Abstract
Background & Aims
Mutational inactivation of APC is an early event in colorectal cancer (CRC) progression that affects the stability and increases the activity of β-catenin, a mediator of Wnt signaling. CRC progression also involves inactivation of signaling via transforming growth factor (TGF)β and bone morphenogenic protein (BMP), which are tumor suppressors. However, the interactions between these pathways are not clear. We investigated the effects of loss of the transcription factor Smad4 loss on levels of β-catenin mRNA and Wnt signaling.
Methods
We used microarray analysis to associate levels of Smad4 and β-catenin mRNA in colorectal tumor samples from 250 patients. We performed oligonucleotide-mediated knockdown of Smad4 in human embryonic kidney (HEK293T) and in HCT116 colon cancer cells and transgenically expressed Smad4 in SW480 colon cancer cells. We analyzed adenomas from (APCΔ1638/+) and (APCΔ1638/+)x(K19CreERT2Smad4lox/lox) mice using laser-capture microdissection.
Results
In human CRC samples, reduced levels of Smad4 correlated with increased levels of β-catenin mRNA. In Smad4-depleted cell lines, levels of β-catenin mRNA and Wnt signaling increased. Inhibition of BMP or depletion of Smad4 in HEK293T cells increased binding of RNA polymerase II to the β-catenin gene. Expression of Smad4 in SW480 cells reduced Wnt signaling and levels of β-catenin mRNA. In mice with heterozygous disruption of Apc(APCΔ1638/+), Smad4-deficient intestinal adenomas had increased levels of β-catenin mRNA and expression of Wnt target genes, compared with adenomas from APCΔ1638/+mice that expressed Smad4.
Conclusions
Transcription of β-catenin is inhibited by BMP signaling to Smad4. These findings provide important information about the interaction among TGF-β, BMP, and Wnt signaling pathways in CRC progression.
Keywords: Colon Cancer, Mouse Model, Intestinal Epithelium, signal transduction, carcinoma
Introduction
The central mediator of downstream Wnt signaling, β-catenin, is normally sequestered at the adherens junctions (E-cadherin, p120, β-catenin, α-catenin). Free cytoplasmic β-catenin is rapidly targeted for degradation through protein complexes that contains adenomatous polyposis coli (APC), Axin, and GSK3β. However, in the presence of Wnt ligand, GSK3β is inhibited resulting in accumulation of cytoplasmic β-catenin. This increase in cytoplasmic β-catenin results in nuclear translocation of β-catenin where it associates with LEF/TCF transcription factors to activate Wnt target genes1.
Tight regulation of Wnt signaling within the normal intestinal tract is critical for maintenance of normal intestinal stem cell function and tissue homeostasis2. Mutational inactivation of APC results in increased β-catenin protein levels with concomitant activation of the downstream Wnt signaling pathway and is an early event in colorectal cancer progression3. Inactivation of APC alone does not appear sufficient to fully activate Wnt signaling or to convey an invasive and metastatic behavior in tumor cells4,5. It is likely that other mutational events or extrinsic factors may be necessary for maximal activation of the Wnt pathway to promote invasion and metastasis. Little is known about regulation of β-catenin levels outside of the paradigm of post-translational regulation. In particular, a role for regulation of β-catenin mRNA expression as an important mechanism to modulate Wnt signaling and colon cancer progression has not been described.
The TGF-β/BMP/Smad4 pathway is a developmentally crucial pathway that is also frequently mutated in colon cancer. BMP antagonists are expressed in the intestinal stem cell niche while BMP and TGF-β signaling activity increases as cells differentiate and migrate along the intestinal gland toward the intestinal lumen2,6–8. Recent reports have included the BMP antagonist Noggin as a requisite factor within media for in vitro culture of isolated intestinal stem cells9. In colon cancer, TGF-β Receptor Type II (TβRII) is mutated in >55% of cases10 and BMPRI/RII is mutated in >70% of cases11 while Smad4 mutations are thought to occur late in 20–30% of cases12,13. In addition, germline mutations in Smad4 and BMPR1A genes are frequently found in patients with Juvenile Polyposis Syndrome, a condition which predisposes patients to developing intestinal adenocarcinoma14,15. Loss of Smad4 function in the presence of Apc mutation in mice markedly accelerates tumor progression16, but the mechanism of this cooperative interaction has not been fully defined.
Both β-catenin activation and Smad4 mutations occur frequently in colon cancer, yet the interaction between these signaling pathways in normal intestinal crypts and in colon cancer biology is unclear. In the present study, we find that decreased expression of Smad4 in human colon cancer is associated with increased expression of β-catenin mRNA. When Smad4 loss is induced in mouse intestinal tumor models, we observe increased expression of β-catenin mRNA and protein and associated increases in the mRNA expression of Wnt target genes, c-Myc and Axin2. In cultured colon cancer and human embryonic kidney (HEK293T) cells, both inhibition of Smad4 expression and inhibition of BMP receptor signaling cause similar increases in β-catenin mRNA expression and canonical Wnt signaling. The increase in β-catenin expression in response to Smad4 depletion or Noggin treatment is associated with increased engagement of RNA polymerase II to ctnnb1 (the β-catenin gene). Thus, in addition to the important role of post-translational modification of β-catenin in canonical Wnt signaling in intestinal neoplasia, up-regulation of β-catenin mRNA expression plays a role in further amplifying the Wnt signal after inhibition of BMP signaling or loss of Smad4 expression.
Results
Inverse correlation of Smad4 and β-catenin expression levels in human colorectal cancer
While loss of Smad4 expression is associated with poor clinical outcomes in colon cancer patients17, its precise role in tumor progression has not been fully determined. To determine whether low Smad4 expression is associated with increased β-catenin expression in colon cancer, we analyzed Smad4 and β-catenin mRNA expression in a microarray dataset representing 250 colorectal cancer patient tumor samples (Stage 1: n=33; Stage 2: n=76; Stage 3: n =82; and Stage 4: n = 59) and ten normal adjacent colorectal tissue specimens (Supplemental Table 1). We observed a significant down-regulation of Smad4 expression in both early and late stage colorectal tumors when compared with normal colon mucosa (Supplemental Figure 1A, P<.0001 for all stages compared to normal [n=10]) and significant up-regulation of β-catenin (Supplemental Figure 1B, P<.002 for all stages compared to normal). To examine if Smad4 and β-catenin mRNA expression levels are inversely correlated on a case by case basis, Pearson correlation tests were performed on the microarray data set. While there was no significant correlation when examining all 250 cases (Supplemental Figure 1C, P<.09), a significant inverse correlation was observed when examining Stage 1 and 2 cases (Supplemental Figure 1D, P<.01). These data suggest that with loss of Smad4 expression in colorectal cancer there is an increase in β-catenin mRNA expression levels.
Smad4 depletion in cultured epithelial cells results in increased β-catenin expression and activation of TOPFlash activity
Since the prevailing paradigm for regulation of β-catenin expression is post-translational, we were surprised to find that increased β-catenin mRNA is associated with Smad4 loss in human colorectal cancer samples. We utilized HCT116 colon cancer cells and Human Embryonic Kidney 293T (HEK293T) cells to determine whether loss of Smad4 expression results in increased expression of β-catenin mRNA and protein in epithelial cells. HCT116 cells are human colon cancer cells that are Smad4, and APC wild type18,19 but have mutations in β-catenin (Ser45 del)18 and TβRII20. HEK293T cells are immortalized human embryonic kidney epithelial cells that have intact Wnt and TGF-β/BMP family signaling pathways. When compared with scrambled siRNA treatment, Smad4 siRNA-treated HCT116 cells showed a modest increase in β-catenin protein levels (Figure 1A), along with a two-fold increase in β-catenin mRNA expression (Figure 1B), and a 6-fold increase in TOPFlash activity (Figure 1C). TOPFlash is a TCF reporter plasmid that measures activation of Wnt signaling21. In HEK293T cells, knock-down of Smad4 expression also caused a modest increase in β-catenin protein expression (Figure 1D), a 4-fold increase in β-catenin mRNA (Figure 1E), and a significant increase in TOPFlash activity (Figure 1F). Both cell lines showed increased TOPFlash activity with Wnt3a treatment, and both cell lines displayed a slight but not significant (P<.15) increase in TOPFlash activity when Smad4 siRNA treatment is combined with Wnt3a treatment. These data show that inhibition of Smad4 expression results in increased β-catenin mRNA along with increased activation of a Wnt/β-catenin-activated reporter plasmid, providing functional evidence of nuclear β-catenin activity, in both HCT116 and HEK293T cell lines. The two-fold increase in TOPFlash activity in HEK293T cells as compared with the 6-fold increase in HCT116 cells is likely due to more rapid turnover of β-catenin in the absence of the stabilizing N-terminal mutation and an intact β-catenin destruction complex in the HEK293T cells.
Figure 1. Smad4 depletion in cultured epithelial cells results in increased β-catenin expression and activation of TOPFlash activity.
Oligonucleotide (RNAi) mediated Smad4 knockdown conducted in (A–C) HCT116 and (D–F) HEK293T human colon cancer cells. (A, D) Results of western blots showing relative expression of Smad4, Smad2, β-catenin and β-actin 24 hours post-transfection with scrambled or Smad4-specific RNAi (B, E) qPCR results showing relative expression of β-catenin mRNA expression 24hours post-transfection with scrambled and Smad4-specific RNAi. The graph shows quantified β-catenin mRNA relative to levels in mock-transfected cells normalized to Pmm1 expression. Triplicate for each condition was performed with mean +/−SEM displayed. Student’s t-tests were performed to determine significance. (C, F) TOPFlash activity 24 hours post-transfection with scrambled or Smad4 specific RNAi, with and without treatment with Wnt3a conditioned media. The graph shows light units from induced TOPFlash activity normalized to constitutive Renilla activity, relative to mock-transfected cells. Significance determined by ANOVA.
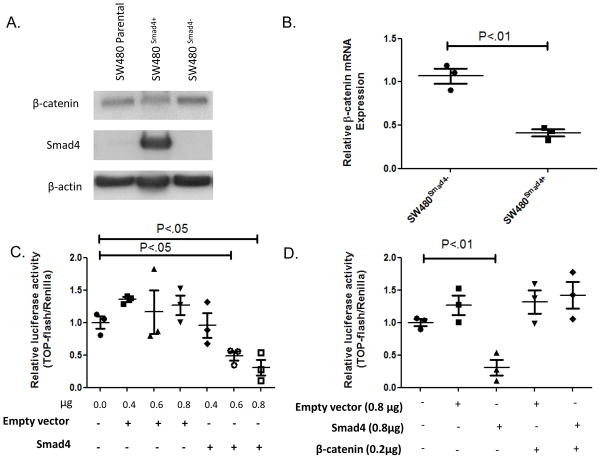
Smad4 restoration suppresses β-catenin mRNA expression and represses TOPFlash activity in β-catenin dependent manner
The converse to blocking cellular Smad4 expression is restoration of Smad4 to cells that lack Smad4 expression. SW480 cells have a deletion in one Smad4 allele and splice site variant Smad4 mutation in the other allele19. We previously reported that restoration of Smad4 into Smad4 deficient SW480 cells suppressed expression of the β-catenin/Wnt target gene, claudin-1, and that Smad4 expression also inhibited TOPFlash activity22. Tian et al.,23 subsequently reproduced our findings and provided data suggesting that constitutive Smad4 expression in SW480 cells reduced β-catenin mRNA levels and decreased nuclear β-catenin immunoreactivity. When Smad4 is transiently transfected into SW480 cells (Figure 2A), we observed a significant (P<.02) decrease in β-catenin mRNA and protein levels (Figures 2A, B), and a dose-dependent repression of TOPFlash activity (Figure 2C). In contrast, Smad4 did not suppress TOPFlash activity in SW480 cells when co-transfected with β-catenin driven by a heterologous promoter (Figure 2D). Thus, these data support a model whereby restoration of the Smad4 signaling pathway inhibits β-catenin expression and that the decrease in β-catenin accounts for the decrease in TOPFlash activation.
Figure 2. BMP signaling suppresses TOPFlash activity and regulates β-catenin mRNA expression levels.
(A) SW480 cells were transiently co-transfected with either BRE-Luc and pCMV-Script or pCMV-Smad4 and then treated with either vehicle 20ng/ml BMP2 and/or 200ng/ml Noggin for 24 hours. Relative luciferase activity is graphed. Significance was determined by ANOVA tests. (B) Western blot of parallel experiments of HEK29T cells. Protein lysate was taken 30 min and 24hours after the indicated treatment to capture the early phosphorylation of R-Smads (p-Smad1, Smad1,p -Smad2, and Smad2) and the downstream effects of activation of the pathways (β-catenin and Id2). Representative β-actin taken at 24 hours is displayed. (C) HEK293T cells were treated with either vehicle, 200ng/ml BMP2, 5ng/ml TGF-β, or Wnt3a conditioned medium (1:1) and assayed for TOPflash activity at 24 hours post-transfection and 12 hours post-treatment. (D) Results of qPCR assays showing relative expression of β-catenin mRNA expression in HEK293T at 12 hours post-treatment with 20ng/ml BMP2, 200ng/ml Noggin or Wnt3a conditioned media. The graph shows quantified β-catenin mRNA relative to untreated cells. Duplicate for each condition was performed with mean +/−SEM displayed for all. Significance determined by student’s t-tests (C&D).
Restoration of Smad4 and BMP signaling is associated with suppression of Wnt signaling
Prior work supports an important role for BMPs 1,2,5, and 7 in the modulation of Wnt signaling and promotion of cellular differentiation in the intestinal crypt8. In order to determine the ligand dependence of TOPFlash activity regulation in SW480 colon cancer cells, we co-transfected Smad4 with a well-characterized BMP-specific Smad reporter plasmid, BRE-Luc24, and assessed BMP-mediated transcriptional activity associated with restoration of Smad4 expression. Previous studies have shown that SW480 and HCT116 cells express BMPR1A receptors and are responsive to BMP2 and BMP725. We selected BMP2 for these experiments because the BMP2 gradient is increased toward the luminal surface of the colonic crypt2. BRE-Luc activity is increased with Smad4 restoration (Figure 3A, Lane 5), and this activity was augmented with the addition of exogenous BMP2 ligand (Figure 3A, Lane6). Conversely, addition of exogenous Noggin, a pan-BMP antagonist, caused a decrease in steady state BRE-Luc activity in SW480 cells with Smad4 expression restored even in the presence of exogenous BMP2 ligand (Figure 3A, Lanes 7, 8). These data suggest that autocrine and paracrine BMP signaling are restored in SW480 cells upon Smad4 re-expression. We and others have found SW480 cells to be refractory to TGF-β treatment13,22, but remain BMP responsive26. To determine whether both BMP- and TGF-β –activated signaling results in suppression of TOPFlash, we used BMP and TGF-β responsive HEK293T cells (Figure 3B). BMP2 treatment of HEK293T cells suppressed TOPFlash activity but TGF-β treatment did not (Figure 3C). In HEK293T cells, we observed a modest, but significant, decrease in β-catenin mRNA levels after treatment with BMP2 and a modest, but significant, increase in β-catenin mRNA levels after Noggin treatment (Figure 3D). Taken together, these experiments support the conclusion that Smad4 restoration/expression enables canonical BMP signaling to decrease β-catenin expression and inhibits Wnt signaling.
Figure 3. Smad4 restoration suppresses β-catenin mRNA expression and represses TOPFlash activity in a β-catenin dependent manner.
((A) Representative results from three biological replicates of FACS sorted SW480 cells co-transfected with pRK-5Smad4 (0.8ug/mL) and pEGFP (1.0ug/mL). Protein isolated from GFP- (Smad4-null) and GFP+ (Smad4+) was run on western blot. (B) qPCR results from Smad4-null and Smad4+cells from (A) showing relative β-catenin expression that was normalized to Pmm1 expression. (C) TOPflash activity 48hrs post-transfection with p-RK5 empty vector or p-RK5-Smad4 vector as indicated. Triplicate for each condition were performed. (D) TOPFlash reporter activity in SW480 cells and in SW480 cells transiently co-transfected with 0.2μg wild-type human β-catenin and 0.8μg p-RK5 empty vector or 0.8μg p-RK5-Smad4 (as indicated). Two biological replicates are displayed. Significance determined using student’s t-test. Mean +/−SEM are displayed with FOP control (C&D).
BMP signaling regulates RNA polymerase II activity of ctnnb1
To determine whether Smad4 regulates transcription of the β-catenin gene, we assessed RNA polymerase II bound to the 2nd exon (+460 to +579) by chromatin immunoprecipitation (ChIP) in HEK293T cells. We observed a significant decrease in RNA polymerase II (RNA Pol II) binding at exon 2 of the ctnnb1 gene in HEK293T cells 12 hours after treatment with BMP2 as compared with untreated cells. Conversely, treatment with the BMP antagonist, Noggin, caused an increase in RNA Pol II engagement with exon 2 of ctnnb1 (Figure 4A). To examine if this release of transcriptional repression occurs through Smad4-dependent signaling, we depleted Smad4 using siRNA. We observed a significant increase in RNA polymerase II (RNA Pol II) pulled down at exon 2 of the ctnnb1 gene upon Smad4 depletion; however, this increase was not augmented with combination of Smad4 depletion and Noggin treatment (Figure 4B). These data support the conclusion that transcription of ctnnb1 is repressed by BMP signaling through Smad4 and that release of transcriptional repression occurs similarly when BMP signaling is inhibited at the receptor level by Noggin or by depletion of Smad4.
Figure 4. BMP signaling regulates RNA polII activity of ctnnb1.
HEK293T cells treated for 12 hours with BMP2, Noggin, or Wnt3a, as labeled, then nuclear lysates were prepared as described followed by immunoprecipitation with anti-RNA pol II antibody. (A) qPCR analysis of bound exon 2 of ctnnb1 using the LightCycler 480 System for analysis. (B) HEK293T cells transfected with either scrambled siRNA or Smad4 siRNA and after 24 hours were treated for 12 hours, as labeled. Nuclear lysates were prepared as described above, followed by immunoprecipitation with anti-RNA pol II antibody and amplification of exon 2 of ctnnb1 for qPCR analysis. Results presented are from two biological replicates run in triplicate.
Smad4 loss promotes carcinogenesis in the presence of mutated tumor suppressor in vivo
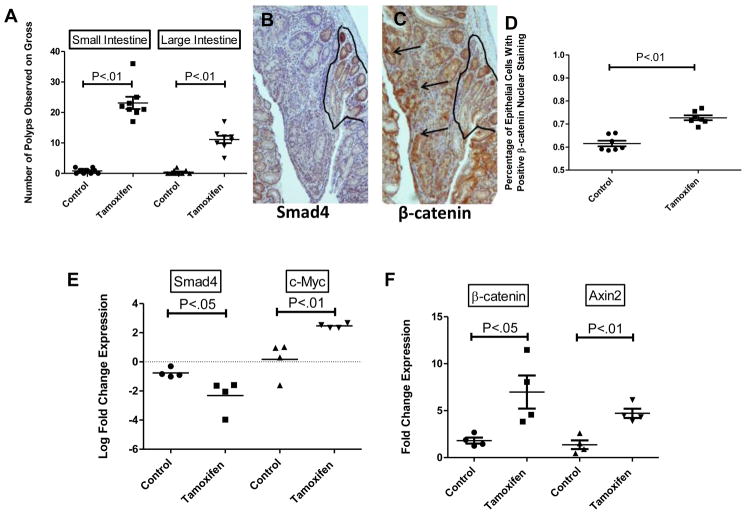
In order to further examine the biological significance of the above observations, we employed genetically defined conditional mouse models of Smad4 depletion. For these experiments, we crossed Smad4lox/lox mice27 with K19CreERT2 mice28, to generate K19CreERT2Smad4lox/lox. These mice can be induced with tamoxifen-treatment to undergo recombination of the Smad4 gene with resultant loss of Smad4 expression, specifically in the stem cell compartments of small intestine, colon, pancreas, and liver. We observed that tissue-specific, conditional loss of Smad4 in colonic epithelium results in expansion of the zone of proliferative cells within the colonic crypt but not in the formation of neoplastic lesions (Supplemental Figure 2A–C). In response to Smad4 depletion induced by tamoxifen treatment in the APCΔ1638/+ backgroundsd, mouse colonoscopy readily detected tumors in the colons of tamoxifen-treated mice by one month after treatment while the colons of vehicle-treated mice appeared entirely normal (data not shown). Smad4 depletion resulted in a ten-fold increase in the tumor burden in both the large and small intestine tumor burden by five weeks after tamoxifen treatment of (K19CreERT2Smad4lox/lox)×(APCΔ1638/+) mice as compared to vehicle-treated control mice with intact Smad4 expression (Figure 5A, n=4 for vehicle and tamoxifen groups). IHC analysis of the polyps from tamoxifen-treated mice showed that all observed adenomatous lesions (n=12) were depleted of Smad4 protein expression and exhibited abundant nuclear β-catenin staining (Figure 5B, C), whereas adenomas from vehicle-treated mice retained Smad4 (Supplemental Figure 2D, E). Since (APCΔ1638/+) mice rarely develop colonic adenomas29, we performed β-catenin immunostaining on the small intestinal polyps from both vehicle-treated and tamoxifen-treated (K19CreERT2Smad4lox/lox)×(APCΔ1638/+) mice. There was a significant (P<.01) increase in the percentage of epithelial cells that displayed nuclear localization of β-catenin by IHC in the tamoxifen-treated mice (Figure 5D and Supplemental Figure 3, black arrows identify cells with nuclear β-catenin immunoreactivity, white arrows identify cells lacking nuclear β-catenin immunoreactivity).
Figure 5. Loss of Smad4 in vivo leads to increased tumor burden, levels of β-catenin mRNA, and downstream targets of Wnt Signaling.
(A) Quantification of polyp burden observed on gross dissection in APCΔ1638/+K19CreERT2 Smad4lox/lox mice five weeks post-treatment (n=8 for both treatments). Serial sections (B, C) of APCΔ1638/+K19CreERT2 Smad4lox/lox polyp resected 5 weeks post-tamoxifen treatment. Photomicrographs were taken at 50x magnification. Scale bars equal 200μm (B) Smad4 immunostaining with outlined areas indicate corresponding Smad4-positive region. (C) β-catenin immunostaining with black arrows indicating areas of nuclear β-catenin staining. (D) Quantification of cells with positive nuclear β-catenin staining (defined as nuclear staining> cytoplasmic staining). Each data point represents the average percentage of positive cells within three high power fields for a single polyp (n=7 control polyps, n=7 tamoxifen polyps). Relative expression of (E) Smad4, c-Myc (F) β-catenin, Axin2 mRNA in vehicle (n=4) or tamoxifen (n=4) treated small bowel adenomas from APCΔ1638/+K19CreERT2Smad4lox/lox mice. Expression of mRNA is displayed as fold changes (2ΔCt) normalizing by the expression of target genes in normal adjacent tissue. Smad4 and c-Myc data are log transformed to display differences in a clear manner due to magnitude of difference. Significance determined by student’s t-tests.
Smad4 loss is associated with increased β-catenin mRNA levels and increased Wnt target gene expression in murine adenomas
qPCR analysis of mRNA from microdissected small intestinal adenomas from vehicle-treated and tamoxifen-treated mice demonstrated a 6-fold increase in β-catenin mRNA levels in adenomas depleted of Smad4 (Figure 5E) when compared with normal adjacent tissue. For adenomatous lesions in vehicle-treated mice, in which Smad4 was retained, there was only a 2-fold increase when compared to normal adjacent tissue (Figure 5F). This represents a significant (P<.05) increase in β-catenin mRNA levels when comparing lesions that have loss of Smad4 expression to those that have retained Smad4 expression. We also observed increased c-Myc (Figure 5E, P<.05) and Axin2 (Figure 5F, P<.05) mRNA expression in lesions that have loss of Smad4 expression when compared to those that retained Smad4 expression. These data indicate that Smad4 loss is associated with increased β-catenin mRNA and Wnt target gene expression levels in murine adenomas.
Murine model parallels β-catenin expression pattern observed in human colorectal cancer
To extend these observations, we examined Smad4 and β-catenin protein expression patterns in tumor tissue serial sections by immunohistochemistry (IHC) (n=24, demographics provided in Supplemental Table 2). Immunostaining for Smad4 and β-catenin was scored by grading intensity as 0-negative, 1-weak, 2-moderate, and 3-strong. Among these samples, 19 (~79%) patients retained Smad4 expression (Immunostaining score [IS]=1–3) while 5 (~21%) exhibited complete tumor cell loss of Smad4 expression (IS = 0). Among those samples exhibiting complete tumor cell Smad4 loss (IS=0), 4 (80%) had higher than median β-catenin IHC staining (IS>2) when compared with the other tumor samples. For those tumor samples retaining Smad4 expression, only 5 (~25%) samples had higher than median β-catenin immunostaining (Supplemental Table 3). Supplemental Figure 4A shows a representative colon tumor section showing predominant Smad4 nuclear staining within the glandular carcinoma cells (black arrows). The serial section (Supplemental Figure 4B) is stained for β-catenin and shows that cells with predominant nuclear staining of Smad4 (black arrows) are associated with membrane-localized β-catenin staining. In contrast, a representative section from a colon tumor lacking Smad4 expression (Figure 1E) shows Smad4 staining exclusively stromal cells (Supplemental Figure 4C) and strong nuclear and cytoplasmic β-catenin staining in the serial section (Supplemental Figure 4D). Similarly, in the tumor retaining Smad4 expression, cells lacking nuclear Smad4 staining (Supplemental Figure 4A, white arrows) correspond with strong nuclear and cytoplasmic β-catenin staining (Supplemental Figure 4B, white arrows).
Discussion
The prevailing paradigm for regulation of β-catenin levels and Wnt signaling activity has been through the ubiquitin-proteasome post-translational regulation of β-catenin protein stability. In this report, we provide experimental evidence for BMP/Smad4-mediated repression of β-catenin mRNA expression as an alternative mechanism for modulating the Wnt signal. We have shown that loss of Smad4 expression results in increased β-catenin mRNA expression and amplification of Wnt signaling both in vitro and in vivo. While other studies have provided evidence for regulatory cross-talk between TGF-β family and Wnt signaling pathways, the mechanisms by which this occurs have not been fully determined22,23. A recent example of this cross-talk implicates up-regulation of a novel Wnt target gene, BAMBI, in the inhibition of TGFβ signaling and poor prognosis in colorectal cancer patients30. Increased BAMBI expression through Wnt signaling is consistent with the concept of a positive feedback loop in the context of colorectal cancer that would suppress TGF-β pathway signaling to further amplify Wnt pathway signaling and augment tumor progression.
In our human colon cancer studies, we observe complete loss of Smad4 expression in ~25% of samples in agreement with others12,13. Our data extend the prior observations to suggest that reduced Smad4 expression correlates with increased expression of both β-catenin mRNA and protein in colorectal cancers. Several prior studies have reported that decreased Smad4 immunoreactivity in colorectal cancer is associated with worse prognosis17,31,32. In fact, based on semiquantitative IHC scoring, Stage III colorectal cancer patients whose tumors exhibited low Smad4 levels had a median overall survival of only 1.7 years, whereas patients with high Smad4 tumor levels had a >9 year median survival17. Our observations that loss of Smad4 has a profound effect on the multiplicity and progression of intestinal adenomas in genetically modified mice raises the question of whether disruption of TGF-β/BMP signaling has a role in the progression of early human intestinal polyps. Mouse models of Juvenile polyposis with BMPR1A deficient6 or Noggin overexpression33 exhibit increased and nuclear localized β-catenin and amplified Wnt signaling in the intestinal polyps. The Vogelstein model places loss of heterozygosity at 18q and loss of Smad4 in the late adenoma phase of tumor progression3. While loss of Smad4 expression has not been demonstrated in early intestinal adenomas associated with Apc mutation, Hardwick et al.34, demonstrated decreased BMP2 expression in early adenomas, which could result in a similar increase in β-catenin. It is likely that loss of Smad4 in more advanced adenomas may further amplify Wnt signaling and drive tumor progression.
Our findings support the conclusion that Smad4 exerts its effect on β-catenin expression at the level of β-catenin mRNA, with important downstream consequences in Wnt signaling. However, this effect of BMP/Smad4 signaling on β-catenin mRNA expression is not mutually exclusive with the well-described mechanism of post-translational regulation of β-catenin by APC, Axin2 and GSK3β. For example, He, et al.6, implicated BMP mediated inhibition of Wnt activity through activation of PTEN, with inhibition of PI3Kinase and Akt activity. Blockade of BMP signaling resulted in inactivation of PTEN, activation of Akt with resultant inactivation of GSK3β and accumulation of β-catenin6. Kosinski et al.2, reported that the BMP inhibitor, Gremlin1, activates Wnt/β-catenin signaling in normal intestinal epithelial cells (IEC-18). While intriguing, the non-canonical pathways implicated by He6 and Kosinski2 do not explain the significant increases in β-catenin mRNA and protein expression that we observe when Smad4 expression is lost. Interestingly, targeted disruption of Smad4 expression in the mouse hair follicle was recently shown to increase active cellular β-catenin and expression of the Wnt target35, c-Myc, without increasing Akt activity or GSK-3β phosphorylation. This is consistent with our observations but is in contrast to the observations by He, et al.6. Our findings are also consistent with recent work showing that conditional Smad4 loss within mouse dental mesenchymal cells results in increased β-catenin mRNA levels along with increased activation of canonical Wnt signaling36. Thus, mounting evidence in multiple experimental systems supports the conclusion that Smad4-dependent regulation of β-catenin mRNA expression is a biologically significant mechanism.
In the mouse, the present study supports the conclusion that induced Smad4 loss in the intestinal epithelium stimulates the progression of intestinal neoplasia. Our mouse tumorigenesis results are consistent with the two-hit hypothesis for carcinogenesis as previously described by Knudson37. Evidence put forth by Takaku, et al.16, demonstrated that combined mutations in Apc and Smad4 accelerated tumor progression in comparison to mutation in Apc alone. Notably, despite mosaic recombination and loss of Smad4 within only 20–30% of the intestinal glands in our studies, we observed loss of Smad4 expression in 100% of colonic and small intestinal adenomas examined in tamoxifen treated APCΔ1638/+K19CreERT2Smad4lox/lox mice. While in vehicle treated isogeneic mice, 100% of the adenomas, almost all of which are located in the small intestine, retained Smad4 expression. Importantly, we also observed a significant increase in expression of β-catenin mRNA, nuclear localization of β-catenin protein and increased expression of Wnt target genes Axin2 and c-Myc38 in Smad4 depleted lesions. Superinduction of c-Myc expression is particularly noteworthy in that expression can be transcriptionally activated by β-catenin/TCF but transcriptionally repressed by TGF-β/Smad signaling39,40.
Our experimental results show that inhibition of BMP signaling or loss of Smad4 can similarly augment β-catenin levels through a transcriptional mechanism, thereby increasing Wnt signaling. Consensus binding elements for NFκB, EGR1, SP1 and AP1 in the proximal β-catenin promoter have been reported41, and we have noted 12 potential consensus Smad binding elements at position within the 3500 bases 5-prime of the transcriptional start site of the ctnnb1 gene. It will be of interest to determine whether Smad4 interacts with the β-catenin promoter to modulate its transcription.
In conclusion, we have shown that the impact of increased β-catenin mRNA associated with Smad4 loss is biologically significant, especially in the setting of Apc mutation, where the augmented Wnt signaling is associated with increased Wnt/β-catenin target gene expression and an increased tumor burden. These findings provide potential insight into the important relationship between Smad4 loss in colon cancer and poor patient prognosis and late-stage disease17,31. Further investigation into the precise mechanisms underlying our observations may provide new avenues to identify therapeutic targets.
Materials and Methods
Cell culture, transfection, and FACS Sort
SW480, HCT116, and Human Embryonic Kidney (HEK) 293T (HEK293-T) cells were obtained from American Type Culture Collection (see Supplementary Methods).
Fluorescence activated cell sorting (FACS) was performed on SW480 cells transiently co-transfected with pRK-5 Smad4 (0.8ug/mL) and pEGFP (1.0ug/mL). Cells were sorted using the FACS Aria System (BD Biosciences, San Jose, CA) gated at 488nm, 48hours post-transfection. The top 30% of GFP expressing cells were selected for RNA and protein isolation.
Immunoblots
RNA Isolation, Preparation, and Analysis
Qiagen RNeasy® kit (Valencia, CA) was utilized for RNA collection. All quantitative PCR reactions were done in quadruplicate and normalized to mock transfected samples for qPCR on HEK293T or HCT116 cells. Statistical analysis of fluorescence was performed using LightCycler 480 SW Version 1.5 (Roche, Indianapolis, IN). (see Supplementary Methods).
Chromatin immunoprecipitation (ChIP) Assay
ChIP assays were performed using the EZ-Magna ChIP (Millipore Cat. #17-408), according to manufacturer instructions. Isolated DNA was subjected to quantitative PCR with a SYBR Green PCR Kit (Applied Biosystems, Carlsbad, CA) using primers for β-catenin Exon 2. (see Supplementary Methods).
Mouse models
Laser Capture Microdissection
Small intestinal polyps from APCΔ1638/+K19CreERT2Smad4lox/lox mice treated with vehicle (n=4) or tamoxifen (n=4) were frozen in OTC. Frozen sections (5μm) sections were cut and dehydrated prior to tissue isolation using the Veritas™704 laser capture microdissection device (Arcturus Engineering, Mountain View, CA). RNA was then isolated from both polyp tissue areas as well as normal adjacent tissue areas using Qiagen Micro RNeasy RNA isolation kit (Valencia, CA). Isolated RNA with RIN values >6.5 was then amplified using WT-Ovation™ Pico RNA Amplification System (NuGEN, San Carlos, CA) prior to qPCR analysis using the LightCycler 480 (Roche, Indianapolis, IN) according to manufacturer instructions. Expression of target genes in Smad4-expressing and Smad-4 deficient polyps was normalized using gene expression in paired normal adjacent tissue.
Immunohistochemistry
Supplementary Material
Acknowledgments
Grant Support:
Supported by CA069457 (R.D.B.) T32 GM07347 (T.J.F, J.J.S.), T32 CA106183 (J.J.S.), CA084239 (A.L.M.), DK052334 (R.D.B.), GM088822 (X.C.), GM088822 (R.D.B.); TL1 RR024978 (J.J.S.); CA112215 (T.J.Y., S.E.); P50CA095103 (N.G.D.); the Vanderbilt-Ingram Cancer Center Support Grant (CA068485); The GI Cancer SPORE Grant (CA095103); and the Vanderbilt Digestive Diseases Center Grant (DK058404), Clinical and Translational Science Award to Vanderbilt (UL1 RR024975); Other sources of funding: Society of University Surgeons-Ethicon Scholarship Award (J.J.S.)
We thank Drs. Mark deCaesteker, Robert Coffey, Ethan Lee, Vivian Siegel, and Chris Williams for providing advice, reagents and for reviewing the manuscript in advance of submission. We are especially grateful for Jenny Zi, Jalal Hamaamen, John Neff, and Christian Kis for their technical assistance. We acknowledge the services of the Vanderbilt Immunohistochemistry (IHC) Core in processing of specimens, the Vanderbilt Functional Genomics Science Resource and the VMC Flow Cytometry Shared Resource. Vanderbilt’s IHC Core is supported by the funding from the Vanderbilt Ingram Cancer Center Support Grant. The Vanderbilt Functional Genomics Sciences Resource is supported by the Vanderbilt Ingram Cancer Center Support Grant and the Vanderbilt Vision Center (P30 EY08126). The VMC Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center and the Vanderbilt Digestive Disease Research Center. We also thank Jarred Tanksley and Moorthy Krishnaan for their technical guidance regarding the cell lines. We gratefully acknowledgeDr. Martin Heslin from the University of Alabama, Birmingham School of Medicine, who contributed human tumor tissue samples.
Abbreviations
- IHC
Immunohistochemistry
- HEK293T
Human Embryonic Kidney cells
- APC
Adenomatous Polyposis
- BMP
Bone Morphogenetic Protein
- TGF-β
Transforming Growth Factor –β
- R-Smads
Receptor associated Smads
- K19
Cytokeratin 19
Footnotes
Disclosures: The authors have nothing to disclose
GEO accession number: GSE17538
Author Contributions: T.J.F., J.J.S., M.K.W., J.T.R., A.L.M., N.G.D. and R.D.B. are responsible for the design and execution of the study and interpretation of the results. R.D.B. supervised the laboratory experiments and wrote the paper with J.J.S., N.G.D., and T.J.F. J.J.S. organized, analyzed and coordinated the clinical and genomic information from all sites. Handling, processing and integrity of the tissue samples were supervised by N.G.D., S.A.E., T.J.Y., and J.J.S. M.K.W. confirmed the pathologic stages of the VMC and UAB samples. X.C. provided statistical support, advice and analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006 Nov 3;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Kosinski C, Li VS, Chan AS, et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci U S A. 2007 Sep 25;104(39):15418–15423. doi: 10.1073/pnas.0707210104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996 Oct 18;87(2):159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 4.Vermeulen L, De Sousa EMF, van der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. May;12(5):468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 5.Brabletz S, Schmalhofer O, Brabletz T. Gastrointestinal stem cells in development and cancer. J Pathol. 2009 Jan;217(2):307–317. doi: 10.1002/path.2475. [DOI] [PubMed] [Google Scholar]
- 6.He XC, Zhang J, Tong WG, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004 Oct;36(10):1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 7.Perreault N, Auclair BA, Benoit YD, Rivard N, Mishina Y. Bone morphogenetic protein signaling is essential for terminal differentiation of the intestinal secretory cell lineage. Gastroenterology. 2007 Sep;133(3):887–896. doi: 10.1053/j.gastro.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 8.Li LH, He XC, Zhang JW, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nature Genetics. 2004 Oct;36(10):1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 9.Sato T, van Es JH, Snippert HJ, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011 Jan 20;469(7330):415. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grady WM, Myeroff LL, Swinler SE, et al. Mutational inactivation of transforming growth factor beta receptor type II in microsatellite stable colon cancers. Cancer Res. 1999 Jan 15;59(2):320–324. [PubMed] [Google Scholar]
- 11.Kodach LL, Bleuming SA, Musler AR, et al. The bone morphogenetic protein pathway is active in human colon adenomas and inactivated in colorectal cancer. Cancer. 2008 Jan 15;112(2):300–306. doi: 10.1002/cncr.23160. [DOI] [PubMed] [Google Scholar]
- 12.Miyaki M, Iijima T, Konishi M, et al. Higher frequency of Smad4 gene mutation in human colorectal cancer with distant metastasis. Oncogene. 1999 May 20;18(20):3098–3103. doi: 10.1038/sj.onc.1202642. [DOI] [PubMed] [Google Scholar]
- 13.Reinacher-Schick A, Baldus SE, Romdhana B, et al. Loss of Smad4 correlates with loss of the invasion suppressor E-cadherin in advanced colorectal carcinomas. J Pathol. 2004 Apr;202(4):412–420. doi: 10.1002/path.1516. [DOI] [PubMed] [Google Scholar]
- 14.Howe JR, Roth S, Ringold JC, et al. Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science. 1998 May 15;280(5366):1086–1088. doi: 10.1126/science.280.5366.1086. [DOI] [PubMed] [Google Scholar]
- 15.Howe JR, Sayed MG, Ahmed AF, et al. The prevalence of MADH4 and BMPR1A mutations in juvenile polyposis and absence of BMPR2, BMPR1B, and ACVR1 mutations. J Med Genet. 2004 Jul;41(7):484–491. doi: 10.1136/jmg.2004.018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin MF, Taketo MM. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell. 1998 Mar 6;92(5):645–656. doi: 10.1016/s0092-8674(00)81132-0. [DOI] [PubMed] [Google Scholar]
- 17.Alazzouzi H, Alhopuro P, Salovaara R, et al. SMAD4 as a prognostic marker in colorectal cancer. Clin Cancer Res. 2005 Apr 1;11(7):2606–2611. doi: 10.1158/1078-0432.CCR-04-1458. [DOI] [PubMed] [Google Scholar]
- 18.Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997 Mar 21;275(5307):1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 19.Woodford-Richens KL, Rowan AJ, Gorman P, et al. SMAD4 mutations in colorectal cancer probably occur before chromosomal instability, but after divergence of the microsatellite instability pathway. Proc Natl Acad Sci U S A. 2001 Aug 14;98(17):9719–9723. doi: 10.1073/pnas.171321498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markowitz S, Wang J, Myeroff L, et al. Inactivation of the Type-Ii Tgf-Beta Receptor in Colon-Cancer Cells with Microsatellite Instability. Science. 1995 Jun 2;268(5215):1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 21.Molenaar M, vandeWetering M, Oosterwegel M, et al. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996 Aug 9;86(3):391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 22.Shiou SR, Singh AB, Moorthy K, et al. Smad4 regulates claudin-1 expression in a transforming growth factor-beta-independent manner in colon cancer cells. Cancer Res. 2007 Feb 15;67(4):1571–1579. doi: 10.1158/0008-5472.CAN-06-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian X, Du H, Fu X, Li K, Li A, Zhang Y. Smad4 restoration leads to a suppression of Wnt/beta-catenin signaling activity and migration capacity in human colon carcinoma cells. Biochem Biophys Res Commun. 2009 Mar 13;380(3):478–483. doi: 10.1016/j.bbrc.2009.01.124. [DOI] [PubMed] [Google Scholar]
- 24.ten Dijke P, Korchynskyi O. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. Journal of Biological Chemistry. 2002 Feb 15;277(7):4883–4891. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- 25.Beck SE, Jung BH, Fiorino A, et al. Bone morphogenetic protein signaling and growth suppression in colon cancer. Am J Physiol-Gastr L. 2006 Jul;291(1):G135–G145. doi: 10.1152/ajpgi.00482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beck SE, Jung BH, Fiorino A, et al. Bone morphogenetic protein signaling and growth suppression in colon cancer. Am J Physiol Gastrointest Liver Physiol. 2006 Jul;291(1):G135–145. doi: 10.1152/ajpgi.00482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bardeesy N, Cheng KH, Berger JH, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006 Nov 15;20(22):3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Means AL, Xu Y, Zhao A, Ray KC, Gu G. A CK19(CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis. 2008 Jun;46(6):318–323. doi: 10.1002/dvg.20397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fodde R, Edelmann W, Yang K, et al. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8969–8973. doi: 10.1073/pnas.91.19.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fritzmann J, Morkel M, Besser D, et al. A colorectal cancer expression profile that includes transforming growth factor beta inhibitor BAMBI predicts metastatic potential. Gastroenterology. 2009 Jul;137(1):165–175. doi: 10.1053/j.gastro.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 31.Isaksson-Mettavainio M, Palmqvist R, Forssell J, Stenling R, Oberg A. SMAD4/DPC4 expression and prognosis in human colorectal cancer. Anticancer Res. 2006 Jan-Feb;26(1B):507–510. [PubMed] [Google Scholar]
- 32.Tanaka T, Watanabe T, Kazama Y, et al. Loss of Smad4 protein expression and 18qLOH as molecular markers indicating lymph node metastasis in colorectal cancer--a study matched for tumor depth and pathology. J Surg Oncol. 2008 Jan 1;97(1):69–73. doi: 10.1002/jso.20896. [DOI] [PubMed] [Google Scholar]
- 33.Haramis AP, Begthel H, van den Born M, et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004 Mar 12;303(5664):1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 34.Hardwick JCH, Van den Brink GR, Bleuming SA, et al. Bone morphogenetic protein 2 is expressed by, and acts upon, mature epithelial cells in the colon. Gastroenterology. 2004 Jan;126(1):111–121. doi: 10.1053/j.gastro.2003.10.067. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Wang L, Yang X. Disruption of Smad4 in mouse epidermis leads to depletion of follicle stem cells. Mol Biol Cell. 2009 Feb;20(3):882–890. doi: 10.1091/mbc.E08-07-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Huang X, Xu X, et al. SMAD4-mediated WNT signaling controls the fate of cranial neural crest cells during tooth morphogenesis. Development. 2011 Apr 13; doi: 10.1242/dev.061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knudson AG. Mutation and Cancer - Statistical Study of Retinoblastoma. P Natl Acad Sci USA. 1971;68(4):820. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costantini F, Jho EH, Zhang T, Domon C, Joo CK, Freund JN. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Molecular and Cellular Biology. 2002 Feb;22(4):1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinzler KW, He TC, Sparks AB, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998 Sep 4;281(5382):1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann FM, Lim SK. Smad4 cooperates with lymphoid enhancer-binding factor 1/T cell-specific factor to increase c-myc expression in the absence of TGF-beta signaling. P Natl Acad Sci USA. 2006 Dec 5;103(49):18580–18585. doi: 10.1073/pnas.0604773103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nollet F, Berx G, Molemans F, van Roy F. Genomic organization of the human beta-catenin gene (CTNNB1) Genomics. 1996 Mar 15;32(3):413–424. doi: 10.1006/geno.1996.0136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.