Abstract
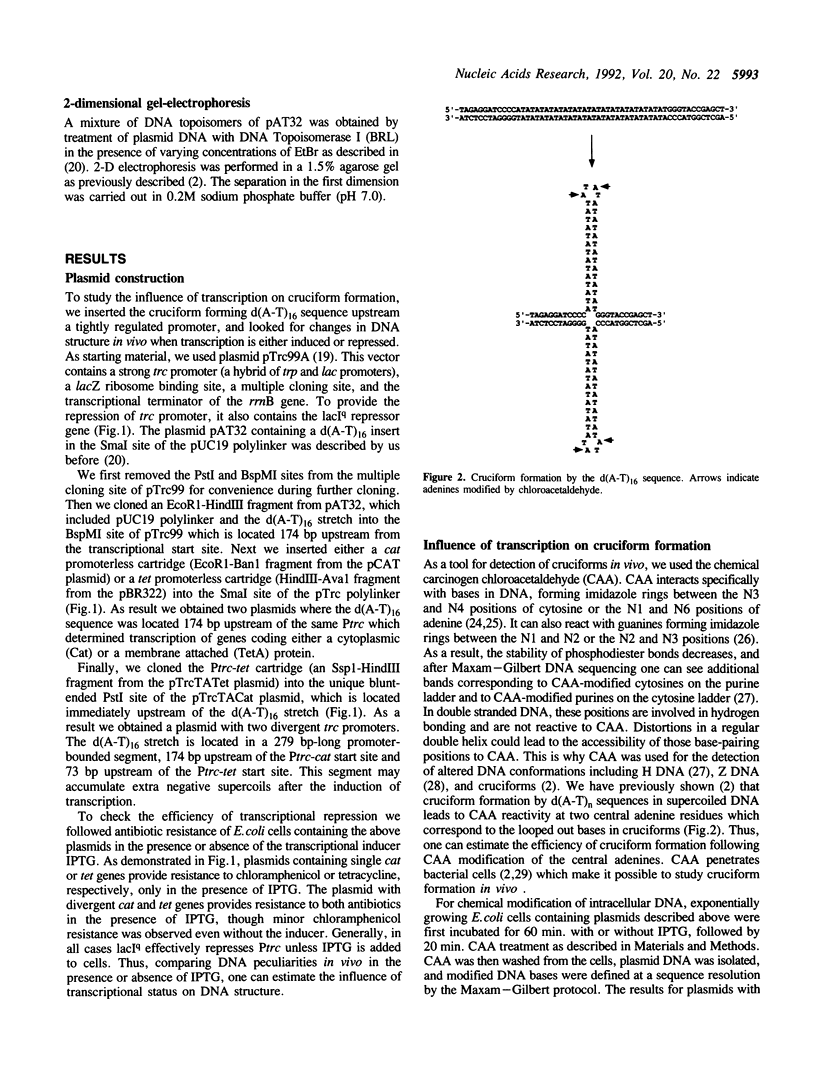
We studied the formation of d(A-T)n cruciforms in E.coli cells by probing intracellular plasmid DNA with chloroacetaldehyde followed by fine analysis of modified DNA bases. d(A-T)16 sequences were inserted into specifically designed plasmids either upstream of a single trc promoter, or between two divergent trc promoters. We found that in both cases, induction of transcription by IPTG leads to the transition of the d(A-T)16 stretch into a cruciform state. In the case of two divergent promoters, we observed cruciform formation even without IPTG. Enhanced cruciform formation correlates with the elevation in promoter activity as defined by the opening of the promoter at the -10 to +2 positions. We conclude that transcriptionally driven negative supercoiling provokes cruciform formation in vivo.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Ochs B., Abel K. J. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988 Sep 30;69(2):301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- Barrio J. R., Secrist J. A., 3rd, Leonard N. J. Fluorescent adenosine and cytidine derivatives. Biochem Biophys Res Commun. 1972 Jan 31;46(2):597–604. doi: 10.1016/s0006-291x(72)80181-5. [DOI] [PubMed] [Google Scholar]
- Chen B., de Crombrugghe B., Anderson W. B., Gottesman M. E., Pastan I., Perlman R. L. On the mechanism of action of lac repressor. Nat New Biol. 1971 Sep 15;233(37):67–70. doi: 10.1038/newbio233067a0. [DOI] [PubMed] [Google Scholar]
- Courey A. J., Wang J. C. Cruciform formation in a negatively supercoiled DNA may be kinetically forbidden under physiological conditions. Cell. 1983 Jul;33(3):817–829. doi: 10.1016/0092-8674(83)90024-7. [DOI] [PubMed] [Google Scholar]
- Dayn A., Malkhosyan S., Duzhy D., Lyamichev V., Panchenko Y., Mirkin S. Formation of (dA-dT)n cruciforms in Escherichia coli cells under different environmental conditions. J Bacteriol. 1991 Apr;173(8):2658–2664. doi: 10.1128/jb.173.8.2658-2664.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge P., Nordheim A. Transcription-induced conformational change in a topologically closed DNA domain. Nucleic Acids Res. 1991 Jun 11;19(11):2941–2946. doi: 10.1093/nar/19.11.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Müller-Hill B. Isolation of the lac repressor. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1891–1898. doi: 10.1073/pnas.56.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S., Loeb L. A. An E. coli promoter that regulates transcription by DNA superhelix-induced cruciform extrusion. Science. 1988 Aug 5;241(4866):703–705. doi: 10.1126/science.2456617. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K., Buc H., Spassky A., Wang J. C. Mapping of single-stranded regions in duplex DNA at the sequence level: single-strand-specific cytosine methylation in RNA polymerase-promoter complexes. Proc Natl Acad Sci U S A. 1983 May;80(9):2544–2548. doi: 10.1073/pnas.80.9.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi Y., Kohwi-Shigematsu T. Magnesium ion-dependent triple-helix structure formed by homopurine-homopyrimidine sequences in supercoiled plasmid DNA. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3781–3785. doi: 10.1073/pnas.85.11.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi Y., Malkhosyan S. R., Kohwi-Shigematsu T. Intramolecular dG.dG.dC triplex detected in Escherichia coli cells. J Mol Biol. 1992 Feb 20;223(4):817–822. doi: 10.1016/0022-2836(92)90242-c. [DOI] [PubMed] [Google Scholar]
- Lee J., Goldfarb A. lac repressor acts by modifying the initial transcribing complex so that it cannot leave the promoter. Cell. 1991 Aug 23;66(4):793–798. doi: 10.1016/0092-8674(91)90122-f. [DOI] [PubMed] [Google Scholar]
- Lilley D. M., Hallam L. R. The interactions of enzyme and chemical probes with inverted repeats in supercoiled DNA. J Biomol Struct Dyn. 1983 Oct;1(1):169–182. doi: 10.1080/07391102.1983.10507433. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockshon D., Morris D. R. Positively supercoiled plasmid DNA is produced by treatment of Escherichia coli with DNA gyrase inhibitors. Nucleic Acids Res. 1983 May 25;11(10):2999–3017. doi: 10.1093/nar/11.10.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge J. K., Kazic T., Berg D. E. Formation of supercoiling domains in plasmid pBR322. J Bacteriol. 1989 Apr;171(4):2181–2187. doi: 10.1128/jb.171.4.2181-2187.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyamichev V., Panyutin I., Mirkin S. The absence of cruciform structures from pAO3 plasmid DNA in vivo. J Biomol Struct Dyn. 1984 Oct;2(2):291–301. doi: 10.1080/07391102.1984.10507568. [DOI] [PubMed] [Google Scholar]
- Majors J. Initiation of in vitro mRNA synthesis from the wild-type lac promoter. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4394–4398. doi: 10.1073/pnas.72.11.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McClellan J. A., Boublíková P., Palecek E., Lilley D. M. Superhelical torsion in cellular DNA responds directly to environmental and genetic factors. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8373–8377. doi: 10.1073/pnas.87.21.8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotatos N., Fontaine A. A native cruciform DNA structure probed in bacteria by recombinant T7 endonuclease. J Biol Chem. 1987 Aug 15;262(23):11364–11368. [PubMed] [Google Scholar]
- Panyutin I., Lyamichev V., Mirkin S. A structural transition in d(AT)n.d(AT)n inserts within superhelical DNA. J Biomol Struct Dyn. 1985 Jun;2(6):1221–1234. doi: 10.1080/07391102.1985.10507634. [DOI] [PubMed] [Google Scholar]
- Pruss G. J., Drlica K. Topoisomerase I mutants: the gene on pBR322 that encodes resistance to tetracycline affects plasmid DNA supercoiling. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8952–8956. doi: 10.1073/pnas.83.23.8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni A. R., Wells R. D. Direct evidence for the effect of transcription on local DNA supercoiling in vivo. J Mol Biol. 1992 Jan 5;223(1):131–144. doi: 10.1016/0022-2836(92)90721-u. [DOI] [PubMed] [Google Scholar]
- Rahmouni A. R., Wells R. D. Stabilization of Z DNA in vivo by localized supercoiling. Science. 1989 Oct 20;246(4928):358–363. doi: 10.1126/science.2678475. [DOI] [PubMed] [Google Scholar]
- Sasse-Dwight S., Gralla J. D. KMnO4 as a probe for lac promoter DNA melting and mechanism in vivo. J Biol Chem. 1989 May 15;264(14):8074–8081. [PubMed] [Google Scholar]
- Sattsangi P. D., Leonard N. J., Frihart C. R. 1,N2-ethenoguanine and N2,3-ethenoguanine. Synthesis and comparison of the electronic spectral properties of these linear and angular triheterocycles related to the Y bases. J Org Chem. 1977 Sep 30;42(20):3292–3296. doi: 10.1021/jo00440a020. [DOI] [PubMed] [Google Scholar]
- Schmitz A., Galas D. J. The interaction of RNA polymerase and lac repressor with the lac control region. Nucleic Acids Res. 1979 Jan;6(1):111–137. doi: 10.1093/nar/6.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden R. R., Broyles S. S., Pettijohn D. E. Perfect palindromic lac operator DNA sequence exists as a stable cruciform structure in supercoiled DNA in vitro but not in vivo. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1797–1801. doi: 10.1073/pnas.80.7.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden R. R., Carlson J. O., Pettijohn D. E. Torsional tension in the DNA double helix measured with trimethylpsoralen in living E. coli cells: analogous measurements in insect and human cells. Cell. 1980 Oct;21(3):773–783. doi: 10.1016/0092-8674(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Straney S. B., Crothers D. M. Lac repressor is a transient gene-activating protein. Cell. 1987 Dec 4;51(5):699–707. doi: 10.1016/0092-8674(87)90093-6. [DOI] [PubMed] [Google Scholar]
- Vogt N., Marrot L., Rousseau N., Malfoy B., Leng M. Chloroacetaldehyde reacts with Z-DNA. J Mol Biol. 1988 Jun 20;201(4):773–776. doi: 10.1016/0022-2836(88)90474-3. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Collier D. A., Hanvey J. C., Shimizu M., Wohlrab F. The chemistry and biology of unusual DNA structures adopted by oligopurine.oligopyrimidine sequences. FASEB J. 1988 Nov;2(14):2939–2949. [PubMed] [Google Scholar]
- Wu H. Y., Shyy S. H., Wang J. C., Liu L. F. Transcription generates positively and negatively supercoiled domains in the template. Cell. 1988 May 6;53(3):433–440. doi: 10.1016/0092-8674(88)90163-8. [DOI] [PubMed] [Google Scholar]
- Yang L., Jessee C. B., Lau K., Zhang H., Liu L. F. Template supercoiling during ATP-dependent DNA helix tracking: studies with simian virus 40 large tumor antigen. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6121–6125. doi: 10.1073/pnas.86.16.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]