Abstract
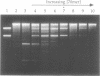
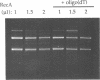
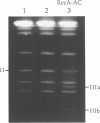
We have developed a novel version of the Achilles' Cleavage (AC) reaction in which virtually any restriction site on DNA of any size can be converted to a unique cleavage site. We first polymerized RecA protein on a synthetic oligodeoxyribonucleotide (oligo) in the presence of a nonhydrolyzable ATP analogue to generate oligo:RecA nucleoprotein filaments. These filament were then incubated with plasmid or intact chromosomal DNA from Saccharomyces cerevisiae to form stable complexes in the yeast LEU2 gene at the target sequence identical (or complementary) to that of the oligo. When HhaII (HinfI) methyltransferase (M.HhaII) was added, all of the recognition sites for HhaII with the exception of the one protected by the RecA filament were methylated and thus no longer cleaved by the cognate restriction endonuclease (HinfI). After inactivation of the RecA and the M.HhaII, HinfI was used to efficiently cleave the plasmid or chromosome specifically at the targeted restriction site. Since oligos specific for any sequence can be easily synthesized and the other reagents necessary to perform RecA-mediated AC (RecA-AC) reactions on both plasmids and intact chromosomes are readily available, this procedure can be applied immediately to the precise dissection and analysis of genomic DNA from any source and to any other research problem requiring efficient, highly specific cleavage of DNA at predetermined sites.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandrasegaran S., Wu L. P., Valda E., Smith H. O. Overproduction and purification of the M.HhaII methyltransferase from Haemophilus haemolyticus. Gene. 1988 Dec 25;74(1):15–21. doi: 10.1016/0378-1119(88)90240-5. [DOI] [PubMed] [Google Scholar]
- Cox M. M., Lehman I. R. Enzymes of general recombination. Annu Rev Biochem. 1987;56:229–262. doi: 10.1146/annurev.bi.56.070187.001305. [DOI] [PubMed] [Google Scholar]
- Cox M. M., Lehman I. R. recA protein of Escherichia coli promotes branch migration, a kinetically distinct phase of DNA strand exchange. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3433–3437. doi: 10.1073/pnas.78.6.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrin L. J., Camerini-Otero R. D. Selective cleavage of human DNA: RecA-assisted restriction endonuclease (RARE) cleavage. Science. 1991 Dec 6;254(5037):1494–1497. doi: 10.1126/science.1962209. [DOI] [PubMed] [Google Scholar]
- Grimes E., Koob M., Szybalski W. Achilles' heel cleavage: creation of rare restriction sites in lambda phage genomes and evaluation of additional operators, repressors and restriction/modification systems. Gene. 1990 May 31;90(1):1–7. doi: 10.1016/0378-1119(90)90432-q. [DOI] [PubMed] [Google Scholar]
- Hanvey J. C., Shimizu M., Wells R. D. Site-specific inhibition of EcoRI restriction/modification enzymes by a DNA triple helix. Nucleic Acids Res. 1990 Jan 11;18(1):157–161. doi: 10.1093/nar/18.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honigberg S. M., Gonda D. K., Flory J., Radding C. M. The pairing activity of stable nucleoprotein filaments made from recA protein, single-stranded DNA, and adenosine 5'-(gamma-thio)triphosphate. J Biol Chem. 1985 Sep 25;260(21):11845–11851. [PubMed] [Google Scholar]
- Honigberg S. M., Rao B. J., Radding C. M. Ability of RecA protein to promote a search for rare sequences in duplex DNA. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9586–9590. doi: 10.1073/pnas.83.24.9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konforti B. B., Davis R. W. The preference for a 3' homologous end is intrinsic to RecA-promoted strand exchange. J Biol Chem. 1990 Apr 25;265(12):6916–6920. [PubMed] [Google Scholar]
- Koob M., Grimes E., Szybalski W. Conferring new specificity upon restriction endonucleases by combining repressor-operator interaction and methylation. Gene. 1988 Dec 25;74(1):165–167. doi: 10.1016/0378-1119(88)90277-6. [DOI] [PubMed] [Google Scholar]
- Koob M., Grimes E., Szybalski W. Conferring operator specificity on restriction endonucleases. Science. 1988 Aug 26;241(4869):1084–1086. doi: 10.1126/science.2842862. [DOI] [PubMed] [Google Scholar]
- Koob M., Szybalski W. Cleaving yeast and Escherichia coli genomes at a single site. Science. 1990 Oct 12;250(4978):271–273. doi: 10.1126/science.2218529. [DOI] [PubMed] [Google Scholar]
- Kur J., Koob M., Burkiewicz A., Szybalski W. A novel method for converting common restriction enzymes into rare cutters: integration host factor-mediated Achilles' cleavage (IHF-AC). Gene. 1992 Jan 2;110(1):1–7. doi: 10.1016/0378-1119(92)90437-t. [DOI] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Wold B., Dervan P. B. Inhibition of DNA binding proteins by oligonucleotide-directed triple helix formation. Science. 1989 Aug 18;245(4919):725–730. doi: 10.1126/science.2549631. [DOI] [PubMed] [Google Scholar]
- Menetski J. P., Bear D. G., Kowalczykowski S. C. Stable DNA heteroduplex formation catalyzed by the Escherichia coli RecA protein in the absence of ATP hydrolysis. Proc Natl Acad Sci U S A. 1990 Jan;87(1):21–25. doi: 10.1073/pnas.87.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddles P. W., Lehman I. R. The formation of plectonemic joints by the recA protein of Escherichia coli. Requirement for ATP hydrolysis. J Biol Chem. 1985 Jan 10;260(1):170–173. [PubMed] [Google Scholar]
- Rigas B., Welcher A. A., Ward D. C., Weissman S. M. Rapid plasmid library screening using RecA-coated biotinylated probes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9591–9595. doi: 10.1073/pnas.83.24.9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca A. I., Cox M. M. The RecA protein: structure and function. Crit Rev Biochem Mol Biol. 1990;25(6):415–456. doi: 10.3109/10409239009090617. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel S. A., Dervan P. B. Single-site enzymatic cleavage of yeast genomic DNA mediated by triple helix formation. Nature. 1991 Mar 14;350(6314):172–174. doi: 10.1038/350172a0. [DOI] [PubMed] [Google Scholar]
- Strobel S. A., Doucette-Stamm L. A., Riba L., Housman D. E., Dervan P. B. Site-specific cleavage of human chromosome 4 mediated by triple-helix formation. Science. 1991 Dec 13;254(5038):1639–1642. doi: 10.1126/science.1836279. [DOI] [PubMed] [Google Scholar]
- Vollrath D., Davis R. W. Resolution of DNA molecules greater than 5 megabases by contour-clamped homogeneous electric fields. Nucleic Acids Res. 1987 Oct 12;15(19):7865–7876. doi: 10.1093/nar/15.19.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. D. The human genome project: past, present, and future. Science. 1990 Apr 6;248(4951):44–49. doi: 10.1126/science.2181665. [DOI] [PubMed] [Google Scholar]