Abstract
To address the role of Tpl2, a MAP3K8 that regulates innate/adaptive immunity and inflammation, in intestinal tumorigenesis, we crossed a Tpl2 KO allele into the Apcmin/+ genetic background. Here, we show that Apcmin/+/Tpl2−/− mice exhibit a fivefold increase in the number of intestinal adenomas. Bone marrow transplantation experiments revealed that the enhancement of polyposis was partially hematopoietic cell-driven. Consistent with this observation, Tpl2 ablation promoted intestinal inflammation. IL-10 levels and regulatory T-cell numbers were lower in the intestines of Tpl2−/− mice, independent of Apc and polyp status, suggesting that they were responsible for the initiation of the enhancement of tumorigenesis caused by the ablation of Tpl2. The low IL-10 levels correlated with defects in mTOR activation and Stat3 phosphorylation in Toll-like receptor-stimulated macrophages and with a defect in inducible regulatory T-cell generation and function. Both polyp numbers and inflammation increased progressively with time. The rate of increase of both, however, was more rapid in Apcmin/+/Tpl2−/− mice, suggesting that the positive feedback initiated by inflammatory signals originating in developing polyps is more robust in these mice. This may be because these mice have a higher intestinal polyp burden as a result of the enhancement of tumor initiation.
Keywords: inflammatory bowel disease, TNF-α, IL-6, Tr1 cells, Th17 cells
Genetic susceptibility to colorectal carcinogenesis has been linked to a host of germ-line mutations that give rise either to familial adenomatous polyposis (FAP) or to hereditary nonpolyposis colorectal cancer. FAP is caused by germ-line mutations of the APC gene and is characterized by the development of large numbers of intestinal polyps early in life. Some of these polyps ultimately progress to give rise to malignant colorectal tumors. APC (adenomatous polyposis coli) gene mutations are detected in at least 95% of human malignant colorectal tumors, including tumors arising in individuals who do not carry germ-line APC mutations, suggesting that it plays a critical role in both familial and sporadic intestinal tumorigenesis. Germ-line mutations of the APC gene in mice give rise to a syndrome that is similar to the FAP syndrome in humans. Mice carrying the Apcmin mutation have been used extensively as an animal model to study intestinal polyposis and cancer (1).
APC mutations promote intestinal tumorigenesis by cell-autonomous as well as stroma-dependent processes. Earlier studies had shown that inflammatory infiltrates consisting of F4/80/CD11b double-positive macrophages, Gr1/CD11b double-positive myeloid-derived suppressor cells, and other types of inflammatory cells (e.g., mast cells) accumulate in the intestinal mucosa of APC mutant mice and contribute to oncogenesis (2–5). Moreover, intestinal inflammation promotes oncogenesis in humans and animals even in the absence of germ-line mutations in the APC locus. Thus, inflammatory bowel disease has been linked to an increased incidence of cancer (6). In addition, high levels of nitric oxide, as well as overexpression of the proinflammatory cytokines TNF-α and IL-6, promote intestinal oncogenesis (7, 8), whereas antiinflammatory agents, such as neutralizing TNF-α antibodies (5), inhibit it. Similarly, inhibitors of the proinflammatory mediators COX-2 and prostaglandins decrease intestinal inflammation and the incidence of colon adenocarcinomas in humans and genetically susceptible mice (9), whereas intestinal bacterial infections, which increase the expression of COX-2, promote both (10). In agreement with these findings, the number of intestinal polyps in ApcΔ716/+/COX-2−/− and ApcΔ716/+/EP2−/− double-mutant mice was lower than in ApcΔ716/+ single mutants (11).
A stroma-derived inhibitor of inflammation and oncogenesis is IL-10, an antiinflammatory cytokine produced by macrophages, dendritic cells, and T cells, including Foxp3+ and Foxp3− regulatory T cells (Tregs). The importance of IL-10 in intestinal homeostasis was confirmed by the finding that Il10 KO mice develop intestinal inflammation (12, 13) and are more susceptible to colonic carcinogenesis induced by APC mutations than WT mice (12). Intestinal IL-10 is produced primarily by macrophages and is essential for the control of inflammation in the colon. One of the functions of macrophage IL-10 is the maintenance of Foxp3 expression in Tregs, which become functionally defective in its absence (14). IL-10 regulation in macrophages and dendritic cells is under the control of Stat3 (15), which is activated by Toll-like receptor (TLR) and cytokine signals (15, 16). Experiments based on either genetic or pharmacological inhibition have shown that Stat3 phosphorylation and IL-10 induction by TLR signals depend on mTOR activation (15). KO of Stat3 in myeloid cells exhibits a similar phenotype to the KO of Il10 (17), underscoring the importance of Stat3 activation in myeloid cells for the induction of IL-10.
Tregs also suppress intestinal inflammation and tumorigenesis in Apcmin/+ mice (3, 18), and they are protective in a variety of human tumors, including sporadic colon cancer (19), colon cancer associated with defects in mismatch repair (20), gastric cancer (21), and head and neck cancer (22). Moreover, WT but not IL-10–deficient Tregs adoptively transferred in Apcmin/+ mice inhibit both intestinal inflammation and tumorigenesis (3, 18, 23). Finally, in the course of progressive polyposis in mice and humans, Tregs tend to lose expression of IL-10, reversing their function from antiinflammatory to proinflammatory (2, 3). The latter two observations are consistent with the finding that Treg-specific ablation of Il10 results in bacteria-dependent inflammatory bowel disease (IBD) in aging mice (24).
The Tpl2 protooncogene encodes a serine-threonine protein kinase that is activated by provirus integration in Moloney MuLV-induced T-cell lymphomas and mouse mammary tumor virus (MMTV)-induced mammary adenocarcinomas in mice (25, 26). When overexpressed in a variety of cell types, Tpl2 activates ERK, JNK, p38MAPK, and the transcription factors NFAT (nuclear factor of activated T cells) and NF-κB (27, 28). Moreover, transgenic mice expressing a constitutively active form of Tpl2 under the control of a T cell-specific promoter develop thymic lymphomas with a mean latency of 3 mo (29). Despite these profound effects of Tpl2 overexpression, Tpl2 KO mice generated to study the physiological role of Tpl2 in intact animals appear normal (30). Studies using these mice revealed that Tpl2 is required for the transduction of signals induced by TLR ligands, IL-1β, TNF-α, CD40L, TCR, and G protein-coupled receptor (GPCR) ligands (30–34). One pathway that is reproducibly impaired by the ablation of Tpl2 in different types of cells following exposure to a variety of stimuli is the ERK pathway. Recent studies have shown that Tpl2 ablation impairs the induction of IL-10 by LPS (TLR4)- and CpG (TLR9)-induced ERK activation signals in both macrophages and dendritic cells (35).
TLR signaling has a critical role in spontaneous tumor development in APC-defective mice. Apcmin/+ mice defective for the myeloid differentiation response gene 88 (MyD88), an obligatory downstream target of TLR4 and TLR2, are protected from polyposis (36). Tpl2, a downstream target of MyD88, transduces ERK activation signals that regulate the expression of a range of proinflammatory and antiinflammatory mediators, including COX-2 (30–33) and IL-10 (35), raising questions about the role of Tpl2 in Apc-dependent intestinal tumorigenesis. To address this question, we crossed the Apcmin mutation into the Tpl2−/− genetic background and examined the latency of tumor induction and the polyp number, size, and distribution in Apcmin/+/Tpl2+/+ and Apcmin/+/Tpl2−/− mice. The results showed that Tpl2 ablation is associated with a fivefold increase in the number of intestinal adenomas. Bone marrow transplantation experiments revealed that the increased susceptibility of Apcmin/+/Tpl2−/− mice to intestinal tumorigenesis is partially driven by hematopoietic cells. Consistent with this observation, the ablation of Tpl2 in Apcmin/+ mice was shown to promote the establishment of a proinflammatory environment that stimulates oncogenesis in the intestinal mucosa. The earliest events in the sequence that leads to inflammation and tumorigenesis appeared to be the secretion of IL-10 and the generation of inducible Tregs (iTregs), both of which were impaired in Tpl2−/− mice. The low levels of IL-10 and the low number and functional impairment of Tregs promote inflammation and tumorigenesis in the context of the Apcmin mutation. Polyp numbers and inflammation increased progressively with time in both Apcmin/+/Tpl2+/+ and Apcmin/+/Tpl2−/− mice, but the rate of increase in both was higher in the Apcmin/+/Tpl2−/− mice. The latter observation suggested that Tpl2 ablation may stimulate positive feedback mechanisms that promote the acceleration of polyposis. Given that polyps give rise to proinflammatory and prooncogenic signals, this may be attributable to the enhancement of tumor initiation, which increases the tumor burden and gives rise to a more robust proinflammatory environment in these mice throughout life. Collectively, these observations provide evidence that Tpl2 has a critical role in the regulation of systemic inflammation and in the susceptibility to intestinal tumorigenesis.
Results
Tpl2 Ablation Promotes Tumor Induction in Apcmin/+ Mice but It Does Not Affect the Distribution or the Histology of the Polyps.
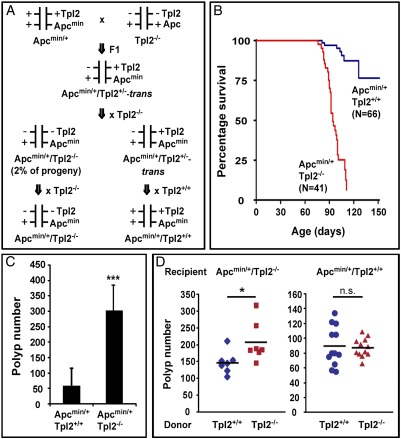
The generation of experimental mice was complicated by the fact that Tpl2 and APC are linked on mouse chromosome 18. To circumvent this problem, we selected for meiotic recombination between the two genes. To this end, we first crossed the Apcmin/+ mice with the Tpl2−/− mice. Subsequently, we mated the F1 mice obtained from the first cross with the Tpl2−/− mice. Two percent of the progeny of the latter cross were Apcmin/+/Tpl2−/−, indicating that they harbored a chromosome 18 that carried both mutations in cis. Experimental mice were generated by crossing Apcmin/+/Tpl2−/− mice with Tpl2−/− mice and Apcmin/+/Tpl2+/+ mice with Tpl2+/+ mice (Fig. 1A). Apcmin/+/Tpl2−/− and Apcmin/+/Tpl2+/+ mice were monitored over a period of up to 150 d for tumor induction. Mice exhibiting signs of systemic illness were killed and autopsied. The survival curves of the two experimental groups are shown in Fig. 1B, which combines survival data of both male and female mice. Plotting the data separately for male and female mice revealed no differences between the sexes.
Fig. 1.
Tpl2 ablation promotes tumor induction in Apcmin/+ mice. The promotion of oncogenesis is partially hematopoietic cell-driven. (A) Generation of Apcmin/+/Tpl2−/− and Apcmin/+/Tpl2+/+ mice. Tpl2 and APC are linked on mouse chromosome 18. To generate Apcmin/+/Tpl2−/− mice, we carried out the indicated crosses and selected for meiotic recombination between the two genes. (B) Kaplan–Meier survival curves comparing Apcmin/+/Tpl2−/− and Apcmin/+/Tpl2+/+ mice. Mice were killed when they developed obvious signs of systemic illness. All surviving mice were killed when they reached the age of 150 d. (C) Apcmin/+/Tpl2−/− mice carried a significantly higher number of polyps than Apcmin/+/Tpl2+/+ mice at the time of euthanasia. (D) Apcmin/+/Tpl2−/− (7 per group) mice and Apcmin/+/Tpl2+/+ (12 per group) mice aged 6–7 wk were transplanted with WT or Tpl2−/− bone marrow, and their tumor burden was examined 7 wk later. *P < 0.05; ***P < 0.001; n.s., not significant.
Evaluation of the number of polyps formed in Apcmin/+/Tpl2−/− and Apcmin/+/Tpl2+/+ mice killed in the 90-d and 110-d time windows showed a dramatic fivefold increase in polyp number in Apcmin/+/Tpl2−/− mice (Fig. 1C and Fig. S1A). Plotting the distribution of polyps along the small intestine (proximal, middle, and distal sections) and the colon revealed that Tpl2 ablation significantly increases the number of polyps in all parts of the intestine but that it does not alter the polyp distribution (Fig. S1B). Histologically, the tumors developing in Apcmin/+/Tpl2+/+ and Apcmin/+/Tpl2−/− mice were similar (Fig. S1C).
Tpl2 Expression in Hematopoietic Cells Is Necessary but Not Sufficient to Inhibit Apcmin-Induced Intestinal Tumorigenesis.
The enhancement of tumorigenesis in Tpl2−/− mice carrying the Apcmin mutation could be the result of a cell-autonomous defect in the intestinal epithelia. Alternatively, it may be attributable to tumor-promoting changes in the intestinal stroma. The latter is supported by the results of earlier studies suggesting that the main function of Tpl2 is the regulation of innate and adaptive immunity (30, 33, 35, 37, 38). To distinguish between these possibilities, Apcmin/+/Tpl2−/− mice were transplanted with Tpl2+/+ or Tpl2−/− bone marrow (Fig. S2). The results (Fig. 1D, Left) revealed that Apcmin/+/Tpl2−/− mice transplanted with the Tpl2−/− bone marrow developed significantly higher numbers of polyps than Apcmin/+/Tpl2−/− mice transplanted with the WT bone marrow. This experiment confirmed that Tpl2 expression in hematopoietic cells is necessary for the Tpl2-mediated inhibition of Apcmin-induced intestinal tumorigenesis. To determine whether it is also sufficient to elicit this phenotype, Apcmin/+/Tpl2+/+ mice were transplanted with Tpl2+/+ or Tpl2−/− bone marrow (Fig. S2). The results showed that if Tpl2 is disrupted only in hematopoietic cells, its ablation is not sufficient to induce the tumor enhancement phenotype (Fig. 1D, Right). Therefore, the ablation of Tpl2 in hematopoietic cells is necessary but not sufficient to elicit the Tpl2−/− intestinal phenotype in Apcmin/+ mice.
Earlier studies had shown that inhibition of TLR signal-induced ERK activation in epithelial cells protects from Apcmin-induced intestinal tumorigenesis (39, 40). Given that Tpl2 is required for ERK activation by TLR signals in different cell types (30, 41), one would expect that Tpl2 ablation would inhibit polyposis. However, Tpl2 ablation had the opposite effect, making the possibility that enhancement of intestinal tumorigenesis depends on the inhibition of TLR signaling in the intestinal epithelia unlikely.
Tpl2 Ablation Promotes the Establishment of a Proinflammatory Environment in the Intestinal Mucosa.
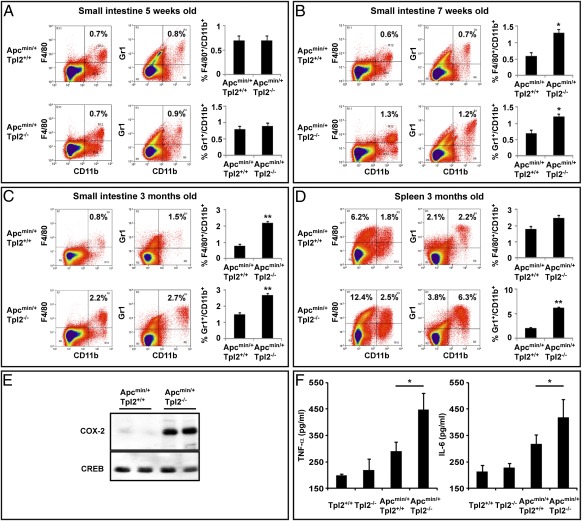
Inflammation plays a critical role in intestinal tumorigenesis (6, 42). This fact, combined with the results of the bone marrow transplantation experiments described above, raised the possibility that Tpl2 ablation may promote Apcmin-induced intestinal tumorigenesis by promoting inflammation. Based on this consideration, we proceeded to determine whether there are differences between the inflammatory infiltrates accumulating in the intestines of Apcmin/+/Tpl2−/− and Apcmin/+/Tpl2+/+ mice. Intestinal infiltrates obtained from 5-wk-old, 7-wk-old, and 3-mo-old Apcmin/+/Tpl2+/+ and Apcmin/+/Tpl2−/− mice were stained with antibodies against F4/80, CD11b, and Gr1, and they were then analyzed by flow cytometry. We chose these antibodies because F4/80/CD11b double-positive macrophages and Gr1/CD11b double-positive tumor-associated myeloid-derived suppressor cells (4, 43) are characteristic of the intestinal inflammatory infiltrates of the Apcmin/+ mice and they have been linked to intestinal carcinogenesis (5). The results showed that the intestinal inflammatory infiltrates of the Apcmin/+/Tpl2−/− mice contained a higher percentage of both the F4/80/CD11b double-positive cells and the Gr1/CD11b double-positive cells, and that the percentages of these cells increased with time (Fig. 2 A–C). Analysis of the splenocytes of the same mice revealed an increase in the F4/80+/CD11b− macrophage progenitors, Gr1+/CD11b− granulocytes, and Gr1+/CD11b+ tumor-associated myeloid suppressor cells (Fig. 2D and Fig. S3).
Fig. 2.
Tpl2 ablation promotes the establishment of a proinflammatory environment in the intestinal mucosa of Apcmin/+ mice. Single-cell suspensions of inflammatory cells isolated from the intestines of 5-wk-old (A), 7-wk-old (B), and 3-mo-old (C) Apcmin/+/Tpl2−/− and Apcmin/+/Tpl2+/+ mice (5 per group) were stained with anti-CD11b, anti-F4/80, and anti-Gr1 antibodies, and they were analyzed by FACS. (D) Single-cell suspensions of splenocytes from 3-mo-old Apcmin/+/Tpl2−/− and Apcmin/+/Tpl2+/+ mice were stained with anti-CD11b, anti-F4/80, and anti-Gr1 antibodies, and they were analyzed by FACS. In addition to a significant increase in the number of CD11b+/Gr1+ tumor-associated myeloid suppressor cells, there was an increase in the number of F4/80+/CD11b− immature monocytes. (E) COX-2 levels were measured by Western blotting in nuclear lysates of intestinal polyps from Apcmin/+/Tpl2+/+ and Apcmin/+/Tpl2−/− mice. (F) TNF-α and IL-6 levels were measured in tissue homogenates from the small intestines of Apc+/+/Tpl2+/+, Apc+/+/Tpl2−/−, Apcmin/+/Tpl2+/+, and Apcmin/+/Tpl2−/− mice by ELISA. *P < 0.05; **P < 0.01.
Polyps developing in Apcmin/+/Tpl2−/− mice expressed higher levels of COX-2 than polyps developing in Apcmin/+/Tpl2+/+ mice (Fig. 2E). Moreover, IL-6 and TNF-α, which are known to promote intestinal oncogenesis (7), were expressed at higher levels in the intestinal mucosa of 3-mo-old Apcmin/+/Tpl2−/− mice (Fig. 2F). In the absence of the Apcmin mutation, the ablation of Tpl2 failed to alter the composition of the intestinal inflammatory infiltrates (Fig. S4) and to enhance the levels of TNF-α and IL-6 in the intestinal mucosa (Fig. 2F). Combined, these data show that Tpl2 ablation promotes the establishment of a proinflammatory environment in the intestinal mucosa of Apcmin/+ but not Apc+/+ mice.
To determine the role of the intestinal microbiota in Apcmin-mediated intestinal inflammation and tumorigenesis, Apcmin/+/Tpl2+/+ and Apcmin/+/Tpl2−/− mice were treated with an antibiotic regimen that inhibits intestinal tumorigenesis in Nod1-deficient mice (44). Pregnant mothers in the last trimester of pregnancy were treated with streptomycin, gentamicin, ciprofloxacin, and bacitracin, as described in SI Materials and Methods. The results showed that the severity of polyposis in the small intestines was not affected by the antibiotics (Fig. S5), in full agreement with the results of earlier studies showing that antibiotic treatment (ampicillin, vancomycin, neomycin, and metronidazole) does not affect the intensity of polyposis in Apcmin/+ mice (45).
Ablation of Tpl2 Is Associated with Low Levels of IL-10 in the Intestinal Mucosa of both Apc+/+ and Apcmin/+ Mice.
The differences in the composition of the intestinal inflammatory infiltrates of the Apcmin/+/Tpl2+/+ and Apcmin/+/Tpl2−/− mice begin to be apparent at the age of 7 wk (Fig. 2), simultaneously with the development of polyposis. Because polyps may stimulate inflammation (3–5), this observation raised the question of whether the differences in intestinal tumorigenesis between Apcmin/+/Tpl2+/+ and Apcmin/+/Tpl2−/− mice precede or follow the differences in intestinal inflammation between the two strains. We therefore embarked on intestinal cytokine measurements at different time points, aiming to identify differences between these mice that occur before the time of detection of the earliest polyps. Similar studies were carried out in Tpl2+/+ and Tpl2−/− mice in the APC WT background, which do not develop polyps.
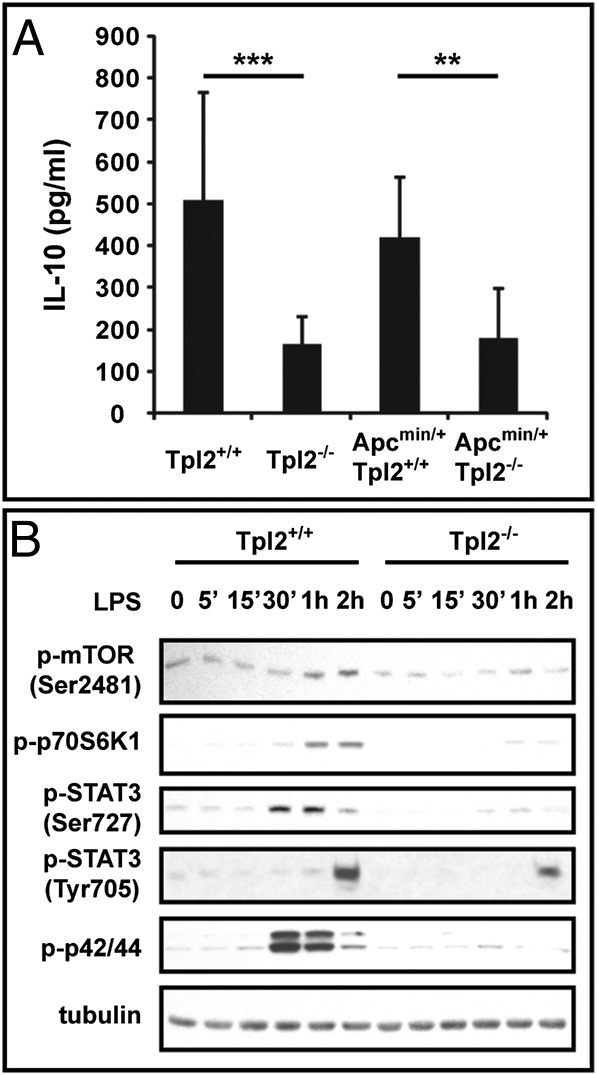
ELISAs on extracts of the intestinal mucosa of Apc+/+/Tpl2+/+, Apc+/+/Tpl2−/−, Apcmin/+/Tpl2+/+, and Apcmin/+/Tpl2−/− mice showed that the levels of intestinal IL-10 were lower in Tpl2−/− mice than in Tpl2+/+ mice, both in the WT and Apcmin/+ backgrounds (Fig. 3A). The difference in IL-10 expression in the intestines of Tpl2+/+ and Tpl2−/− mice was observed in both young and older mice. The observation that Tpl2 ablation is associated with low levels of intestinal IL-10 before the detection of inflammation and polyp formation suggests that the reduced IL-10 expression in these mice may be one of the initiating factors for inflammation and tumorigenesis, even though it may not be sufficient to induce these processes by itself.
Fig. 3.
IL-10 levels in the intestinal mucosa. Role of Tpl2 in the induction of IL-10 by TLR signals in macrophages. (A) Homogenates of the small intestines of sets of age-matched Apc+/+/Tpl2+/+ (n = 11), Apc+/+/Tpl2−/− (n = 11), Apcmin/+/Tpl2+/+ (n = 8), and Apcmin/+/Tpl2−/− (n = 8) mice were analyzed for IL-10 by ELISA. **P < 0.01; ***P < 0.001. (B) LPS-induced phosphorylation of mTOR and Stat3, which control IL-10 induction in macrophages, is Tpl2-dependent. BMDMs from Tpl2+/+ and Tpl2−/− mice were stimulated with LPS. Cell lysates harvested at the indicated time points were probed with anti–phospho-mTOR, anti–phospho-p70S6K, anti–phospho-STAT3, anti–phospho-p42/44, and antitubulin antibodies. Results are representative of three independent experiments.
IL-10 is produced by a variety of cell types, including macrophages, dendritic cells, and T cells. It has been proposed that F4/80+/CD11b+ macrophages are mostly responsible for IL-10 production in the intestines (14, 46). Previous studies had shown that Tpl2 ablation gives rise to a macrophage and dendritic cell defect in the induction of IL-10 by TLR signals (35). Following confirmation of this finding (Fig. S6), we embarked on studies to determine the mechanism by which Tpl2 regulates IL-10 expression in macrophages in response to TLR signals.
IL-10 induction by TLR signals is blocked by mTOR inhibitors, such as rapamycin. The latter inhibits IL-10 induction by inhibiting the phosphorylation of Stat3, an upstream regulator of IL-10 (15). To examine whether Tpl2 ablation inhibits the induction of IL-10 by TLR signals, also by inhibiting mTOR activation and Stat3 phosphorylation, lysates of Tpl2+/+ and Tpl2−/− bone marrow-derived macrophages (BMDMs) harvested before and after stimulation with LPS were probed with phosphoantibodies specific for mTOR, p70S6K, Stat3, and ERK (control). The results show that, similar to mTOR inhibition, Tpl2 ablation in macrophages inhibits mTOR, p70S6K, and Stat3 phosphorylation by TLR signals (Fig. 3B). These findings suggest that the defect in IL-10 induction by TLR signals in Tpl2−/− macrophages may be attributable to inhibition of mTOR activation in these cells.
Tpl2 Ablation Is Associated with Low Numbers of Tregs in the Intestinal Mucosa and Peripheral Lymphoid Organs.
Intestinal IL-10 deficiency in Tpl2−/− mice may be attributable, at least in part, to a decrease in the number of Tregs in the intestines of these mice. Consistent with this possibility are observations that Tregs exert a protective role in intestinal tumorigenesis and that their dysregulation contributes to Apcmin-mediated intestinal polyposis (2, 3, 18).
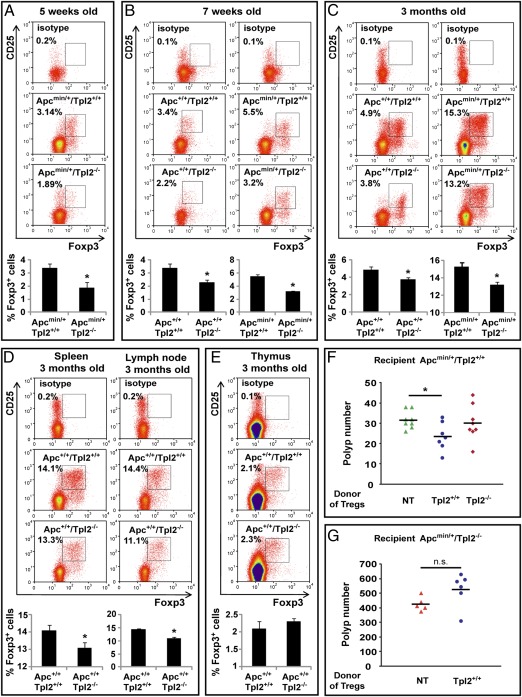
To determine the relative number of Tregs in the intestines of Apc+/+/Tpl2+/+, Apc+/+/Tpl2−/−, Apcmin/+/Tpl2+/+, and Apcmin/+/Tpl2−/− mice, mononuclear cells prepared from collagenase-digested gut tissue were stained with antibodies that recognize T-cell surface markers and intracellular Foxp3, and they were analyzed by flow cytometry. The results (Fig. 4 A–C) revealed that the intestines of Tpl2−/− mice contain lower numbers of Foxp3+ Tregs than the intestines of Tpl2+/+ mice and that the difference is independent of polyposis. Moreover, the correlation between Tpl2 ablation and the reduction in the number of intestinal Tregs was observed in both young (5- and 7-wk-old) Apcmin/+ mice, which carry only a small number of micropolyps, and older (3-mo-old) polyp-bearing Apcmin/+ mice. The Treg defect extended also in the spleen and the lymph nodes of Tpl2−/− mice (Fig. 4D). We conclude that the ablation of Tpl2 in both the Apc+/+ and Apcmin/+ genetic backgrounds gives rise to a generalized regulatory T-cell defect that is not limited to the intestines.
Fig. 4.
Ablation of Tpl2 results in the reduction of Tregs in the intestinal mucosa and peripheral lymphoid organs. (A–C) Single-cell suspensions of intestinal mononuclear cells from Apc+/+/Tpl2+/+, Apc+/+/Tpl2−/−, Apcmin/+/Tpl2+/+, and Apcmin/+/Tpl2−/− mice were stained with anti-CD4, anti-CD25, and anti-Foxp3 antibodies, and they were analyzed by flow cytometry for the abundance of CD4+/CD25+/Foxp3+ Treg cells. Five-week-old mice (A; n = 5 per genotype), 7-wk-old mice (B; n = 5 per genotype), and 3-mo-old mice (C; n = 7 per genotype) were included in the analysis. Single-cell suspensions of splenocytes and lymph node lymphocytes (D) and thymocytes (E) from 3-mo-old mice (n = 7 per genotype) were analyzed for the abundance of CD4+/CD25+/Foxp3+ Treg cells as in A–C. Ablation of Tpl2 resulted in the reduction of Tregs in the spleen, lymph nodes, and intestine (iTregs) but not in the thymus (nTregs). The histograms show the mean value for each group. *P < 0.05. (F) Whereas Tpl2+/+ Tregs partially protect Apcmin/+/Tpl2+/+ mice from the development of intestinal polyps, Tpl2−/− Tregs do not. Seven 9-wk-old Apcmin/+/Tpl2+/+ mice were inoculated with 4.5 × 105 CD4+/CD45RBlow/CD25+ Tpl2+/+ Tregs, and eight age-matched Apcmin/+/Tpl2+/+ mice were inoculated with 4.5 × 105 CD4+/CD45RBlow/CD25+ Tpl2−/− Tregs. Eight age-matched Apcmin/+/Tpl2+/+ mice were used as controls. All mice were killed at the age of 13 wk. The difference in the number of polyps between the control mice and the mice inoculated with the Tpl2+/+ Tregs was statistically significant (P = 0.01534). NT, not transplanted. (G) Six 9-wk-old Apcmin/+/Tpl2−/− mice were inoculated with Tpl2+/+ Tregs as in F. Five age-matched Apcmin/+/Tpl2−/− mice were used as controls.
Foxp3+ Tregs are generated in the thymus [natural Tregs (nTregs)], and they are dispersed in the periphery. Alternatively, they are induced extrathymically in the course of the T-cell response to external signals (iTregs) (47). The preceding data showed that Tpl2−/− mice have reduced numbers of extrathymic Tregs. Staining thymocytes from Apc+/+/Tpl2+/+ and Apc+/+/Tpl2−/− mice with a Foxp3 antibody revealed no Tpl2-dependent differences in the number of thymic nTregs (Fig. 4E). Therefore, Tpl2 ablation gave rise to a Treg defect that was limited to extrathymic tissues. We conclude that the ablation of Tpl2 does not affect the generation of nTregs in the thymus.
Tpl2+/+ Tregs Protect Apcmin/+/Tpl2+/+ Mice from Intestinal Polyposis, Whereas Tpl2−/− Tregs Do Not.
Reanalyzing the above data for the expression of Foxp3 in CD4+/CD25+ Tregs revealed that the expression of Foxp3 was lower in cells isolated from Apc+/+/Tpl2−/− mice (Fig. S7). Because the levels of Foxp3 correlate with the functional activity of Tregs (48), we proceeded to determine whether Tpl2+/+ and Tpl2−/− Tregs differ in their ability to inhibit polyp formation in Apcmin/+/Tpl2+/+ mice. To this end, Apcmin/+/Tpl2+/+ mice were inoculated with 4.5 × 105 CD4+/CD45RBlow/CD25+ Tregs from WT or Tpl2−/− mice. The results revealed that Tpl2+/+ Tregs were more efficient in protecting the Apcmin/+/Tpl2+/+ mice from intestinal polyposis than Tpl2−/− Tregs, which appeared to be functionally inactive in this assay (Fig. 4F). However, although Tpl2+/+ Tregs inhibited tumorigenesis when transplanted into Apcmin/+/Tpl2+/+ mice, they failed to inhibit tumorigenesis when transplanted into Apcmin/+/Tpl2−/− mice (Fig. 4G), suggesting that the expression of Tpl2 in the intestinal microenvironment is required to support normal Treg function.
Tpl2 Contributes to the Generation of iTregs in Response to TGF-β.
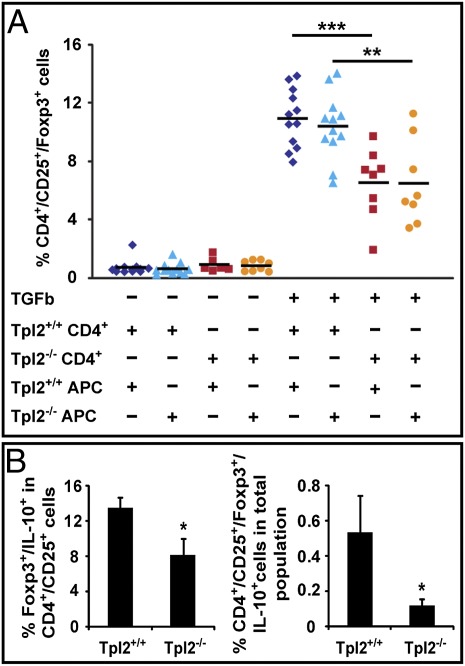
The generation of iTregs can be modeled in culture by stimulating naive CD4+ T cells with anti-CD3 and anti-CD28, in combination with TGF-β. Cells subjected to this treatment differentiate into Foxp3+ Tregs within a period of 4–5 d. To determine whether Tpl2 is required for the generation of iTregs, CD4+ naive T cells from Apc+/+/Tpl2+/+ and Apc+/+/Tpl2−/− mice were stimulated with anti-CD3, anti-CD28, and TGF-β in the presence of antigen-presenting cells from Apc+/+/Tpl2+/+ or Apc+/+/Tpl2−/− mice. The results (Fig. 5A) revealed that the generation of CD4+/CD25+/Foxp3+ iTregs was significantly impaired in Tpl2−/− T cells and that the origin of the antigen-presenting cells did not significantly affect the outcome. The independence of the outcome from the genetic identity of the antigen-presenting cells suggests that the defect in iTreg induction in Tpl2−/− T cells is T cell-intrinsic. Analysis of GolgiStop (BD Biosciences)-treated parallel cultures for the expression of IL-10 revealed that the percentage of IL-10–expressing Foxp3+ Tregs among the CD4+/CD25+ cells and among the CD4+ cells was also reduced (Fig. 5B, Left and Right, respectively). We conclude that Tpl2 is required for the generation of iTregs and that the defect caused by the ablation of Tpl2 may be responsible for the decreased numbers of iTregs in Tpl2−/− mice.
Fig. 5.
Ablation of Tpl2 suppresses the generation of Tregs in culture. (A) Tpl2+/+ and Tpl2−/− CD4+ naive T cells were cocultured with irradiated antigen-presenting cells from Tpl2+/+ or Tpl2−/− mice. Cultures with all four combinations of CD4+ T cells and antigen-presenting cells were stimulated with anti-CD3 and anti-CD28 plus TGF-β1, and they were analyzed by flow cytometry 5 d later. The figure shows the percentage of CD4+/CD25+/Foxp3+ Treg cells in cultures of CD4+ T cells derived from 12 Tpl2+/+ and 8 Tpl2−/− mice. APC, antigen-presenting cells. (B) Tpl2+/+ CD4+ naive T cells cocultured with irradiated Tpl2+/+ antigen-presenting cells and Tpl2−/− CD4+ naive T cells cocultured with irradiated Tpl2−/− antigen-presenting cells as in A were treated with GolgiStop before harvesting, and they were stained with anti-CD4, anti-CD25, anti-Foxp3, and IL-10 antibodies. The figure shows the mean of the percentage of Foxp3+/IL-10+ cells within the population of CD4+/CD25+ T cells (Left) and the mean of the percentage of CD4+/CD25+/Foxp3+/IL-10+ cells within the total cell population in these cultures (Right). *P < 0.05; **P < 0.01; ***P < 0.001.
Accumulation of Tregs, CD4+/CD25+/Foxp3−/IL-10+ T Cells, and CD4+/Foxp3−/IL-17+ Cells in the Intestinal Mucosa and the Rate of Appearance of New Polyps Change with Age Disproportionately in Apcmin/+/Tpl2+/+ and Apcmin/+/Tpl2−/− Mice.
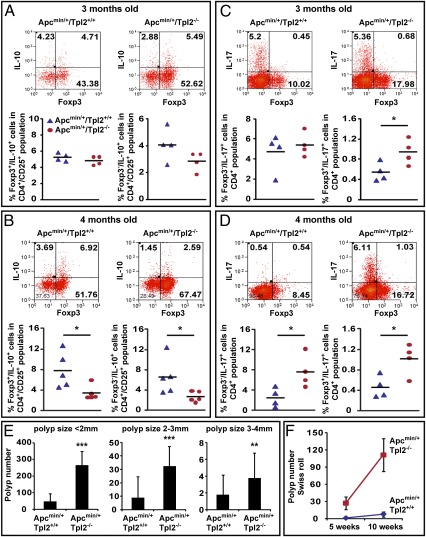
The preceding in vitro studies raised the question of whether Tpl2 modulates the secretion of IL-10 from intestinal T cells. To address this question, we focused on two age groups of Apcmin/+/Tpl2+/+ and Apcmin/+/Tpl2−/− mice: a younger (3-mo-old) group and an older (4-mo-old) group. The younger mice were generally healthy, whereas the older mice were showing signs of systemic illness. Mononuclear cells from the intestinal mucosa of these mice were cultured in the presence of phorbol 12-myristate 13-acetate, ionomycin, and GolgiStop. The harvested cells were stained with anti-CD4, anti-CD25, anti-Foxp3, and anti–IL-10. The results showed that in the younger age group, the numbers of CD4+/CD25+/Foxp3+/IL-10+ T cells (Tregs) and CD4+/CD25+/Foxp3−/IL-10+ T (Tr1) cells tended to be lower in Apcmin/+/Tpl2−/− mice than in Apcmin/+/Tpl2+/+ mice, but the differences were not statistically significant (Fig. 6A). In the older age group however, the differences were significant (Fig. 6B).
Fig. 6.
Foxp3+ and Foxp3− CD4+/CD25+/IL-10+ and Foxp3+ and Foxp3− CD4+/IL-17+ cells in the intestinal mucosa of Apcmin/+/Tpl2+/+ and Apcmin/+/Tpl2−/− mice. Percentage of Foxp3+ and Foxp3− CD4+/CD25+/IL-10+ T cells in the intestinal mucosa of younger (A; 3-mo-old) and older (B; 4-mo-old) Apcmin/+/Tpl2+/+ and Apcmin/+/Tpl2−/− mice. Percentage of Foxp3+ and Foxp3− CD4+/IL-17+ T cells in the intestinal mucosa of younger (C; 3-mo-old) and older (D; 4-mo-old) Apcmin/+/Tpl2+/+ and Apcmin/+/Tpl2−/− mice. (E) Size distribution of intestinal polyps in the two experimental mouse groups. The figure shows the mean number of polyps in Apcmin/+/Tpl2+/+ (n = 47) and Apcmin/+/Tpl2−/− (n = 28) mice. The difference in the number of polyps between the two groups of mice is larger in the small polyp size (<2 mm) category than in the large polyp size (3–4 mm) category. (F) Rate of polyp formation increases more rapidly in Apcmin/+/Tpl2−/− mice than in Apcmin/+/Tpl2+/+ mice. *P < 0.05; **P < 0.01; ***P < 0.001.
The intestinal inflammatory infiltrates developing in Apcmin/+ mice also contain proinflammatory CD4+/IL-17+ T cells (49). To determine whether the ablation of Tpl2 alters the abundance of these cells as well, we repeated the preceding experiment but replaced staining with anti–IL-10 by staining with anti–IL-17. The results in Fig. 6C showed that the numbers of CD4+/Foxp3−/IL-17+ (Th17) cells tended to be higher in the Apcmin/+/Tpl2−/− mice in both age groups. However, the difference was statistically significant only in the older age group (Fig. 6D), suggesting that the accumulation of the proinflammatory Th17 cells increases with age but that the increase is disproportionately more pronounced in Apcmin/+/Tpl2−/− mice. It is noteworthy that CD4+/Foxp3+/IL-17+ cells, which appear in small numbers and may represent Tregs undergoing a transition to Th17 cells (3, 50), were also increased disproportionally with time in the Apcmin/+/Tpl2−/− mice. These data suggest that proinflammatory signals triggered by the developing polyps are more robust in Apcmin/+/Tpl2−/− mice, perhaps because the enhancement of polyp initiation in these mice results in a higher tumor burden throughout life. Alternatively, polyps developing in Apcmin/+/Tpl2−/− mice may produce a more robust proinflammatory environment than polyps developing in Apcmin/+/Tpl2+/+ mice.
Given the importance of inflammation in intestinal tumorigenesis, the preceding data prompted us to examine the rates by which the numbers of polyps increase with time in Apcmin/+/Tpl2−/− and Apcmin/+/Tpl2+/+ mice. We predicted that if the number of polyps correlated positively with the percentage of Th17 cells and negatively with the percentage of Tregs, the polyp number would increase more rapidly in Apcmin/+/Tpl2−/− mice. Initial support of the aforementioned prediction was provided from measurements of the size of the polyps. These measurements revealed small (<2 mm), medium (2–3 mm), and large (3–4 mm) polyps. Very large polyps (>4 mm) were few in number, they were only detected in the colon, and they were not included in this analysis. The number of polyps was higher in Apcmin/+/Tpl2−/− mice than in Apcmin/+/Tpl2+/+ mice across all size categories. However, the difference was more pronounced in the small size category rather than in the large size category (Fig. 6E). We reasoned that if the large polyps were the earliest to develop and the small polyps were the latest, the rate of development of new polyps should increase with time and the increase should be more rapid in the Apcmin/+/Tpl2−/− mice than in the Apcmin/+/Tpl2+/+ mice. To address this hypothesis, we measured the number of polyps in the two mouse groups at different time points. Plotting the change in polyp number over time confirmed the prediction (Fig. 6F).
The correlation between the rates by which the numbers of Treg, Tr1, and Th17 cells in the intestinal mucosa change with time and the rates by which the numbers of intestinal polyps increase with time in Apcmin/+/Tpl2−/− and Apcmin/+/Tpl2+/+ mice provided additional support for the concept that the promotion of intestinal tumorigenesis by the ablation of Tpl2 is attributable to the proinflammatory effects of the Tpl2 ablation.
Discussion
Data presented in this paper revealed that the ablation of Tpl2 enhances the oncogenic potential of the Apcmin mutation. Apcmin/+/Tpl2−/− mice indeed developed at least fivefold more polyps and succumbed to the polyposis syndrome at a younger age than Apcmin/+/Tpl2+/+ mice. Bone marrow transplantation experiments showed that the ablation of Tpl2 in hematopoietic cells is necessary, although not sufficient, to elicit the protumorigenic Tpl2−/− phenotype in Apcmin/+ mice. Therefore, the increased susceptibility of Apcmin/+/Tpl2−/− mice to intestinal tumorigenesis is, at least in part, hematopoietic cell-driven. In agreement with this observation, Tpl2 ablation was shown to promote the establishment of a proinflammatory environment in the intestinal mucosa, suggesting that it promotes polyp formation by enhancing intestinal inflammation. Experiments in Tpl2+/+ and Tpl2−/− mice in the WT APC background provided evidence that the initiating events for the enhancement of tumorigenesis in Apcmin/+/Tpl2−/− mice are the low expression of IL-10 and a T-cell defect in the generation and function of iTregs and Tr1 cells.
The initial characterization of the intestinal inflammatory infiltrates focused on F4/80+/CD11b+-activated macrophages, Gr1+/CD11b+ tumor-associated myeloid-derived suppressor cells, F4/80+/CD11b− immature monocytic cells, and Gr1+/CD11b− granulocytes. The results showed that by as early as the 7 wk of age, there is increased accumulation of F4/80+/CD11b+ and Gr1+/CD11b+ cells in the intestinal mucosa of the Apcmin/+/Tpl2−/− mice. F4/80+/CD11b+-activated macrophages are a source of proinflammatory mediators, and an increase in this population has been associated with inflammation-induced tumorigenesis (43, 51). Similarly, Gr1+/CD11b+ myeloid-derived suppressor cells have been linked to the production of proinflammatory and proangiogenic factors as well as to tumor induction (43, 51). The finding of high COX-2, IL-6, and TNF-α expression in the intestines of the Apcmin/+/Tpl2−/− mice was consistent with the higher percentage of F4/80+/CD11b+ and Gr1+/CD11b+ cells in the intestinal mucosa of these mice.
Although inflammation promotes polyp formation in Apcmin/+ mice, developing polyps themselves also trigger inflammatory reactions that act in a positive feedback loop to promote polyposis (3–5). Therefore, the question was raised of whether the different composition of the inflammatory infiltrates and the high levels of proinflammatory cytokines in the intestines of aging Apcmin/+/Tpl2−/− mice were attributable to the higher number of polyps in these mice. If this was the case, the enhanced inflammatory environment associated with the higher number of polyps in Apcmin/+/Tpl2−/− mice would perhaps promote the development of additional polyps. However, it would not explain how the interdependent processes of inflammation and tumorigenesis were initiated in the first place. Given the known role of Tpl2 in the regulation of innate immunity and the results of the bone marrow transplantation experiment, we hypothesized that Tpl2 ablation induced inflammation and that the enhanced tumorigenesis observed in the Apcmin/+/Tpl2−/− mice was secondary to the more severe intestinal inflammation in these mice. To address this hypothesis, we embarked on studies aiming to determine whether intestinal inflammation can be detected in the intestinal mucosa of Apc+/+/Tpl2−/− mice that do not develop polyps, and whether it precedes polyp formation in young Apcmin/+/Tpl2−/− mice. The results did not reveal any significant differences in the composition of the intestinal inflammatory infiltrates between Tpl2+/+ and Tpl2−/− mice and 5-wk-old Apcmin/+/Tpl2+/+ and Apcmin/+/Tpl2−/− mice. However, they showed that the ablation of Tpl2 is associated with low levels of IL-10 and low numbers of Tregs in the intestinal mucosa of both the Tpl2−/− mice and the 5-wk-old Apcmin/+/Tpl2−/− mice. Given the importance of both IL-10 and Tregs in intestinal inflammation and tumorigenesis, we conclude that Tpl2 ablation stimulates both by inhibiting the expression of IL-10 and the generation or survival of Tregs. Despite the low levels of IL-10 and the low numbers of Tregs in the intestinal mucosa of the Apc+/+/Tpl2−/− mice, we could not detect evidence of intestinal inflammation. The difference between these mice and Apcmin/+/Tpl2−/− mice suggests that by down-regulating IL-10 and Tregs, Tpl2 ablation may enhance the response to inflammatory signals triggered by polyps, which develop only in Apcmin/+ mice. Therefore, minor proinflammatory changes triggered by the ablation of Tpl2 may be magnified with time in Apcmin/+/Tpl2−/− mice because of the differential response of Tpl2+/+ and Tpl2−/− mice to polyp-induced proinflammatory signals.
IL-10 is produced by a variety of cell types, including macrophages and dendritic cells. More importantly, IL-10 induction by TLR signals in these cells depends on Tpl2 (35). Experiments reported here confirmed these data. In addition, they showed that LPS-stimulated Tpl2−/− macrophages express higher levels of IL-12p40 and IFN-β than LPS-stimulated Tpl2+/+ macrophages. Combined, these data indicate that Tpl2 ablation in macrophages gives rise to an LPS stimulation phenotype that is similar to the phenotype of LPS-stimulated and TORC1-inhibited WT macrophages (15, 16). Based on these considerations, we examined whether Tpl2 ablation in macrophages interferes with the activation of mTOR and the phosphorylation of the Stat3 in LPS-stimulated macrophages. The results confirmed that Tpl2 is required for both mTOR activation and Stat3 phosphorylation. The relevance of the down-regulation of Stat3 phosphorylation to the observed phenotype of intestinal inflammation in the Tpl2−/− genetic background is underscored by earlier findings showing that the ablation of Stat3 in myeloid cells exhibits the same proinflammatory phenotype in the intestinal mucosa as the ablation of Il10 (17).
IL-10 is also secreted by CD4+/CD25+/Foxp3+ Tregs and CD4+/Foxp3−/IL-10+ Tr1 cells (23, 52). Tregs are either nTregs or iTregs. The nTregs are produced in the thymus during positive selection, whereas the iTregs are produced in the periphery from T cells responding to antigen (53). The study presented in the present paper shows that Tpl2 is required for the maintenance of the physiological number of Tregs in the periphery but not in the thymus and that transplantation of WT Tregs inhibits intestinal tumorigenesis in Apcmin/+/Tpl2+/+ mice, whereas Tpl2−/− Tregs do not. Based on these observations, we conclude that Tpl2 regulates the production and/or survival of iTregs but not nTregs and that Tpl2−/− Tregs are functionally defective. However, WT Tregs did not inhibit polyposis in Apcmin/+/Tpl2−/− recipients, suggesting that Treg function is intact only in the Tpl2+/+ intestinal microenvironment. Because the Tpl2+/+ bone marrow inhibits tumorigenesis in the Tpl2−/− genetic background but the Tpl2+/+ Tregs do not, we propose that the cells responsible for the functional integrity of Tregs in this microenvironment are of hematopoietic origin. The most likely candidates are the macrophages, which are defective in IL-10 production in the absence of Tpl2. This is in agreement with the results of a recent paper showing that Foxp3 expression and functional activity of Tregs in the intestines of Rag−/− mice inoculated with CD4+ T cells depend on IL-10 produced by CD11b+ macrophages (14).
The number of Tregs in peripheral lymphoid and nonlymphoid organs, such as the intestines, is determined by the balance between Treg production and death. Treg production can be modeled in culture by stimulating naive CD4+ T cells with anti-CD3, anti-CD28, and TGF-β, in the presence of irradiated antigen-presenting cells. The results showed that Tpl2 ablation interferes with the production of Tregs and that the defect in iTreg induction in Tpl2−/− T cells is T-cell intrinsic. The same experiment revealed that the induction of CD4+/CD25+/Foxp3+/ IL-10+ T cells in the Tpl2−/− cultures is also defective.
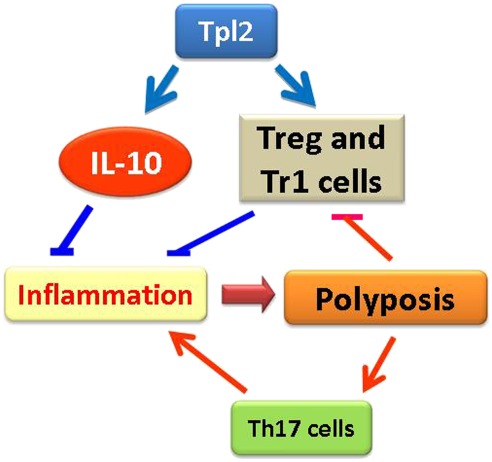
The findings in this report support the model that Tpl2 ablation promotes inflammation in Apcmin/+ mice by down-regulating the expression of IL-10 in myeloid cells in the intestinal mucosa and by interfering with the development and function of regulatory T cells. Developing polyps produce proinflammatory molecules that suppress Tregs and Tr1 cells, and stimulate the generation of Th17 cells. Such molecules may include IL-6 and other proinflammatory cytokines (54, 55). The higher polyp number in Apcmin/+/Tpl2−/− mice, which is caused by the enhancement of polyp initiation, results in accentuation of a positive feedback loop that promotes inflammation and polyposis (Fig. 7).
Fig. 7.
Model of the role of Tpl2 in intestinal tumorigenesis in Apcmin/+ mice. The initiating event in the enhancement of tumorigenesis caused by the ablation of Tpl2 is the down-regulation of IL-10 and the decrease in the number of Tregs and Tr1 cells in the intestinal mucosa. This causes inflammation, which promotes tumorigenesis. A positive feedback loop induced by signals originating in the polyps promotes inflammation and tumorigenesis by regulating the abundance of intestinal Tregs and Th17 cells. This results in progressive acceleration of polyposis, which is more rapid in the Apcmin/+/Tpl2−/− mice because of the higher tumor burden or because the polyp-induced proinflammatory signals are regulated by Tpl2.
In summary, the data in this report provide a previously undescribed genetic link between inflammation and cancer. Moreover, the Tpl2 ablation model provides a valuable tool to address the pathophysiology of IBD and colorectal cancer mechanistically in animals and humans.
Materials and Methods
Mice.
Tpl2 KO mice were described previously (30). Apcmin/+ mice were purchased from The Jackson Laboratory. Both strains of mice were in the C57BL/6 background.
Polyp Counting and Histopathological Analysis.
Intestines were removed, washed with PBS, and cut into four pieces (proximal, middle, and distal small intestine and colon). The number and size of the macroscopic polyps were measured in longitudinally opened intestines under a dissecting microscope. For the Swiss roll preparations, intestines were removed, flushed with PBS, opened longitudinally, rolled, and fixed in 10% (vol/vol) buffered formalin. Paraffin sections were stained with H&E and examined microscopically.
Mononuclear Cell Isolation and FACS Analysis.
Isolation of mononuclear cells from the intestines and antibody staining were carried out using standard procedures (3). Staining was monitored by flow cytometry. (Details are provided in SI Materials and Methods.)
Splenocytes, lymph node cells, and thymocytes were isolated as previously described (30), and they were stained with the antibodies also used to stain the mononuclear cells isolated from the intestinal mucosa. (Details are provided in SI Materials and Methods.)
Western Blotting.
BMDMs were prepared as described previously (30). Serum-starved BMDMs were stimulated with 1 μg/mL LPS (Escherichia coli 0:111; Sigma), and they were harvested at the indicated time points. Western blots of the BMDM lysates and lysates of adenomas removed from the mouse small intestines were probed with the indicated antibodies. (Details are provided in SI Materials and Methods.)
Bone Marrow Transplantation.
Bone marrow transplantation was carried out using standard procedures. Briefly, Apcmin/+/Tpl2−/− and Apcmin/+/Tpl2+/+ mice were irradiated once with 1,000 rad (J. L. Shepherd Irradiator). Irradiated mice were injected i.v. through the tail vein with 5 × 106 bone marrow cells from male Tpl2−/− or Tpl2+/+ mice. Polyps developing in all experimental mice were counted 7 wk later using Swiss roll intestinal preparations. All the irradiated nontransplanted mice died within 14 d from the time of irradiation, confirming that surviving mice were effectively transplanted. The repopulation of the recipients with the donor bone marrow was additionally confirmed by PCR of the SRY gene (male bone marrow transplanted into female recipients) or the Tpl2 gene (WT bone marrow transplanted into Tpl2−/− mice and Tpl2−/− bone marrow transplanted into Tpl2+/+ mice) (Fig. S2).
Measurement of Cytokine Levels.
BMDMs were stimulated with LPS (1 μg/mL) for 3–12 h. Selected cytokines were measured in the culture media by ELISA, using kits from BD Biosciences (IL-10), R&D Systems (IL-12p70), and the PBL (Pestka Biomedical Laboratories) interferon source (IFN-β). mRNA levels of IL-10, IL-12p35, IL-12p40, and IFN-β were measured in the same cells by real-time RT-PCR with GAPDH as the internal control (the primers are shown in Table S1). Cytokine levels were measured, also by ELISA, in homogenates of the small intestines. (Details are provided in SI Materials and Methods.)
Generation of Tregs in Culture.
Naive CD4+ T cells (CD4+/CD8−/CD25−/CD62Lhi) and antigen-presenting cells (CD4−/CD8−) were FACS-sorted, using a MoFlow sorter (DakoCytomation). Mixed cultures of naive CD4+ T cells (2 × 105 cells) and irradiated antigen-presenting cells (8 × 105 cells) were stimulated with soluble anti-CD3 and anti-CD28 antibodies and with recombinant TGF-β1 for 5 d. GolgiStop was added to the cultures that were to be used for intracellular staining 3 h before harvesting. For intracellular staining, cells were fixed with 0.5% paraformaldehyde, permeabilized with 0.5% saponin and stained with the indicated antibodies. (Details are provided in SI Materials and Methods.)
Supplementary Material
Acknowledgments
We thank Dr. Cinzia Sevignani for the technical assistance with the initial Apcmin and Tpl2 genotyping. We thank Monica Bentacur for technical assistance with the bone marrow transplantation experiment and the Tufts Medical Center Cancer Center flow cytometry facility for all the experiments requiring cell sorting. We also thank Drs. Philip Hinds, Alexander Poltorak, and Henry Wortis for helpful discussions and for reviewing the manuscript. This work was supported by National Institutes of Health Grant R01 CA124835 (to P.N.T.) and by the European Commission (EC) research program INFLA-CARE (EC contact no. 223151) (A.G.E.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.H. is a guest editor invited by the Editorial Board.
See Author Summary on page 6802 (volume 109, number 18).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115098109/-/DCSupplemental.
References
- 1.Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- 2.Blatner NR, et al. In colorectal cancer mast cells contribute to systemic regulatory T-cell dysfunction. Proc Natl Acad Sci USA. 2010;107:6430–6435. doi: 10.1073/pnas.0913683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gounaris E, et al. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res. 2009;69:5490–5497. doi: 10.1158/0008-5472.CAN-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gounaris E, et al. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci USA. 2007;104:19977–19982. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gounaris E, et al. Live imaging of cysteine-cathepsin activity reveals dynamics of focal inflammation, angiogenesis, and polyp growth. PLoS ONE. 2008;3:e2916. doi: 10.1371/journal.pone.0002916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol. 2009;9:405–410. doi: 10.1016/j.coph.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erdman SE, et al. Nitric oxide and TNF-alpha trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc Natl Acad Sci USA. 2009;106:1027–1032. doi: 10.1073/pnas.0812347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elder DJ, Paraskeva C. COX-2 inhibitors for colorectal cancer. Nat Med. 1998;4:392–393. doi: 10.1038/nm0498-392. [DOI] [PubMed] [Google Scholar]
- 10.Newman JV, Kosaka T, Sheppard BJ, Fox JG, Schauer DB. Bacterial infection promotes colon tumorigenesis in Apc(Min/+) mice. J Infect Dis. 2001;184:227–230. doi: 10.1086/321998. [DOI] [PubMed] [Google Scholar]
- 11.Seno H, et al. Cyclooxygenase 2- and prostaglandin E(2) receptor EP(2)-dependent angiogenesis in Apc(Delta716) mouse intestinal polyps. Cancer Res. 2002;62:506–511. [PubMed] [Google Scholar]
- 12.Berg DJ, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson NJ, et al. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med. 1996;184:241–251. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murai M, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weichhart T, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeda K, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 18.Erdman SE, et al. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- 19.Salama P, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 20.Frey DM, et al. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010;126:2635–2643. doi: 10.1002/ijc.24989. [DOI] [PubMed] [Google Scholar]
- 21.Haas M, et al. Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterol. 2009;9:65. doi: 10.1186/1471-230X-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badoual C, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 23.Maloy KJ, et al. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Erny KM, Peli J, Lambert JF, Muller V, Diggelmann H. Involvement of the Tpl-2/cot oncogene in MMTV tumorigenesis. Oncogene. 1996;13:2015–2020. [PubMed] [Google Scholar]
- 26.Patriotis C, Makris A, Bear SE, Tsichlis PN. Tumor progression locus 2 (Tpl-2) encodes a protein kinase involved in the progression of rodent T-cell lymphomas and in T-cell activation. Proc Natl Acad Sci USA. 1993;90:2251–2255. doi: 10.1073/pnas.90.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patriotis C, Makris A, Chernoff J, Tsichlis PN. Tpl-2 acts in concert with Ras and Raf-1 to activate mitogen-activated protein kinase. Proc Natl Acad Sci USA. 1994;91:9755–9759. doi: 10.1073/pnas.91.21.9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsatsanis C, Patriotis C, Tsichlis PN. Tpl-2 induces IL-2 expression in T-cell lines by triggering multiple signaling pathways that activate NFAT and NF-kappaB. Oncogene. 1998;17:2609–2618. doi: 10.1038/sj.onc.1202460. [DOI] [PubMed] [Google Scholar]
- 29.Ceci JD, et al. Tpl-2 is an oncogenic kinase that is activated by carboxy-terminal truncation. Genes Dev. 1997;11:688–700. doi: 10.1101/gad.11.6.688. [DOI] [PubMed] [Google Scholar]
- 30.Dumitru CD, et al. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–1083. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 31.Eliopoulos AG, Wang CC, Dumitru CD, Tsichlis PN. Tpl2 transduces CD40 and TNF signals that activate ERK and regulates IgE induction by CD40. EMBO J. 2003;22:3855–3864. doi: 10.1093/emboj/cdg386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatziapostolou M, Polytarchou C, Panutsopulos D, Covic L, Tsichlis PN. Proteinase-activated receptor-1-triggered activation of tumor progression locus-2 promotes actin cytoskeleton reorganization and cell migration. Cancer Res. 2008;68:1851–1861. doi: 10.1158/0008-5472.CAN-07-5793. [DOI] [PubMed] [Google Scholar]
- 33.Tsatsanis C, et al. Tpl2 and ERK transduce antiproliferative T cell receptor signals and inhibit transformation of chronically stimulated T cells. Proc Natl Acad Sci USA. 2008;105:2987–2992. doi: 10.1073/pnas.0708381104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatziapostolou M, et al. Tumor progression locus 2 mediates signal-induced increases in cytoplasmic calcium and cell migration. Sci Signal. 2011;4:ra55. doi: 10.1126/scisignal.2002006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaiser F, et al. TPL-2 negatively regulates interferon-beta production in macrophages and myeloid dendritic cells. J Exp Med. 2009;206:1863–1871. doi: 10.1084/jem.20091059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317(5834):124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 37.Watford WT, et al. Tpl2 kinase regulates T cell interferon-gamma production and host resistance to Toxoplasma gondii. J Exp Med. 2008;205:2803–2812. doi: 10.1084/jem.20081461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watford WT, et al. Ablation of tumor progression locus 2 promotes a type 2 Th cell response in Ovalbumin-immunized mice. J Immunol. 2010;184:105–113. doi: 10.4049/jimmunol.0803730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukata M, et al. Innate immune signaling by Toll-like receptor-4 (TLR4) shapes the inflammatory microenvironment in colitis-associated tumors. Inflamm Bowel Dis. 2009;15:997–1006. doi: 10.1002/ibd.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SH, et al. ERK activation drives intestinal tumorigenesis in Apc(min/+) mice. Nat Med. 2010;16:665–670. doi: 10.1038/nm.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das S, et al. Tpl2/cot signals activate ERK, JNK, and NF-kappaB in a cell-type and stimulus-specific manner. J Biol Chem. 2005;280:23748–23757. doi: 10.1074/jbc.M412837200. [DOI] [PubMed] [Google Scholar]
- 42.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114.e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 43.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen GY, Shaw MH, Redondo G, Núñez G. The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 2008;68:10060–10067. doi: 10.1158/0008-5472.CAN-08-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao H, et al. Loss of single immunoglobulin interlukin-1 receptor-related molecule leads to enhanced colonic polyposis in Apc(min) mice. Gastroenterology. 2010;139:574–585. doi: 10.1053/j.gastro.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murai M, Krause P, Cheroutre H, Kronenberg M. Regulatory T-cell stability and plasticity in mucosal and systemic immune systems. Mucosal Immunol. 2010;3:443–449. doi: 10.1038/mi.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: More of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 49.Chae WJ, et al. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc Natl Acad Sci USA. 2010;107:5540–5544. doi: 10.1073/pnas.0912675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: Regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 51.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: Limiting collateral damage. J Clin Invest. 2004;114:1372–1378. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 54.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 55.McGeachy MJ, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]