TEF5 | 100 | 100 | 1.0 | 1 | NM_011566 |
| CXCR4 | 100 | 166 | 1.7 | 1 | D87747 |
| Dlx3 | 100 | 100 | 1.0 | 1 | U79738 |
A remarkable effect of the 400mM sorbitol is the high amount (76.6+/−6%) of cell death that it causes after 24hr. This is similar to the level of cell death seen in somatic cells under similar sorbitol doses and durations [23]. However, there were very few TGC during the unstressed state (Supplemental figure 1A) and 400mM sorbitol stress suppresses further DNA synthesis in TSC and embryos [3; 4], so no generation of TGC occurred during the 24hr of hyperosmolar stress.
Upregulated mRNA produced upregulated proteins in placental cells and embryos
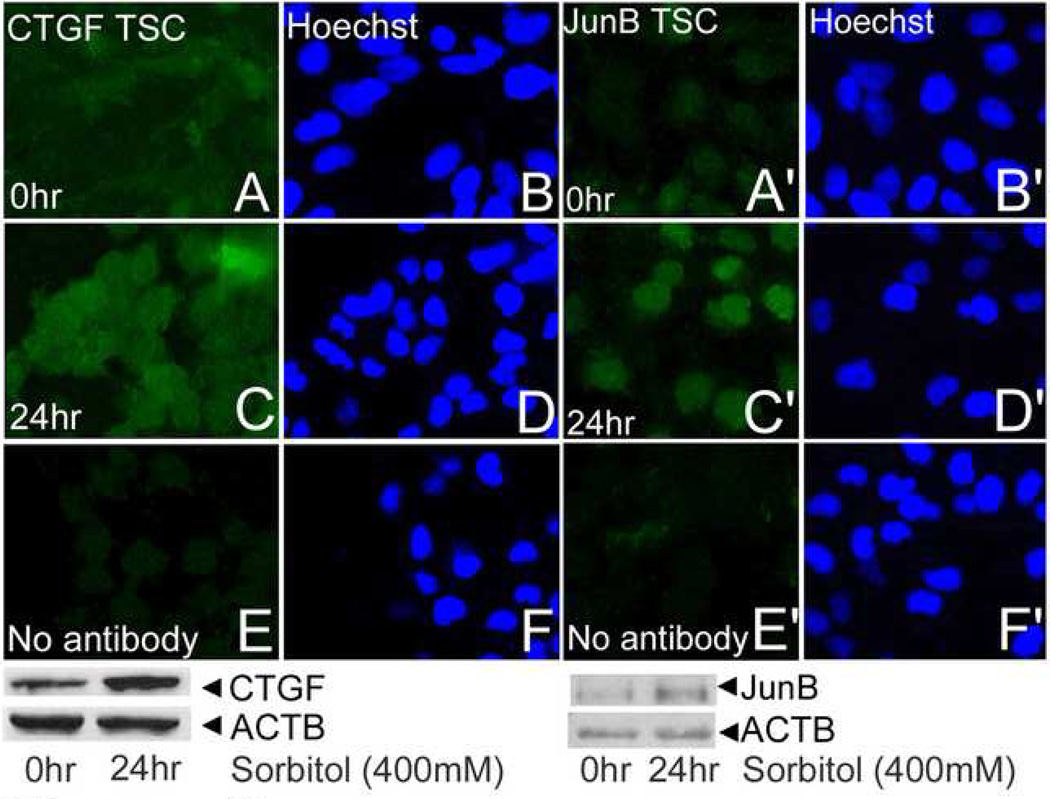
To follow up the studies on global mRNA expression several genes were studied for protein expression (Figure 3). CTGF/CCN2, GADDβ/γ and p21 were upregulated by hyperosmolar stress in TSC. JunB protein was upregulated by hyperosmolar stress in TSC and embryos. These data suggest that stress-induced mRNA are translated into proteins.
Figure 3.
Two proteins, CTGF/CCN2 and JunB, increase similarly to the increase in their mRNA transcripts 24hr. TSC were cultured without stress (A, B; A’, B’), for 24hr with stress (C, D; C’, D’) and probed with antibodies to CTGF/CCN2 or JunB as indicated. TSC were cultured for 24hr of 400mM sorbitol and then probed with no first antibody (E, F; E’, F’). Paired micrographs in the first and second and in the third and fourth columns show protein immunofluorescence and Hoechst-stained nuclei, respectively. At the bottom of each micrograph series is an immunoblot derived from TSC cultured without stress or after 24hr of 400mM sorbitol. Immunoblots were probed with antibodies to CTGF/CCN2 or JunB and then reprobed with antibodies to Actin (ACTB) as a loading control. Bands for CTGF/CCN2 and JunB are both detected at the correct size of 38kDa.
Discussion
Hyperosmolar stress causes differentiation of TSC
Hyperosmolar stress induces the first spatial and temporal differentiation events after implantation. Previous reports of TSC differentiation have used a variety of developmentally relevant stimuli in vitro and in vivo. These include leukemia inhibitory factor [LIF, [24]], retinoic acid [RA, [25]], diethylstilbestrol [DES, [26]], and the FGF4 removal [27] emulating migration away or loss of contact with FGF4 sources in vivo [28]. A surprising finding is how similar stress-mediated differentiation is to these normal stimuli.
Primary TGC arise from the abembryonic pole of the implanting blastocyst from cells that have migrated away from the inner cell mass and the source of FGF4 that maintains TSC in the undifferentiated state [27; 29; 30]. Primary TGC are induced by 24hr of hyperosmolar stress, characterized by the induction of transcription factors Stra13 and HES1 and the placental hormone PL1. Stra13 is sufficient to override FGF4 and cause differentiation of TSC into primary TGC [31]. Similarly, stress is dominant over FGF4 for inducing PL1 mRNA (shown here) and protein (Zhong et al, submitted).
Hyperosmolar stress induces a subset of markers of early secondary TGC, characterized by the transcription factor GATA2 and the placental hormones proliferin, PLPM, and PLPE. Secondary TGC are those that arise later from a second center of TSC proliferation, the ectoplacental cone. However, stress did not induce the placental hormones PLII or PLPA of secondary TGC [32]. Other later placental lineages including spongiotrophoblasts (MASH2 and TPBPa/4311) and syncytiotrophoblasts (GCM1 and TEF5)[33]. These lineages are the final ones developing at E11.5, when the placenta becomes essential for mouse fetal life [34]. These markers for these three placental lineages are not induced by 24hr of stress or by FGF4 removal [25; 31; 35]. Therefore, hyperosmolar stress does not accelerate differentiation to produce later placental lineages. GCM1 mRNA can be induced within 24hr of FGF4 removal from TSC [31], but is not expressed after sorbitol addition to media containing FGF4. Thus stress may suppress later differentiation events.
In contrast, expression of one marker of differentiation is speeded up during stressed differentiation. PL1 mRNA arises 5 days after FGF4 removal [31], but PL1 mRNA (and protein) is induced by 24hr after sorbitol addition. Thus, stress actively modulates differentiation.
TSC potency under stress appears to be preserved, although signs of differentiation occur. Although, TSC have differentiated into primary and secondary TGC, they maintain levels of Id2 mRNA that are unchanged after 24hr of hyperosmolar stress. Of nine genes investigated five remained at nearly the same level of expression as in unstressed TSC (Eed/107%, Id2/92%, AP2Gamma/94%, CyclinE1/92% and CyclinD3/81%). An additional four genes had decreased to around half the level of expression of unstressed TSC (Cdx2/60%, Eomesodermin/53%, ErrBeta/47%, and CyclinA2/60%). Although, stressed TSC express transcription factors that mediate differentiation to TGC, they still express substantial levels of mRNA transcripts mediating the undifferentiated state. If stress subsided, proteins maintaining reestablishing TSC potency could be translated without new transcription in energy-depleted cells.
The homeostatic response to hyperosmolar stress
The homeostatic genes include ontologic groups that mediate suppression of apoptosis (GADD45β/γ), cell cycle arrest (p15, p21), and heat shock proteins (HSP1a/1b/2/8), and AP1 transcription factors (JunB/C, ATF3/4). The downregulated genes include RNA processing factors that decrease immediately at 0.5hr and remain low through 24hr. Thus the stressed TSC stops growth and prepares for survival.
The stress response is highly dynamic with differences between the rapid changes at 0.5hr and the maturing response at 24hr
A very small specific number of 31 mRNA change highly at 0.5hr and the change is all downregulation. By 24hr, over half of the highly changing mRNA are upregulated. These genes are developmental and homeostatic.
Hyperosmolar 400mM sorbitol causes an 80% decrease in new translation in somatic cells after 0.5hr [36]. Although translation was not measured here, there were no new highly upregulated mRNA at 0.5hr in TSC stressed by 400mM sorbitol. Of the 158 mRNA highly upregulated at 24hr, these were still not significantly upregulated at 6hr. Thus, stressed TSC respond to stress for many hours in a post-transcriptional, and probably post-translational mode.
Stress enzymes and their substrates are modified to stress-induced function by phosphorylation and change in cell location [23]. For example, SAPK is phosphorylated and localized to the nucleus in mouse preimplantation embryos and TSC, and human first trimester placental cell lines [1; 3; 4; 15]. This suggests that SAPK may be responsible for new transcription of homeostatic and developmental transcripts.
Approximately 3/4th of the cells died during 24hr of 400mM sorbitol and the function of the persistent cells needs clarification. The die off at 24hr is seen in adult somatic cells exposed to similar levels of sorbitol [37; 38]. The initial die off is followed by establishment of a persistent cell population that can actually increase in cell number from 48–72hr. We have also seen that 22.4+/−6.1% of TSC persist at 48hr of 400mM sorbitol after 23.8+/−4.4% persisted at 24hr (Zhong et al, submitted). Also, nearly 100% of TSC stressed for 24hr express PL1 protein, suggesting nearly total differentiation. Differentiation in persistent cells could result for two reasons. One is that the differentiated state allows a more efficient response to hyperosmolar stress during culture. Alternately, differentiation may emulate the organismal survival response by TSC as a model for the stressed implanting embryo. This second hypothesis is supported by observations that the stress enzymes maintain HAND1 protein and decrease ID2, and the two stress enzymes are required to upregulate maternal-produced placental lactogen (PL)1 (Zhong, manuscript submitted). PL1 and proliferin are not known to have effects on the placenta or TSC themselves and would not be part of survival program for the TSC. Thus for stress that kills most TSC, surviving stressed TSC differentiate as part of an organismal survival strategy.
The high cell loss after 24hr at 400mM sorbitol also suggests the possibility that differentiation could be an artifact of survival of stress by a fraction of differentiated TGC existing before stress. Several lines of evidence argue against this. First, no increase in TGC was seen in micrographs of stressed TSC prior to mRNA preparation. Second, many mRNA mediating differentiation were at background levels in unstressed cells. Third, 400mM causes 100% of TSC to express PL1 protein (Zhong et al., submitted). Thus, hyperosmolar stress causes differentiation rather than selecting for a differentiated subpopulation.
Placental hormones mediating maternal signaling are important during stress. For example, PLPA is essential for embryonic survival if the gestational female is placed under transient hypobaric hypoxia [39]. Thus placental hormones induced by hypoxic stress mediate a survival strategy.
Sorbitol was used here because it activates stress and stress enzyme responses in all cell types and embryos, because stress enzymes were cloned using hyperosmolar stress, and this continues as the standard stressor for enzymologists [8]. However, sorbitol and other polyols may play a significant normal role during first trimester placentation under hypoxic conditions [40]. But, the level of sorbitol used as a stressor here is about three orders of magnitude higher than the levels detected during human placental development. suggesting that stress and stress enzyme function are the primary consequences of sorbitol. Using lower stress doses and other stressors like TNFα [that activate SAPK similarly to sorbitol [7]] is an important future area of research.
The studies here did not test for dependency of the induction of developmental and homeostatic genes on stress enzymes. Stress enzymes such as SAPK and AMPK have overlapping and distinct functions during the stress response of mouse embryos and the stem cells derived from them [8; 23]. It will be important to test whether stress enzymes regulate homeostatic and developmental genes. It will also be important to test for the induction of differentiation of TSC under lower hyperosmolar stresses that do not produce apoptosis, but may reflect lower levels of stress caused by IVF, malnutrition, or exposure to toxins such as cigarette smoke.
Supplementary Material
TSC were cultured for 0–24hr with 400mM sorbitol and microgaphed before cells were lysed and RNA isolated. TSC were micrographed using phase contrast and transmitted light after exposure to 400mM sorbitol for the durations indicated. Bar in (A) shows 20micron.
Acknowledgements
We thank Mike Kruger for advice on statistical analysis, Drs Mike Diamond for comments on the manuscript, and Drs Mike Diamond and Ghassan Saed for help with QPCR. This research was supported by a grant from the National Institute of Child Health and Human Development, NIH, (R01-HD40972).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Wang Y, Puscheck EE, Wygle DL, Lewis JJ, Trostinskaia AB, Wang F, Rappolee DA. Increases in phosphorylation of SAPK/JNK and p38MAPK correlate negatively with mouse embryo development after culture in different media. Fertility and Sterility. 2005;83:1144–1154. doi: 10.1016/j.fertnstert.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 2.Maekawa M, Yamamoto T, Tanoue T, Yuasa Y, Chisaka O, Nishida E. Requirement of the MAP kinase signaling pathways for mouse preimplantation development. Development. 2005;132:1773–1783. doi: 10.1242/dev.01729. [DOI] [PubMed] [Google Scholar]
- 3.Xie Y, Zhong W, Wang Y, Trostinskaia A, Wang F, Puscheck EE, Rappolee DA. Using hyperosmolar stress to measure biologic and stress-activated protein kinase responses in preimplantation embryos. Mol Hum Reprod. 2007;13:473–481. doi: 10.1093/molehr/gam027. [DOI] [PubMed] [Google Scholar]
- 4.Zhong W, Xie Y, Wang Y, Lewis J, Trostinskaia A, Wang F, Puscheck EE, Rappolee DA. Use of hyperosmolar stress to measure stress-activated protein kinase activation and function in human HTR cells and mouse trophoblast stem cells. Reprod Sci. 2007;14:534–547. doi: 10.1177/1933719107307182. [DOI] [PubMed] [Google Scholar]
- 5.Hamatani T, Daikoku T, Wang H, Matsumoto H, Carter MG, Ko MS, Dey SK. Global gene expression analysis identifies molecular pathways distinguishing blastocyst dormancy and activation. Proc Natl Acad Sci U S A. 2004;101:10326–10331. doi: 10.1073/pnas.0402597101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Y, Wang F, Zhong W, Puscheck E, Shen H, Rappolee DA. Shear stress induces preimplantation embryo death that is delayed by the zona pellucida and associated with stress-activated protein kinase-mediated apoptosis. Biol Reprod. 2006;75:45–55. doi: 10.1095/biolreprod.105.049791. [DOI] [PubMed] [Google Scholar]
- 7.Drake PM, Red-Horse K, Fisher SJ. Reciprocal chemokine receptor and ligand expression in the human placenta: implications for cytotrophoblast differentiation. Dev Dyn. 2004;229:877–885. doi: 10.1002/dvdy.10477. [DOI] [PubMed] [Google Scholar]
- 8.Rappolee DA. Impact of transient stress and stress enzymes on development. Dev Biol. 2007;304:1–8. doi: 10.1016/j.ydbio.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 9.Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51:970–975. doi: 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]
- 10.Bose P, Kadyrov M, Goldin R, Hahn S, Backos M, Regan L, Huppertz B. Aberrations of early trophoblast differentiation predispose to pregnancy failure: lessons from the anti-phospholipid syndrome. Placenta. 2006;27:869–875. doi: 10.1016/j.placenta.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- 12.Ecker DJ, Stein P, Xu Z, Williams CJ, Kopf GS, Bilker WB, Abel T, Schultz RM. Long-term effects of culture of preimplantation mouse embryos on behavior. Proc Natl Acad Sci U S A. 2004;101:1595–1600. doi: 10.1073/pnas.0306846101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Gonzalez R, Moreira P, Bilbao A, Jimenez A, Perez-Crespo M, Ramirez MA, Rodriguez De Fonseca F, Pintado B, Gutierrez-Adan A. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. Proc Natl Acad Sci U S A. 2004;101:5880–5885. doi: 10.1073/pnas.0308560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- 15.Xie Y, Puscheck EE, Rappolee DA. Effects of SAPK/JNK inhibitors on preimplantation mouse embryo development are influenced greatly by the amount of stress induced 20 by the media. Mol Hum Reprod. 2006;12:217–224. doi: 10.1093/molehr/gal021. [DOI] [PubMed] [Google Scholar]
- 16.Xie Y, Wang F, Puscheck EE, Rappolee DA. Pipetting causes shear stress and elevation of phosphorylated stress-activated protein kinase/jun kinase in preimplantation embryos. Mol Reprod Dev. 2007;74:1287–1294. doi: 10.1002/mrd.20563. [DOI] [PubMed] [Google Scholar]
- 17.Johnson MR, Riddle AF, Grudzinskas JG, Sharma V, Campbell S, Collins WP, Lightman SL, Mason B, Nicolaides KH. Endocrinology of in-vitro fertilization pregnancies during the first trimester. Hum Reprod. 1993;8:316–322. doi: 10.1093/oxfordjournals.humrep.a138043. [DOI] [PubMed] [Google Scholar]
- 18.Surveyor GA, Wilson AK, Brigstock DR. Localization of connective tissue growth factor during the period of embryo implantation in the mouse. Biol Reprod. 1998;59:1207–1213. doi: 10.1095/biolreprod59.5.1207. [DOI] [PubMed] [Google Scholar]
- 19.Hogan B, Beddington R, Constantini F, Lacy B. Manipulating the mouse embryo, A laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory; 20002. [Google Scholar]
- 20.Zhong W, Sun T, Wang Q, Wang Y, Xie Y, Johnson A, Leach R, Puscheck EE, Rappolee DA. SAPK/JNK1, 2, but not SAPK/JNK3 mRNA transcripts, are expressed in early gestation human placenta and mouse eggs, preimplantation embryos, and trophoblast stem cells. Fertility and Sterility. 2004;82:1140–1148. doi: 10.1016/j.fertnstert.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci U S A. 2001;98:31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang B, Schmoyer D, Kirov S, Snoddy J. GOTree Machine (GOTM): a web-based platform for interpreting sets of interesting genes using Gene Ontology hierarchies. BMC Bioinformatics. 2004;5:16. doi: 10.1186/1471-2105-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie Y, Liu J, Proteasa S, Proteasa G, Zhong W, Wang Y, Wang F, Puscheck EE, Rappolee DA. Transient stress and stress enzyme responses have practical impacts on parameters of embryo development, from IVF to directed differentiation of stem cells. Mol Reprod Dev. 2008;75:689–697. doi: 10.1002/mrd.20787. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi Y, Carpino N, Cross JC, Torres M, Parganas E, Ihle JN. SOCS3: an 20 essential regulator of LIF receptor signaling in trophoblast giant cell differentiation. Embo J. 2003;22:372–384. doi: 10.1093/emboj/cdg057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan J, Tanaka S, Oda M, Makino T, Ohgane J, Shiota K. Retinoic acid promotes differentiation of trophoblast stem cells to a giant cell fate. Dev Biol. 2001;235:422–432. doi: 10.1006/dbio.2001.0300. [DOI] [PubMed] [Google Scholar]
- 26.Tremblay GB, Kunath T, Bergeron D, Lapointe L, Champigny C, Bader JA, Rossant J, Giguere V. Diethylstilbestrol regulates trophoblast stem cell differentiation as a ligand of orphan nuclear receptor ERR beta. Genes Dev. 2001;15:833–888. doi: 10.1101/gad.873401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- 28.Uy GD, Downs KM, Gardner RL. Inhibition of trophoblast stem cell potential in chorionic ectoderm coincides with occlusion of the ectoplacental cavity in the mouse. Development. 2002;129:3913–3924. doi: 10.1242/dev.129.16.3913. [DOI] [PubMed] [Google Scholar]
- 29.Chai N, Patel Y, Jacobson K, McMahon J, McMahon A, Rappolee DA. FGF is an essential regulator of the fifth cell division in preimplantation mouse embryos. Dev Biol. 1998;198:105–115. doi: 10.1006/dbio.1997.8858. [DOI] [PubMed] [Google Scholar]
- 30.Rappolee DA, Basilico C, Patel Y, Werb Z. Expression and function of FGF-4 in peri-implantation development in mouse embryos. Development. 1994;120:2259–2269. doi: 10.1242/dev.120.8.2259. [DOI] [PubMed] [Google Scholar]
- 31.Hughes M, Dobric N, Scott IC, Su L, Starovic M, St-Pierre B, Egan SE, Kingdom JC, Cross JC. The Hand1, Stra13 and Gcm1 transcription factors override FGF signaling to promote terminal differentiation of trophoblast stem cells. Dev Biol. 2004;271:26–37. doi: 10.1016/j.ydbio.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 32.Simmons DG, Fortier AL, Cross JC. Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev Biol. 2007;304:567–578. doi: 10.1016/j.ydbio.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Cross JC, Anson-Cartwright L, Scott IC. Transcription factors underlying the development and endocrine functions of the placenta. Recent Prog Horm Res. 2002;57:221–234. doi: 10.1210/rp.57.1.221. [DOI] [PubMed] [Google Scholar]
- 34.Copp AJ. Death before birth: clues from gene knockouts and mutations. Trends Genet. 1995;11:87–93. doi: 10.1016/S0168-9525(00)89008-3. [DOI] [PubMed] [Google Scholar]
- 35.Hemberger M, Hughes M, Cross JC. Trophoblast stem cells differentiate in vitro into invasive trophoblast giant cells. Dev Biol. 2004;271:362–371. doi: 10.1016/j.ydbio.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 36.Patel J, McLeod LE, Vries RG, Flynn A, Wang X, Proud CG. Cellular stresses 25 profoundly inhibit protein synthesis and modulate the states of phosphorylation of multiple translation factors. Eur J Biochem. 2002;269:3076–3085. doi: 10.1046/j.1432-1033.2002.02992.x. [DOI] [PubMed] [Google Scholar]
- 37.Galvez A, Morales MP, Eltit JM, Ocaranza P, Carrasco L, Campos X, Sapag-Hagar M, Diaz-Araya G, Lavandero S. A rapid and strong apoptotic process is triggered by hyperosmotic stress in cultured rat cardiac myocytes. Cell Tissue Res. 2001;304:279–285. doi: 10.1007/s004410100358. [DOI] [PubMed] [Google Scholar]
- 38.Morales MP, Galvez A, Eltit JM, Ocaranza P, Diaz-Araya G, Lavandero S. IGF-1 regulates apoptosis of cardiac myocyte induced by osmotic-stress. Biochem Biophys Res Commun. 2000;270:1029–1035. doi: 10.1006/bbrc.2000.2550. [DOI] [PubMed] [Google Scholar]
- 39.Ain R, Dai G, Dunmore JH, Godwin AR, Soares MJ. A prolactin family paralog regulates reproductive adaptations to a physiological stressor. Proc Natl Acad Sci U S A. 2004;101:16543–16548. doi: 10.1073/pnas.0406185101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jauniaux E, Hempstock J, Teng C, Battaglia FC, Burton GJ. Polyol concentrations in the fluid compartments of the human conceptus during the first trimester of pregnancy: maintenance of redox potential in a low oxygen environment. J Clin Endocrinol Metab. 2005;90:1171–1175. doi: 10.1210/jc.2004-1513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TSC were cultured for 0–24hr with 400mM sorbitol and microgaphed before cells were lysed and RNA isolated. TSC were micrographed using phase contrast and transmitted light after exposure to 400mM sorbitol for the durations indicated. Bar in (A) shows 20micron.