Abstract
How receptors catalyze exchange of GTP for GDP bound to the Gα subunit of trimeric G proteins is not known. One proposal is that the receptor uses the G protein's βγ heterodimer as a lever, tilting it to pull open the guanine nucleotide binding pocket of Gα. To test this possibility, we designed a mutant Gα that would bind to βγ in the tilted conformation. To do so, we excised a helical turn (four residues) from the N-terminal region of αs, the α subunit of GS, the stimulatory regulator of adenylyl cyclase. In the presence, but not in the absence, of transiently expressed β1 and γ2, this mutant (αsΔ), markedly stimulated cAMP accumulation. This effect depended on the ability of the coexpressed β protein to interact normally with the lip of the nucleotide binding pocket of αsΔ. We substituted alanine for an aspartate in β1 that binds to a lysine (K206) in the lip of the α subunit's nucleotide binding pocket. Coexpressed with αsΔ and γ2, this mutant, β1-D228A, elevated cAMP much less than did β1-wild type; it did bind to αsΔ normally, however, as indicated by its unimpaired ability to target αsΔ to the plasma membrane. We conclude that βγ can activate αs and that this effect probably involves both a tilt of βγ relative to αs and interaction of β with the lip of the nucleotide binding pocket. We speculate that receptors use a similar mechanism to activate trimeric G proteins.
Located on the cytoplasmic face of the plasma membrane, heterotrimeric G proteins relay extracellular signals (hormones, neurotransmitters, photons, and odorants) from transmembrane receptors to effector enzymes and ion channels that mount appropriate cellular responses (1). G protein activation is initiated by the receptor-stimulated replacement by GTP of GDP bound to the α subunit of the G protein trimer; bound GTP induces Gα-GTP to dissociate from the Gβγ heterodimer, generating two signals for regulation of downstream effectors. Hydrolysis of GTP by Gα and reassociation of Gα-GDP with Gβγ terminate these signals. The molecular mechanism that releases bound GDP, the rate-limiting step in transmitting the signal from receptor to G protein trimer (2), remains poorly understood.
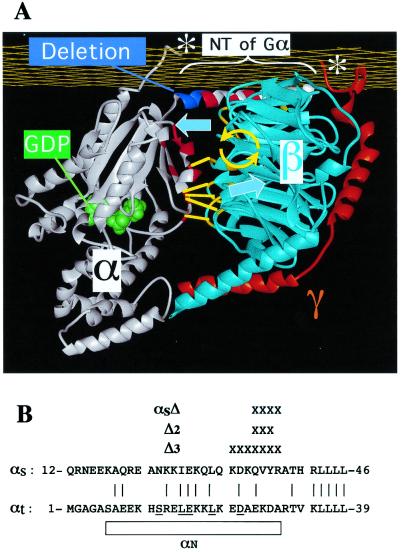
Possible molecular explanations of GDP release must take account of the 30-Å distance, in crystal structures of G protein trimers (3, 4), between bound GDP and surfaces of the trimer that are known to interact with receptors (1, 5, 6)—a distance too long for loops of many G protein-coupled receptors (GPCRs) to touch Gα near its guanine nucleotide binding pocket (5, 7). One explanation (8) of this “action-at-a-distance,” depicted in Fig. 1A, proposes that receptors use the βγ dimer as a lever to pry open the nucleotide binding pocket. The lever hypothesis depends on the fact that Gβγ interacts with two distinct surfaces of Gα. One of these is located on an N-terminal α-helix of Gα; one side of the helix binds Gβ, whereas the other is thought to interact with the cytoplasmic surface of the plasma membrane. The other Gα surface that contacts Gβ involves two regions of Gα that are called Switch 1 and Switch 2 (Sw1 and Sw2) because their conformations differ dramatically in the protein's GDP- and GTP-bound conformations. Sw1 connects the α-helical domain of Gα to its Ras-like domain; Sw2 includes an α-helix and the loop preceding it. In addition to contacting Gβ—and most important for the lever hypothesis—Sw1, along with the loop and first part of the α-helix of Sw2, forms a lip for a potential exit route for GDP from the nucleotide binding pocket (Fig. 1A). According to the lever hypothesis, a modest tilt of Gβ relative to Gα-GDP would use this second interaction surface to pull open the lip of the nucleotide binding pocket.
Figure 1.
Structure of the G protein trimer and N-terminal deletions of Gα used to test the lever hypothesis. (A) Residues of Gα subunit (white) that interact with Gβ (light blue) in the trimer are shown in red, and the residues of Gβ that contact Gα are yellow. The α-helical turn that was deleted from the N-terminal helix of αs to form αsΔ is colored dark blue. The carboxyl termini of Gα and Gγ (white asterisks) interact with the receptor and presumably with the cytoplasmic face of the plasma membrane (yellow grid). According to the lever hypothesis, the GPCR (not shown) uses these carboxyl termini to tilt Gβγ relative to αs; the postulated movement of Gβγ, indicated by the large blue arrows, induces a tilt (yellow arrows) about an axis parallel to the plasma membrane. Interactions of β with the lip of Gα's nucleotide binding pocket (short yellow lines) allow this tilt to pull red residues in the lip away from the GDP binding pocket, so that GDP (green) can exit. The deletion in αsΔ was designed to induce a similar tilt of Gβγ relative to Gα and thus to promote βγ-dependent activation of αsΔ. The trimer structure is based on coordinates of the crystal structure of transducin (3). (B) Alignment of the N termini of αs and αt, showing the residues removed (X) to form αsΔ and two other deletion mutants, Δ2 and Δ3. Residues that are identical or conserved between the two Gα proteins are connected by a vertical line. The N-terminal helix (αN) of αt in the transducin trimer is indicated by the rectangle, and αt residues that interact with β in the crystal structure (3) are underscored .
Several observations are in keeping with the lever hypothesis: (i) Gβγ is required for the photoreceptor, rhodopsin, to activate its trimeric G protein target, Gt (9); (ii) an activated GPCR could induce the postulated tilt by inducing a small movement toward one another of the two parts of the G protein trimer, the C-terminal 10 residues of Gα and the prenylated C terminus of Gγ, that are known to interact with the active forms of rhodopsin and other GPCRs (5, 10, 11); (iii) alanine substitutions for several Gβ residues located at its interface with Sw1 and Sw2 of Gαt impair activation of Gt by rhodopsin but are not required for strong association between Gβγ and αt (12); (iv) guanine nucleotide exchange factors for monomeric GTPases [elongation factor Tu, Ras, ADP-ribosylation factor (ARF)-1, and Rac1] open the nucleotide binding pockets of their targets by interacting with and distorting their Sw1 and Sw2 regions (13–17), just as βγ is postulated to do in receptor-activated G protein trimers.
To mimic the hypothetical levering action of receptors, we designed a mutant Gα that should bind preferentially to βγ in a tilted conformation. To do so, we excised four residues (one helical turn) from the N-terminal α-helix of a Gα (Fig. 1A). If removal of these residues preserves a stable association between βγ and the mutant Gα, membrane-apposed portions of the two subunits will be pulled ≈6 Å closer to one another, inducing the relative tilt that is postulated to trigger GDP release. As predicted, the effect of the transiently expressed mutant Gα is markedly increased by coexpressed Gβγ, thereby strengthening the Gβγ lever hypothesis for Gα activation.
Materials and Methods
Construction of Gαs and β1 Mutants.
A cDNA encoding αs-wild type (WT) with an internal hemagglutinin (HA) epitope (18) was subcloned between the HindIII and XbaI sites of pcDNA3, by using PCR. cDNAs in the same vector, encoding β1-WT and γ2-WT, including a Glu-Glu (epitope for monoclonal antibody) and a myc epitope, respectively, attached to the N terminus, were also previously described (19). Deletions were introduced by Kunkel site-directed mutagenesis (Muta-Gene in vitro Mutagenesis Kit, Bio-Rad), and single site mutations were generated by using PCR-based mutagenesis (Quickchange site-directed mutagenesis kit, Stratagene).
Cell Culture and Transfection.
COS-7 and HEK-293 cells were maintained in DMEM H21 containing 10% FCS. COS-7 cells were transiently transfected by the adenovirus DEAE-dextran method (20) with pcDNA3 containing DNA encoding either HA-tagged mutant or WT αs and cotransfected with DNA for epitope-tagged β1 or γ2. HEK-293 cells were transfected by the calcium phosphate method (CalPhos Maximizer transfection kit, CLONTECH).
Membrane Preparation and Immunoblotting.
Membranes were prepared from one 150-mm culture dish containing 20 × 106 cells, as described (20). Cells were washed once with 20 ml PBS (Ca2+- and Mg2+-free) containing 10 mM EDTA, 4 mM EGTA, 40 μg/ml bacitracin, 20 μg/ml aprotinin, and 1 mM PMSF. Cells were then scraped off the plate and resuspended in 25 ml of the same buffer by pipetting up and down several times and collected by centrifugation for 5 min at 1000 rpm. The cell pellet was resuspended in 1 ml ice-cold lysis buffer (50 mM Tris⋅HCl, pH 7.8/1 mM EDTA/1 mM DTT/20 μg/ml aprotinin/0.5 mM PMSF) and homogenized by passing the suspension 20 times although a 27 1/2-gauge needle. Cellular debris was discarded by centrifugation twice at 3000 rpm for 10 min at 4°C. The supernatant fraction was then centrifuged at 60,000 rpm for 30 min at 4°C in a Beckman fixed angle TL100.3 ultracentrifuge rotor, and the membranes were recovered in the pellet fraction. Membranes were resuspended in 200 μl resuspension buffer (20 mM Hepes, pH 8.0/50 mM NaCl/10 mM MgCl2/1 mM EDTA/1 mM β-mercaptoethanol/10 μM GDP/proteases inhibitors) by using a 27 1/2-gauge needle, and then diluted to a concentration of 3.0 mg/ml in resuspension buffer as described (21). Lubrol was added to a final concentration of 0.64%, and samples were agitated by rotation for 1 h at 4°C on a rotative system. Samples were then centrifuged at 40,000 rpm for 30 min at 4°C, and the Lubrol-soluble fractions were mixed with 6× loading buffer (300 mM Tris⋅HCl, pH 7.0/600 mM DTT/12% SDS/0.6% bromophenol blue/60% glycerol) and frozen at −80°C for later analysis by Western blotting. Aliquots of each were subjected to SDS/PAGE by using 12% polyacrylamide (Criterion Precast System, Bio-Rad), transferred to poly(vinylidene difluoride) (PVDF; Immobilon-P, Millipore) by using a Criterion Blotter (Bio-Rad), and probed with 12CA5 monoclonal antibody (0.6 μg/ml). Proteins were visualized by chemiluminescence (femtoLUCENT, Chemicon), and quantified with the Storm 860 PhosphorImager (Molecular Dynamics) by using enhanced chemifluorescence (ECF) Western blotting reagent (Amersham Pharmacia).
Immunofluorescence.
Forty-eight hours after transfection, HEK-293 cells were plated onto glass coverslips, fixed in 3.7% formaldehyde, and permeabilized in 1% Triton X-100, both in PBS, as described (22). Localization of the HA tag associated with recombinant mutant or WT αs was assessed by using mAb 12CA5 at 12 μg/ml and donkey anti-mouse fluorescein isothiocyanate conjugate at 1 μg/ml.
cAMP Assay.
cAMP accumulation in intact cells was assayed as described (23, 24). Briefly, 24 h after transfection, cells were replated in 24-well plates at 1.5 × 105 cells/well and labeled with [3H]adenine (4 μCi/ml, Amersham Pharmacia) for an additional 24 h. Cells were washed once with Hepes-buffered DMEM and then immediately broken by addition of a cold solution of 5% trichloroacetic acid plus 1 mM each of ATP and cAMP, for 30 min at 4°C. cAMP and ATP fractions were resolved on columns, and cAMP accumulation estimated by determining the ratio of cAMP radioactivity to the sum of radioactivity of cAMP and ATP.
Results
We tested the lever hypothesis by transient coexpression of mutant Gα and Gβγ in COS-7 cells. We chose to study GS, the stimulatory regulator of adenylyl cyclase, rather than other trimeric G proteins for two reasons: (i) activation of mutant αs can be conveniently assessed in intact cells because cAMP accumulation is readily measured; (ii) because our strategy required coexpression of βγ with mutant α, it was important to choose a cellular response, cAMP accumulation, that is stimulated by αs-GTP rather than by free βγ. In addition, αs-WT binds GDP with low affinity in vitro (25, 26), relative to other Gα subunits, and this affinity is increased by association with βγ; thus we imagined that a properly designed αs mutant (hereafter called αsΔ) might be more amenable than other Gα proteins to activation by Gβγ.
In shortening the N-terminal α-helix of αs, we avoided extreme N-terminal residues, which are essential for lipid modification and for association with the plasma membrane (27–29). To create αsΔ, we removed from αs four residues, 35-QVYR-38, which are cognate to four residues in the N terminus of αt (24-EKDA-27; Fig. 1B). We chose this sequence because it corresponds to positions of residues in αt that do not contact βγ (3); moreover, mutational replacement of αt residues at these positions by alanine failed to reduce apparent affinity for βγ (6). Deletion of these residues should remove one turn of the α-helix, shortening the N terminus of αs by about 6.0 Å.
Gβγ Increases cAMP Elevation in Cells Coexpressing αsΔ.
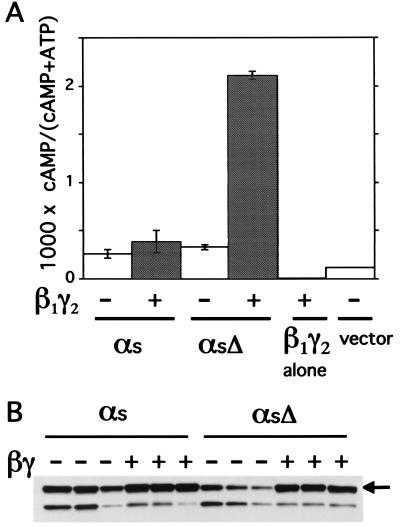
Transient coexpression of αsΔ with β1 and γ2 stimulates cAMP accumulation in COS-7 cells to a level at least 5-fold higher than in controls transfected with empty vector, αsΔ alone, αs-WT alone, αs-WT plus βγ, or βγ alone (Fig. 2A). Thus, together, overexpressed αsΔ and βγ induce cAMP accumulation, but neither protein by itself does so. In other experiments (not shown), two αs deletion mutants similar to αsΔ also produced βγ-dependent increases in cAMP accumulation; these mutations deleted either three or seven residues from the N-terminal α helix of αs (Fig. 1B). We do not know why endogenous Gβγ in COS-7 cells does not suffice to elevate cAMP when αsΔ is expressed alone. One possibility is that endogenous βγ is largely associated with endogenous Gα (or other proteins) and therefore is unavailable for association with αsΔ. Alternatively, in the absence of excess Gβγ, αsΔ may be thermally labile or poorly targeted to the plasma membrane.
Figure 2.
Gβγ increases cAMP accumulation in COS-7 cells coexpressing αsΔ, but not αs-WT, and increases the amount of both WT and mutant proteins in membrane fractions. (A) cAMP accumulation. COS-7 cells were transiently transfected with control plasmid (pcDNA3) or plasmids encoding αs-WT or αsΔ, with or without β1 and γ2, as indicated. cAMP was measured as indicated in Materials and Methods. Values represent means ± SD of three independent transfections. This set of results is representative of three or more additional experiments. (B) Immunoblots showing relative amounts of HA-tagged recombinant αs-WT or αsΔ in Lubrol extracts of particulate fractions of cells expressing the indicated αs construct, with or without coexpressed βγ. For each condition, immunoblots representing three independent transfections are shown. The arrow indicates bands corresponding to intact αs; the lower band probably corresponds to a proteolytic fragment.
The latter possibility suggested the disturbing notion that the βγ-dependent cAMP increase in αsΔ-expressing cells exceeds that induced by αs-WT (plus or minus βγ) simply because βγ recruits much more αsΔ than αs-WT to the plasma membrane. To test this notion, we assessed amounts of recombinant αsΔ or αs-WT found in membrane fractions of COS-7 cells and solubilized in Lubrol, a non-ionic detergent. The detergent serves to separate normally folded recombinant αs from αs that may be aggregated and nonfunctional (21). Immunoblots showed that β1γ2 increased membrane content of Lubrol-soluble αsΔ more than that of αs-WT, but that in cells coexpressing β1γ2, Lubrol-soluble fractions contained similar amounts of the two proteins (Fig. 2B). Specifically, in three independent transfections, intensities of immunoblot signals (in arbitrary PhosphorImager units) of αsΔ or αs-WT were 2.8 ± 0.1 or 2.9 ± 0.2, respectively, in the presence of β1γ2, and 0.8 ± 0.2 and or 2.1 ± 0.4 in its absence. The ability of Gβγ to increase membrane content of αsΔ indicates that the mutant, like αs-WT, can associate with Gβγ. The equivalent membrane amounts of αsΔ and αs-WT in the presence of β1γ2 indicate that the much greater relative stimulation of cAMP accumulation by the mutant does not simply reflect a higher concentration (relative to αs-WT) in membranes.
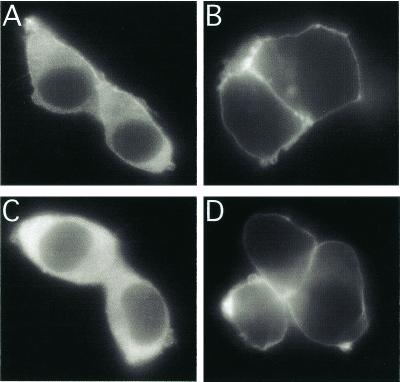
The effect of β1γ2 on membrane content of αsΔ reflects primarily the ability of Gβγ to target and to stabilize Gα at the plasma membrane (22, 30, 31), as shown by patterns of αsΔ immunofluorescence in transiently expressing HEK-293 cells (Fig. 3). In the absence of β1γ2, αsΔ was seen in cytoplasm as well as the plasma membrane; in the presence of β1γ2, however, αsΔ appeared to associate almost exclusively with the plasma membrane—as did αs-WT. Thus, the β1γ2-dependent increase in the membrane content of αsΔ (Fig. 2D) is probably due to membrane targeting by Gβγ rather than to stabilization of αsΔ against denaturation and proteolysis.
Figure 3.
Cellular localization of WT and mutant αs. HEK-293 cells transiently expressed αs-WT (A and B) or αsΔ (C and D), in the absence (A and C) or presence (B and D) of coexpressed β1γ2. Fixation and immunofluorescent detection of HA epitopes incorporated into the recombinant αs proteins are described in Materials and Methods.
β1 Mutations Reduce the Ability of Gβγ To Activate αsΔ.
The immunofluorescence results suggested an additional possibility to explain Gβγ-induced cAMP accumulation in cells expressing αsΔ: that is, Gβγ merely targets to the plasma membrane a mutant αs with intrinsic constitutive activity. We therefore examined effects of β1 mutations designed to impair its interaction with αsΔ (Fig. 4). The phenotype of one β1 mutant, D228A, ruled out an explanation based on recruitment of a constitutively active αsΔ to membranes; this β1 mutant was defective in its ability to stimulate cAMP accumulation but targeted αsΔ to the membrane normally.
Figure 4.
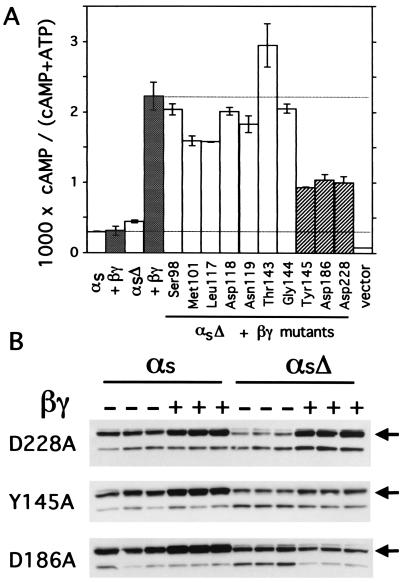
Effects of Gβ mutants on activity and membrane amounts of αsΔ. (A) cAMP accumulation was measured (as described in Materials and Methods) in COS-7 cells expressing the indicated αs construct (WT or αsΔ), with βγ-WT (filled columns) or γ2 plus the β1 mutant indicated; β1 mutants activated αsΔ normally (open columns) or weakly (cross-hatched columns). Columns represent means ± SD of three independent transfections. This set of results is representative of two additional experiments. (B) Amounts of recombinant αs-WT or αsΔ detected by the 12CA5 antibody in Lubrol extracts from membranes of COS-7 cells expressing γ2, the indicated αs construct, and the indicated mutant β1. The arrows indicate bands corresponding to intact αs; the lower bands probably correspond to a proteolytic fragment.
We tested 10 β1 mutants in each of which alanine replaced an amino acid whose side chain contacts Sw1 or Sw2 of Gαt in crystals of the Gt trimer (3). These mutations should not impair interaction with Gγ, because the substituted amino acids are located on the Gα-facing surface of β1, opposite to the surface that interacts with Gγ. When coexpressed with γ2 and αsΔ, three of the ten β1 mutants activated cAMP accumulation quite weakly—less than 30% as strongly as β1-WT; the other seven mutants supported normal (or near-normal) stimulation of cAMP accumulation (Fig. 4A).
To determine whether the three loss-of-function β1 mutants interacted with αsΔ, we assessed their abilities to target αsΔ to the Lubrol-extractable fraction of COS-7 membranes (Fig. 4B). Two of these mutants properly targeted αs-WT to the membrane fraction, but targeted αsΔ only weakly (Y145A) or not at all (D186A). In contrast, the β1-D228A mutant appeared to target αsΔ in a manner similar to that observed with β1-WT, and to an extent similar to that observed with αs-WT. Thus, the D228A mutation shows that substitution of a single amino acid can impair the ability of β1 to activate αsΔ without altering its targeting to membranes.
Comparison of αsΔ to GTPase-Deficient αs Mutants.
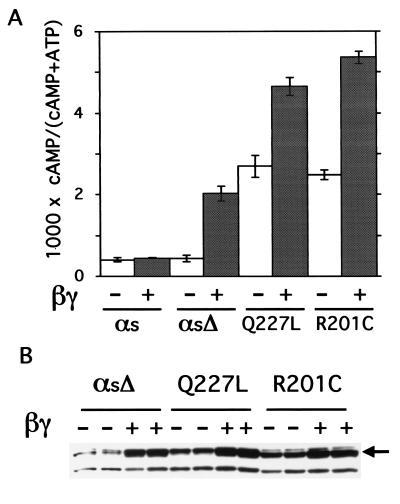
Stimulation of cAMP accumulation by αsΔ was robust and comparable to that induced by two previously described (32–34) GTPase-deficient αs mutants, Q227L and R201C (Fig. 5). In the presence of transfected Gβγ, αsΔ caused ≈75% of the cAMP elevation seen with the GTPase mutants alone (Fig. 5A). Coexpression of Gβγ also increased strikingly the cAMP accumulation induced by the GTPase mutants, although the fold increase because of Gβγ was considerably less than that seen with αsΔ (1.8- to 2.1-fold for the GTPase mutants vs. 4.7-fold for αsΔ). Because it seems unlikely that Gβγ directly activates GTPase-deficient Gα proteins, we asked whether βγ increased targeting of these proteins to the Lubrol-extractable fraction of COS-7 membranes (Fig. 5B). Immunoblots from cells coexpressing β1γ2 showed membrane amounts of the GTPase-deficient mutants equal to or greater than those of αsΔ in parallel transfections (Fig. 5B). Overall, normalizing for amounts of protein apparently targeted to the membrane, we estimate that βγ-activated αsΔ molecules stimulate adenylyl cyclase about half as well as do GTPase-deficient αs mutants studied here.
Figure 5.
Comparison of cAMP accumulation stimulated by αsΔ plus βγ vs. that stimulated by GTPase-deficient αs mutants. (A) cAMP accumulation by αs-WT (αs), αsΔ, αs-Q227L, and αs-R201C, in the absence or presence of coexpressed βγ. Transfections and cAMP assays were as described in Materials and Methods. Values represent means ± SD of three independent transfections. This set of results is representative of two additional experiments. (B) Amounts of recombinant αs constructs detected by the 12CA5 antibody in Lubrol extracts from membranes of COS-7 cells expressing β1γ2 and the indicated αs construct. The arrow indicates bands corresponding to intact αs; the lower band probably corresponds to a proteolytic fragment.
Discussion
In contrast to previously described gain-of-function αs mutants (32–35), αsΔ is inactive as a stimulator of cAMP accumulation in the absence of coexpressed Gβγ. Each of the previously described mutants is constitutively active because it hydrolyzes GTP slowly (32–34) or because (like normal αs stimulated by a GPCR) it releases GDP at a much faster rate, allowing it to associate more frequently with GTP (35). In both cases, increased activity reflects residence of the mutant αs in its GTP-bound state for a larger proportion of time than that seen with αs-WT. From the results presented here, we infer that αsΔ similarly spends more of its time in the GTP-bound state, but only when coexpressed Gβγ elevates the rate at which it releases GDP and becomes available for binding GTP.
βγ-Dependent Activation of αsΔ Is Consistent with the Lever Hypothesis.
As outlined below, the phenotype of the β1-D228A mutant justifies a more specific and surprising inference: that βγ increases the proportion of GTP-bound αsΔ by increasing the rate at which αsΔ releases bound GDP, rather than by inhibiting its hydrolysis of GTP. This inference is surprising because Gβγ substantially slows spontaneous GDP release from αs-WT (29, 36). In doing so, Gβγ is thought to act by contacting and stabilizing Sw1 and Sw2 in positions that keep the lip of the GDP binding pocket firmly closed. In contrast, the βγ lever hypothesis (Fig. 1A) proposes that βγ acts on αsΔ by precisely the opposite mechanism: that is, by pulling and deforming the lip of the GDP binding pocket to open an exit route for GDP.
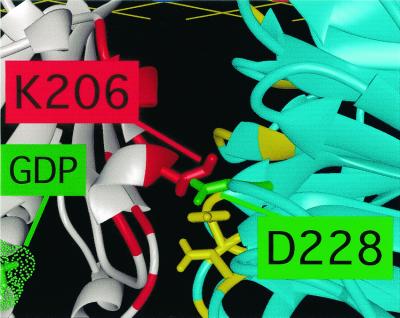
According to the lever hypothesis, Gβγ can open the nucleotide binding pocket only if it binds to the lip of the binding pocket tightly enough to pull it open—that is, only if its contacts with Sw1 and Sw2 remain intact. A mutation that sufficiently weakens the interaction between the surface of β and either switch region will inevitably prevent activation of αsΔ. This is exactly the phenotype produced by alanine substitution for D228 in β1 (Fig. 4). Crystal structures of two G protein trimers (3, 4) show that the carboxylate of D228 participates in a highly conserved ionic interaction with the ɛ-amino group of a conserved lysine in the Sw2 region of Gα (Fig. 6). This lysine (K206 in αt, K233 in αs) is located in the lip of the GDP binding pocket, precisely where its interaction with D228 of β1 can help to mediate opening of the pocket by a tilted Gβγ.
Figure 6.
Close-up view of part of the interaction of Gβ with Gα in the G protein trimer, based on coordinates of the crystal structure of transducin (3). Gβ is light blue, Gα white; as in Fig. 1, positions of interacting residues are indicated in red (Gα) or yellow (Gβ). The β1 side chains, whose replacement by alanine reduced activation of αsΔ (see Fig. 4), are represented as sticks. The carboxylate of D228 in β1 (green) forms an ionic bond with the ɛ-amino group of a lysine residue in Sw2 of Gα (K206 in αt, K233 in αs).
Does Gβγ Normally Act as a Lever To Mediate Gα Activation by GPCRs?
We listed above several observations that fit with the idea that GPCRs use βγ as a lever to open the GDP-binding site; these include well-documented interactions of the C termini of both Gα and Gγ with GPCRs (5, 10, 11), the fact that guanine nucleotide exchange factors act directly on Sw1 and Sw2 regions of their small GTPase targets (13–17), and the fact that several alanine substitutions in β1 inhibited activation of Gt by rhodopsin (12). Indeed, in the latter study, one of the β1 mutations that blocked Gt activation by rhodopsin was D228A, which similarly inhibited activation of αsΔ by βγ in our experiments (Fig. 4). The high degree of conservation of residues at the interface between β and the Sw1 and Sw2 regions of Gα proteins is also in keeping with the βγ lever hypothesis; of the 12 β1 residues that interact with Sw1 or Sw2, 10 are strictly conserved in all isoforms of Gβ (37). The latter include the aspartate residue corresponding to D228 in β1; its Gα partner, K206 in αt, is also virtually invariant in all Gα sequences (3).
We recognize, of course, that these observations and our experiments do not directly test the βγ lever hypothesis as a mechanism for explaining GPCR activation. We did perform one more direct test, but the result was not conclusive. Thus, we reasoned that if the Gβγ lever hypothesis were correct for GPCRs in vivo, β1-D228A mutant might exert a dominant negative effect in intact cells, preventing GPCR activation of normal Gα proteins. In transient expression experiments, however, β1-D228A (with or without coexpressed γ2) did not inhibit hormonal stimulation of cAMP accumulation (results not shown). This negative result does not necessarily disprove the βγ lever hypothesis, for a number of reasons. For instance, the β1-D228A mutant did stimulate αsΔ, albeit to an extent less than β1-WT; this defect may not have been severe enough to prevent GPCR activation of αs-β1-D228A-γ trimers. In addition, β1-D228A may not interact normally with both WT Gα subunits and GPCRs, as would be required for it to exert a dominant negative effect. Convincing evidence that GPCRs do use βγ as a lever will require rigorous experiments with purified GPCRs and G proteins to obtain evidence for the proposed tilt of Gβγ relative to Gα—ideally by crystallizing a GPCR-G trimer complex in the “empty” state (lacking bound nucleotide).
While awaiting results of these demanding experiments, it may be useful to consider other aspects of the Gβγ-lever hypothesis. For instance, it is important to recognize that the lever mechanism does not exclude use by GPCRs of a second proposed route for conducting conformational change to the guanine nucleotide binding pocket of Gα. In this alternative scenario (5, 6), a GPCR contacting the C-terminal tail of Gα, an extension of the α5 helix, induces the α5 helix to move in a way that alters conformation of the β6-α5 loop at its other end. A mutation in this loop (A366S in αs), which forms an important part of the guanine nucleotide-binding pocket, activates the G protein by accelerating release of bound GDP (35).
In addition, we should consider whether it is plausible to imagine that a single GPCR can interact with and pull together the C termini of both Gα and Gγ, as the lever hypothesis requires. The “footprint” of a G protein trimer is large relative to that of GPCRs. Indeed, the crystal structure of rhodopsin (38) reveals a cytoplasmic face that is just barely broad enough (longest dimension ≈43 Å) to touch the α and γ C termini, which lie ≈40 Å distant from one another in the crystal structure of rhodopsin's target, the Gt trimer (39). Although many different G-protein-coupled receptors have been shown to form dimers or oligomers in vivo (40–45), it is not yet clear whether these larger structures are necessary for signaling to G proteins.
Acknowledgments
We thank Sonia Pollitt, Fei Wang, Guy Servant, Orion Weiner, and Véronique Perrier for helpful discussion. This work was supported by Grant GM-27800 from the National Institutes of Health to the laboratory of H.R.B. Support for P.R. was provided by a Long-Term Fellowship Human Frontier Science Program and a fellowship from Association pour la Recherche sur le Cancer (France).
Abbreviations
- αsΔ
mutant αs lacking residues 35–38
- WT
wild type
- GPCR
G protein- coupled receptor
- Sw1 and Sw2
switch 1 and switch 2 regions of Gα proteins
- HA
hemagglutinin epitope for monoclonal antibody
References
- 1.Hamm H E. J Biol Chem. 1998;273:669–672. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson K M, Higashijima T, Smigel M D, Gilman A G. J Biol Chem. 1986;261:7393–7399. [PubMed] [Google Scholar]
- 3.Lambright D G, Sondek J, Bohm A, Skiba N P, Hamm H E, Sigler P B. Nature (London) 1996;379:311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 4.Wall M A, Coleman D E, Lee E, Iñiguez-Lluhi J A, Posner B A, Gilman A G, Sprang S R. Cell. 1995;83:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 5.Bourne H R. Curr Opin Cell Biol. 1997;9:134–142. doi: 10.1016/s0955-0674(97)80054-3. [DOI] [PubMed] [Google Scholar]
- 6.Onrust R, Herzmark P, Chi P, Garcia P D, Lichtarge O, Kingsley C, Bourne H R. Science. 1997;275:381–384. doi: 10.1126/science.275.5298.381. [DOI] [PubMed] [Google Scholar]
- 7.Bohm A, Gaudet R, Sigler P B. Curr Opin Biotechnol. 1997;8:480–487. doi: 10.1016/s0958-1669(97)80072-9. [DOI] [PubMed] [Google Scholar]
- 8.Iiri T, Farfel Z, Bourne H R. Nature (London) 1998;394:35–38. doi: 10.1038/27831. [DOI] [PubMed] [Google Scholar]
- 9.Fung B K. J Biol Chem. 1983;258:10495–10502. [PubMed] [Google Scholar]
- 10.Kisselev O G, Kao J, Ponder J W, Fann Y C, Gautam N, Marshall G R. Proc Natl Acad Sci USA. 1998;95:4270–4275. doi: 10.1073/pnas.95.8.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kisselev O G, Meyer C K, Heck M, Ernst O P, Hofmann K P. Proc Natl Acad Sci USA. 1999;96:4898–4903. doi: 10.1073/pnas.96.9.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford C E, Skiba N P, Bae H, Daaka Y, Reuveny E, Shekter L R, Rosal R, Weng G, Yang C S, Iyengar R, et al. Science. 1998;280:1271–1274. doi: 10.1126/science.280.5367.1271. [DOI] [PubMed] [Google Scholar]
- 13.Kawashima T, Berthet-Colominas C, Wulff M, Cusack S, Leberman R. Nature (London) 1996;381:511–518. doi: 10.1038/379511a0. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Jiang Y, Meyering-Voss M, Sprinzl M, Sigler P B. Nat Struct Biol. 1997;4:650–656. doi: 10.1038/nsb0897-650. [DOI] [PubMed] [Google Scholar]
- 15.Boriack-Sjodin P A, Margarit S M, Bar-Sagi D, Kuriyan J. Nature (London) 1998;394:337–343. doi: 10.1038/28548. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg J. Cell. 1998;95:237–248. doi: 10.1016/s0092-8674(00)81754-7. [DOI] [PubMed] [Google Scholar]
- 17.Worthylake D K, Rossman K L, Sondek J. Nature (London) 2000;408:682–688. doi: 10.1038/35047014. [DOI] [PubMed] [Google Scholar]
- 18.Levis M J, Bourne H R. J Cell Biol. 1992;119:1297–1307. doi: 10.1083/jcb.119.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fishburn C S, Pollitt S K, Bourne H R. Proc Natl Acad Sci USA. 2000;97:1085–1090. doi: 10.1073/pnas.97.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia P D, Onrust R, Bell S M, Sakmar T P, Bourne H R. EMBO J. 1995;14:4460–4469. doi: 10.1002/j.1460-2075.1995.tb00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berlot C H, Bourne H R. Cell. 1992;68:911–922. doi: 10.1016/0092-8674(92)90034-a. [DOI] [PubMed] [Google Scholar]
- 22.Morales J, Fishburn C S, Wilson P T, Bourne H R. Mol Biol Cell. 1998;9:1–14. doi: 10.1091/mbc.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iiri T, Bell S M, Baranski T J, Fujita T, Bourne H R. Proc Natl Acad Sci USA. 1999;96:499–504. doi: 10.1073/pnas.96.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong H Y. Methods Enzymol. 1994;238:81–94. doi: 10.1016/0076-6879(94)38008-2. [DOI] [PubMed] [Google Scholar]
- 25.Graziano M P, Freissmuth M, Gilman A G. J Biol Chem. 1989;264:409–418. [PubMed] [Google Scholar]
- 26.Sprang S R. Annu Rev Biochem. 1997;66:639–678. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- 27.Wedegaertner P B, Chu D H, Wilson P T, Levis M J, Bourne H R. J Biol Chem. 1993;268:25001–25008. [PubMed] [Google Scholar]
- 28.Wedegaertner P B, Bourne H R. Cell. 1994;77:1063–1070. doi: 10.1016/0092-8674(94)90445-6. [DOI] [PubMed] [Google Scholar]
- 29.Iiri T, Backlund P S, Jones T L Z, Wedegaertner P B, Bourne H R. Proc Natl Acad Sci USA. 1996;93:14592–14597. doi: 10.1073/pnas.93.25.14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degtyarev M Y, Spiegel A M, Jones T L. J Biol Chem. 1994;269:30898–30903. [PubMed] [Google Scholar]
- 31.Evanko D S, Thiyagarajan M M, Wedegaertner P B. J Biol Chem. 2000;275:1327–1336. doi: 10.1074/jbc.275.2.1327. [DOI] [PubMed] [Google Scholar]
- 32.Masters S B, Miller R T, Chi M H, Chang F H, Beiderman B, Lopez N G, Bourne H R. J Biol Chem. 1989;264:15467–15474. [PubMed] [Google Scholar]
- 33.Landis C A, Masters S B, Spada A, Pace A M, Bourne H R, Vallar L. Nature (London) 1989;340:692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- 34.Graziano M P, Gilman A G. J Biol Chem. 1989;264:15475–15482. [PubMed] [Google Scholar]
- 35.Iiri T, Herzmark P, Nakamoto J M, van Dop C, Bourne H R. Nature (London) 1994;371:164–168. doi: 10.1038/371164a0. [DOI] [PubMed] [Google Scholar]
- 36.Higashijima T, Ferguson K M, Sternweis P C, Smigel M D, Gilman A G. J Biol Chem. 1987;262:762–766. [PubMed] [Google Scholar]
- 37.Sondek J, Bohm A, Lambright D G, Hamm H E, Sigler P B. Nature (London) 1996;379:369–374. doi: 10.1038/379369a0. [DOI] [PubMed] [Google Scholar]
- 38.Palczewski K, Kumasaka T, Hori T, Behnke C A, Motoshima H, Fox B A, Le Trong I, Teller D C, Okada T, Stenkamp R E, et al. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 39.Bourne H R, Meng E C. Science. 2000;289:733–734. doi: 10.1126/science.289.5480.733. [DOI] [PubMed] [Google Scholar]
- 40.Jones K A, Borowsky B, Tamm J A, Craig D A, Durkin M M, Dai M, Yao W J, Johnson M, Gunwaldsen C, Huang L Y, et al. Nature (London) 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 41.Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, et al. Nature (London) 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 42.White J H, Wise A, Main M J, Green A, Fraser N J, Disney G H, Barnes A A, Emson P, Foord S M, Marshall F H. Nature (London) 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 43.Jordan B A, Devi L A. Nature (London) 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ginés S, Hillion J, Torvinen M, Le Crom S, Casadó V, Canela E I, Rondin S, Lew J Y, Watson S, Zoli M, et al. Proc Natl Acad Sci USA. 2000;97:8606–8611. doi: 10.1073/pnas.150241097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rocheville M, Lange D C, Kumar U, Patel S C, Patel R C, Patel Y C. Science. 2000;288:154–157. doi: 10.1126/science.288.5463.154. [DOI] [PubMed] [Google Scholar]