Abstract
In all organisms tRNAs play the essential role of connecting the genetic information found in DNA with the protein synthesis machinery ensuring fidelity during translation. Following transcription tRNAs undergo a number of processing events including numerous post-transcriptional modifications that render a tRNA molecule fully functional. The effects of some modifications go beyond simply affecting tRNA structure and can alter the meaning of the tRNA. This review will summarize the current state of the tRNA editing field, highlighting how editing affects tRNA structure and function in various organisms. It will also discuss recent data that hints at connections between editing and modification that may be exploited by cells to modulate a tRNA’s role in translation.
Keywords: tRNA, editing, modification, post-transcriptional, processing, mitochondria
1. Introduction
In all organisms tRNAs are transcribed as precursor molecules containing extra sequences at their 5′ and 3′ ends and in a few cases introns. These have to be removed by a series of maturation steps that generate a full-length tRNA required for protein synthesis. However, tRNA maturation is not limited to trimming of these extra sequences but also includes the addition of numerous post-transcriptional chemical modifications that ensure proper folding and function of the mature tRNA. These can target the ribose sugar as well as any position on the bases. On average, each tRNA molecule contains 12 modifications, with over 100 different naturally occurring chemical modifications described so far [1]. In fact, it has been suggested that the tRNA modifications carry more genetic information than tRNA genes themselves [2]. Although the positions of some modifications are conserved among tRNAs from all domains of life, many are unique to specific tRNAs.
In 1965 the modified nucleotides inosine and 1-methyl inosine were among 9 unusual nucleotides found in yeast tRNAAla [3, 4]. Francis Crick suggested in his wobble rules that inosine could form sufficiently stable non-Watson and Crick base pairs providing critical flexibility, without sacrificing translational fidelity [5]. Inosine could thus allow a single tRNA to base pair with multiple codons for the same amino acid, expanding a tRNA’s decoding capacity and obviating the need for additional tRNAs to be encoded in genomes. Post-transcriptional modifications that alter or expand decoding capacity are now part of an ever-growing number of events known as tRNA editing. This review will mostly focus on examples of tRNA editing described in eukaryotic organisms. These include nucleotide additions and single nucleotide deamination events that may affect both the structure and function of tRNAs. We will also highlight a number of recent examples, where editing and modification are closely intertwined and occur as interrelated reactions. It is our view that all these events are carefully orchestrated within cells to not only ensure overall tRNA function but at times to also modulate it, thus having direct impact on decoding and translational efficiency.
2. A brief history of tRNA editing: “Unconventional tRNA alterations”
Thirteen of the sixteen tRNA genes encoded in the mitochondrial genome of the protozoan Acanthamoeba castellanii are unusual in that they naturally contain nucleotide mismatches in the aminoacyl acceptor stem [6, 7](Fig. 1). The precedent set by the discovery of mRNA editing in trypanosomes by Benne and co-workers [8], led to the suggestion that these non-canonical tRNAs could be rendered functional by an editing type mechanism, which will effectively restore base pairing in the acceptor stem and allow aminoacyl tRNA synthetase recognition. Direct sequencing of the gene products of this “defective” tRNAs revealed that the observed mismatches had been post-transcriptionally corrected at the RNA level, whereby purine to purine (A to G) and pyrimidine to purine (U to A/G) base-replacement editing had restored full base pairing of the stems [7]. Likewise Spizellomyces punctatus, a chytridomycete fungus, phylogenetically distant and unrelated to A. castellanii, exhibits an analogous system of mitochondrial tRNA editing (Fig. 1). Similar changes in the acceptor stem have also been noted in tRNAs of land snail, Physarum and squid tRNAThr [9-12].
Fig. 1.
Editing of the amino acid acceptor stems of tRNA. Editing repairs mismatched nucleotides naturally occurring in the encoding genes, resulting in a fully functional tRNA. Edited positions are denoted by colored circles with all the corresponding editing events listed in the corresponding boxes.
Since the observed nucleotide replacements described above restore base pairing, all the genetic information required for editing must be contained within the tRNAs itself, and unlike trypanosome editing, these types of nucleotide additions do not seem to require small trans-acting guide RNAs to provide the editing information. Gray and co-workers proposed a nucleotide addition mechanism, whereby the mismatched nucleotides are removed from the 5′ end of the tRNA and replaced by nucleotides that recreate canonical base pairs [13, 14]. This mode of nucleotide insertion is of course unusual in that it requires the 3′ to 5′ templated addition of nucleotides, where the sequences on the 3′ side of the acceptor stem will provide the editing information. In support of this model, they showed that A. castellanii mitochondrial extracts could indeed edit a synthetic tRNA [14]. A similar activity was also described with Spizellomyces punctatus extracts, [13].
To date, the only example of naturally occurring addition of a nucleotide in the 3′ to 5′ direction in tRNA occurs during the incorporation of guanosine in tRNAHis [15]. A reaction that creates an unpaired G at the 5′ end of tRNAHis that is absolutely required for synthetase recognition [16]. Phizicky and co-workers while studying the enzyme responsible for G-1 addition to tRNAHis of yeast (tRNAHis guanylyltransferase, Thg1), first made the connection that the non-templated reaction was akin to the editing required of A. castellani [15, 17]. They found that indeed this enzyme could polymerize nucleotides in the 3′ to 5′ direction, but unlike the G-1 addition, reverse polymerization occurred in a templated fashion [15, 17].
Recently, genes that encode tRNAs with mismatches at the 3′ end of the acceptor stems have been reported in the mitochondria of the centipede Lithobius forficatus [18] and Seculamonas ecuadoriensis (a single-cell protist) [19]. In these systems, mismatched nucleotides are replaced with nucleotides that regenerate canonical base pairs. Although neither the activity nor an in vitro assay has been established for this system. It has been proposed that due to the templated nature of this 3′ addition, an RNA-dependent RNA polymerase could represent a perfect candidate enzyme for this type of editing.
3. Editing by deamination: avoiding conundrums
3.1. Adenosine to inosine (A-to-I) editing
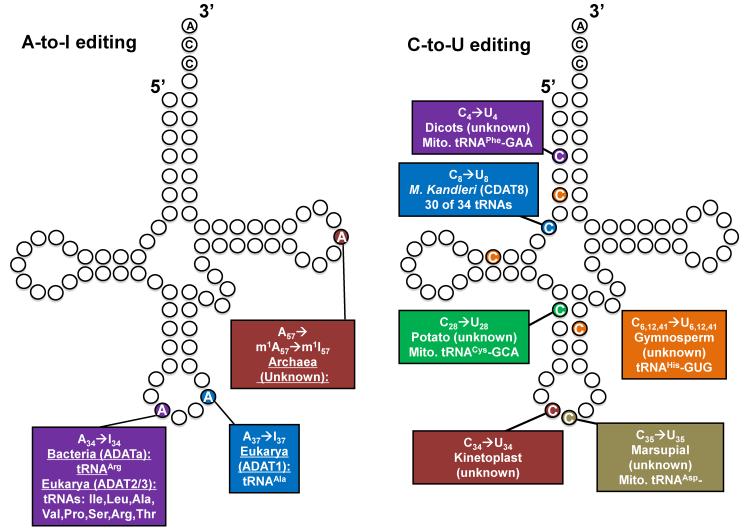
Despite its early discovery, it took over 30 years before the mechanism of inosine formation in tRNA was clearly understood. Initially it was proposed that inosine was the result of direct replacement of adenine for hypoxanthine involving a transglycosylation-type mechanism but attempts to reconstitute this activity proved futile. It is now well established that, just like with free nucleotide interconversions and inosine formation in mammalian mRNAs, inosine in tRNA is the result of a hydrolytic deamination reaction catalyzed by ADATs (Adenosine Deaminases Acting on tRNA) (Fig 2). Unlike mRNA A-to-I editing, however, which can target many sites in an mRNA, A-to-I editing in tRNA is so far restricted to three positions [20] (Fig 2).
Fig. 2.
A-to-I editing and C-to-U editing of tRNA. Only few examples of C-to-U editing of tRNAs have been described and in all cases, with the exception of archaea, the editing enzyme has not been identified. Edited nucleotides are shown in colored circles with numbers denoting the specific tRNA nucleotide position. The enzymes responsible for a particular editing event is shown in parentheses, unless the enzyme has not been identified which is denoted as “unknown”. The colored boxes also show the tRNAs and organisms where editing has been described.
Editing of tRNA at position 37 is a non-essential event observed solely in tRNAAla of eukaryotes [21, 22] and is catalyzed by ADAT1, alternatively known as Tad1p. ADAT1 is homodimeric enzymes and shares conserved sequence motifs with other well-characterized adenosine deaminases, including the mammalian mRNA deaminases (ADARs) (Fig. 2). Also, like most members of the deaminase superfamily, a Zn2+ ion is coordinated by a conserved histidine and two cysteines. The fourth ligand is an actived water molecule that acts as the nucleophile. A highly conserved glutamate then aids the reactions by shuttling a proton from water to the N1 position of inosine during the deamination reaction. These motifs constitute the canonical deaminase signature (C/H)xExnPCxxC found in most nucleotide deaminases [21-24]. The ADAT1 homodimer is very specific for tRNA, and unlike ADARs, is inactive on double stranded DNA (dsDNA) or pre-mRNA, likely due to the absence of a double stranded RNA binding domain (dsRBD) [22, 25]. The biological significance of I37 in tRNAAla is still not fully clear but since it does not affect the anticodon sequences directly, it does not expand decoding capacity or alters the meaning of tRNAAla in decoding.
In eukarya, 7-8 cytoplasmic tRNAs (depending on the organism) contain inosine in the first position of the anticodon (I34) [26, 27], which is required for decoding the C-ending codons for the amino acids Ile, Ala, Leu, Pro, Val, Ser, Arg, and Thr (Fig. 2). Since no G34-containing tRNAs for these codons exist in eukarya that could potentially read these codons, unlike I37, A-to-I editing at position 34 is essential for viability. Inosine 34 is also an important determinant for aminoacyl tRNA synthetase [23, 28].
In eukaryotes, A-to-I editing at position 34 is catalyzed by the heterodimeric enzyme ADAT2/ADAT3 (alternatively known as Tad2p and Tad3p) (Fig. 2). Although the mechanism for A34 deamination is similar to that of ADAT1, the two enzymes show marked differences in terms of sub-unit composition as well as at the primary sequence level. The arrangement of active site residues of ADAT2 and ADAT3 resemble those of a cytidine deaminase while residues of ADAT1 resemble those of classical adenosine deaminases [23, 29]. Paradoxically, these enzymes that catalyze A to I editing at position 34 possess all the features of cytosine-specific deaminases. Additionally, the ADAT3 active site has evolved such that the catalytic glutamate (in the HAE signature) has been naturally replaced by a non-catalytic residue (a valine in T. brucei and Saccharomyces cerevisiae). This evolutionary change led to the proposal that the ADAT3 subunit only serves a structural role [23]. Recent work from our laboratory, however, has shed some light on residues of the T. brucei enzyme that are important for substrate recognition and catalysis. This work led to an explanation for the unusual cytidine deaminase similarity as well as the role played by T. brucei ADAT3 (TbADAT3) in the context of a heterodimer. We showed that the C-terminal residues in T. brucei ADAT2 (TbADAT2) play a critical role in tRNA binding [30]. Since this C-terminal motif (KR domain) is conserved among other eukaryotic ADAT2 proteins, but absent in the bacterial counterparts, we suggest a similar role in all the eukaryotic A34-specific deaminases. Beyond this, the crystal structure of any eukaryotic ADAT2/3 enzyme, alone or in complex with tRNA, has not been reported thus a clearer picture of how tRNA is recognized is still lacking.
As for the observed similarity between A34-specific deaminases and cytidine deaminases, once again TbADAT2/3 leads the way. We showed that the T. brucei enzyme is capable of catalyzing in vitro C-to-U deamination of DNA, an argument that strongly supports the evolution of this group of ADATs from pre-existing a cytidine deaminases as previously proposed [30]. Recent work with the T. brucei enzyme also showed that TbADAT3 is not solely a structural component of the enzyme but actively partakes in catalysis [29]. Whether these observations with the trypanosomatid enzyme can be extrapolated to other A34-specific deaminases, it is not currently clear but given the degree of aminoacid conservation in their active sites, it would not be surprising if the same is true for all members of this sub-group of tRNA deaminases.
In bacteria, the C-ending codons for the amino acids Ile, Ala, Leu, Pro, Val, Ser, and Thr are decoded by naturally occurring G34-containing tRNAs encoded in their genome. However, since no G34-containing tRNA gene exists that can decode the C-ending codon for arginine, tRNAArgACG has to also undergo essential A-to-I editing, a reaction catalyzed by ADATa (TadA), a homolog of ADAT2 with similar active site residues [31, 32]. Unlike eukaryotic ADAT2/3, bacterial ADATa is a homodimer [33-35]. One major difference between bacterial ADATa and eukaryotic ADAT2/3 is their ability to efficiently deaminate in vitro a substrate as small as the anticodon stem-loop of tRNAArg, while ADAT2/3 requires a full-length tRNA for activity. This suggests that the two enzymes differ at least in the manner they bind their substrates. In line with this observation, the C-terminal residues indentified by us in TbADAT2/3 are missing from most, if not all, bacterial ADATs [36]. Additionally, although inosine has not been reported in mitochondrial tRNAs, it was shown recently that a similar situation as in bacteria occurs in plant chloroplasts, with a homolog of the bacterial ADATa catalyzing the reaction and tRNAArg2 as the only tRNA species carrying the edited nucleotide [37, 38].
3.2. Cytosine to Uridine (C-to-U) editing
Deamination of cytosine to uracil in tRNA has been documented in kinetoplastids, marsupials, plants and archaea [39-44]. There were also early reports of editing events at positions 32 and 33 of tRNAAsp in the rat liver, but attempts at data replication have not been successful [45, 46] (Beier et al 1992, Tomita et al 1996). In all instances so far of C-to-U editing of tRNAs in eukaryotes, the editing enzyme has not been identified, but given the similarity of this reaction to that described for mammalian mRNA editing mediated by Apobec, it is safe to assume that this type of editing also proceeds by a deamination mechanism. In support of this view, recently a cytidine deaminase homolog, CDAT8, was identified in Methanocryptus kandlerii [43]. It is well known that canonical tRNAs contain a U8 residue to form Hoogsteen base pairing with A14, an important tertiary contact for maintaining a tRNA’s L-shaped structure [47] (Fig. 2). Thirty of the 34 M. kandleri tRNA genes contain a C at position 8 in place of the conserved U8 (T8 in DNA). It was shown that C8 is efficiently deaminated to U8 by CDAT8. The enzyme is active on a substrate consisting of only the C8 containing acceptor stem and the 3′CCA alone, while the anticodon loop and D-arm are dispensable for activity. The developmental and evolutionary purpose of editing such a large number of tRNA transcripts, which could just as easily be encoded within the genome is not clear. However, it is possible that the extreme temperatures at which M. kandleri lives demand the genomic stability inherent in abundant G:C pairing forcing the maintenance of C8 in the tRNA genes of this organism [43]. In terms of mechanism, just like ADATs, CDAT8 has an N-terminal cytidine deaminase domain. Additionally, CDAT8 has a central ferredoxin like domain of unknown function and a C-terminal THUMP domain (THioUridine synthases, RNA Methyltransferases and Pseudouridine synthases) presumably involved in tRNA binding [43].
The first example of C to U editing at the anticodon of a tRNA was described in marsupial mitochondria, where no tRNA is encoded in the genome that can translate the aspartate codons. This particular decoding conundrum is elegantly solved by C-to-U editing of a tRNAGly encoded with the anticodon GCC where editing at position 35 generates a GUC anticodon [40, 48]. The edited tRNA then has all the aminoacylation and translational properties that permits it to function as a bona fide tRNAAsp ensuring proper reading of the otherwise orphan aspartate codons [40] (Fig. 2). Since the levels of the edited and unedited tRNA are relatively equivalent through the initial stages of marsupial development, it has been suggested that both tRNAs are functional and necessary for translation [48]. Importantly, the unedited tRNAGlyGCC is aminoacylated in vivo with glycine, while the edited tRNAAspGUC is aminoacylated with aspartate [40]. Thus, charging is highly specific for the final edited anticodon sequence and two charged tRNA species are produced from one tRNA gene in the marsupial mitochondria.
The only other known example of C-to-U editing in the anticodon of a tRNA was shown in Leishmania tarentolae [39] and later in T. brucei [49] (Fig. 2). The mitochondrial genomes of these organisms do not encode any tRNA genes needed for translation and, consequently, mitochondrial protein synthesis relies on an active import pathway to bring in nucleus-encoded tRNAs from the cytoplasm [50]. Once again in this system, a potential problem results from the fact that the mitochondrial genetic code, like in most organisms, is not universal and 88% of all tryptophan codons are encoded as UGA [39]. The only nucleus-encoded tryptophanyl tRNA has the canonical CCA anticodon which can decode UGG but not UGA codons. Once again, C-to-U editing of the first position of the anticodon to generate tRNATrpUCA prevents a potential problem. Thus in this particular case, the cell encodes a tRNATrpCCA for cytoplasmic UGG decoding and avoids encoding a potential suppressor tRNATrpUCA by using a mitochondria-localized activity to solve the riddle of UGA mitochondrial decoding. In parity to the marsupial example, a single tRNA gene is again used to encode two different tRNAs.
Although not directly affecting decoding, numerous C-to-U editing events have also been described in plants, where mitochondrial tRNAs undergo editing to correct, as well as introduce, mismatches in their acceptor stems [44, 51]. In the dicot mitochondria a C to U editing event corrects a mismatched C4:A69 pair in the acceptor stem of mitochondrial tRNAPhe GAA into a U4:A69. [51, 52]. By restoring proper tRNA precursor folding, this C-to-U editing at position 4 is a prerequisite for processing and efficient excision of the 5′ and 3′ extension of the pre-tRNA [53] (Fig. 2).
Recent secondary structure modeling of Isoetes engelmannii tRNA sequences suggested that 43 possible C to U editing sites could exist to restore evolutionarily conserved residues or basepairs in stem regions, a higher degree of editing than seen previously in any plant species. Furthermore, cDNA sequence data was obtained for 36 of the predicted sites of which 29 were confirmed, revealing C-to-U and 4 instances of U-to-C editing events all directed at precursor tRNAs prior to processing [41].
From this report it would appear that unlike A-to-I editing, C-to-U editing of tRNAs is the sole realm of the organelles. However our laboratory showed that tRNAThr undergoes C-to-U editing at position 32 of the anticodon loop [54], representing the first example of C-to-U editing in a non-organellar tRNA (Fig. 2). The biological significance of this editing event is not currently clear but due to the importance of C32 in anticodon loop structure, we suggest that it may play a role in translational efficiency and/or fidelity.
4. When editing and modification join forces: affecting function
It has been suggested that tRNA modifications offer a fine-tuning mechanism to help cells deal with changing environmental demands. Along these lines, we have previously proposed an “interdependence model” to explain the connection between tRNA editing and modification in trypanosomatid mitochondria [55] (Fig. 3).
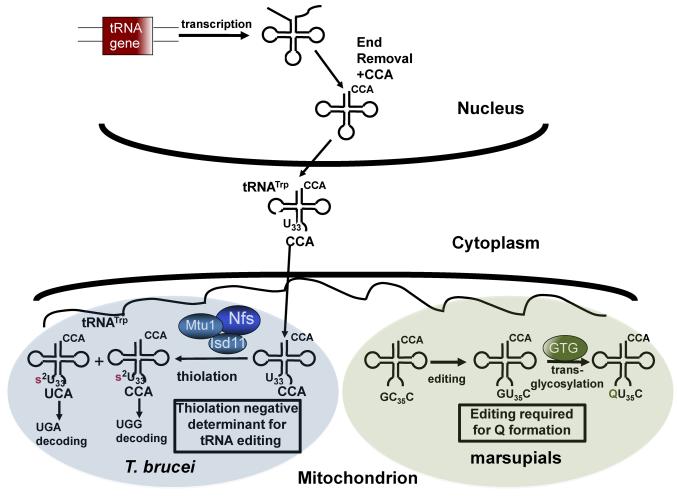
Fig. 3.
Connection between editing and modification. Following import into the mitochondria of kinetoplasts (T. brucei and Leishmania), tRNAs are edited and thiolated. In T. brucei thiolation at the unusual position 33 serves as a negative determinant for editing. In marsupials editing creates the recognition motif for the enzyme guanosine transglycosylase (GTG) required for the biosynthesis of the nucleotide queosine (Q). Mtu1, Nfs and Isd11 refer to factors of the FeS-cluster biosynthetic pathway that also required for thiolation.
In trypanosomatids such as T. brucei and Leishmania spp., besides RNA editing, tRNATrp undergoes extensive mitochondria-specific posttranscriptional modifications, including the first example of thiolation of U33 (s2U33), the universally unmodified uridine [56]. Since the levels of editing and thiolation in Leishmania were similar (~50 %), and due to the proximity of these two events on the anticodon loop, it was suggested that either editing serves as a determinant for thiolation or that thiolation may be required for editing [56]. However, while in the closely related T. brucei similar levels of edited tRNATrp were detected as in Leishmania, about 85% of tRNATrp was thiolated [49]. Demonstrating that both edited and unedited tRNAs were thiolated and suggesting that editing is not a thiolation determinant (Fig. 3). Taking advantage of the availability of a robust RNAi pathway in T. brucei, recent studies showed that after silencing of key components of the mitochondria-specific thiolation pathway involving members of the Iron-sulfur cluster assembly, the editing levels of tRNATrp reach nearly 100% [57, 58]. Based on this result it has been proposed that thiolation of tRNATrp serves as a negative determinant for C to U editing at C34 (Fig. 3). This regulation may be important in maintaining the ratios of edited and unedited tRNAs suggesting some biological role in the T. brucei life cycle. These observations also provide a possible link between the tRNA editing, modification and the iron-sulfur assembly pathway. Several mitochondrial respiratory proteins require iron–sulfur clusters for function and their expression coincides with developmental changes. Similarly, some tRNA modification enzymes either require iron-sulfur clusters or use components of the iron sulfur cluster assembly pathway. It has been suggested that by the possible integration of these pathways T. brucei carefully coordinates energy demands to translational rates in response to environmental changes [59].
In the marsupial mitochondria tRNA editing example using a thin layer chromatography approach it was shown that this tRNA undergoes several post-transcriptional modifications. Three pseudouridines (positions 27,32 and 55) and two methylations (m1A9 and m2G10) were found in both unedited and edited tRNA. However presence of the conserved queosine, a hypermodified base, was only detected at the first position of the anticodon of the edited form [60] (Fig. 3). This may be of biological importance given that queosine hypomodification is closely associated with defects in cell proliferation and malignancy [61].
Although few examples currently exist for the potential interconnection between editing and modification, this relationship may be in fact widespread and common among very divergent organisms. For example in archaea, tRNAs contain 1-methylinosine at position 57 (m1I57), where formation of 1-methyadenosine (m1A57) is an obligatory intermediate step for deamination [20] (Fig. 2). Currently, the importance of coupling editing and methylation at one site in these systems is not clear and will remain an open question. It is however provocative to think that cells are using these types of mechanisms to modulate tRNA function and provide yet one more layer of complexity to the tangled web of regulatory genetic circuitry.
Highlights.
- tRNA editing for structure and function
- 5′ editing by potential 3′-5′ polymerase
- interdependence of editing and modification
- editing and modification modulate tRNA function
- tRNA editing is phylogenetically widespread
Acknowledgements
We wish to thank all members of Alfonzo laboratory for helpful discussions and suggestions. This work was supported in part by grant GM084065 (NIGMS) to JDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dunin-Horkawicz S, Czerwoniec A, Gajda MJ, Feder M, Grosjean H, Bujnicki JM. MODOMICS: a database of RNA modification pathways. Nucleic Acids Res. 2006;34:D145–9. doi: 10.1093/nar/gkj084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bjork GR. Genetic dissection of synthesis and function of modified nucleosides in bacterial transfer RNA. Prog Nucleic Acid Res Mol Biol. 1995;50:263–338. doi: 10.1016/s0079-6603(08)60817-x. [DOI] [PubMed] [Google Scholar]
- [3].Holley RW, Apgar J, Everett GA, Madison JT, Marquisee M, Merrill SH, et al. Structure of a Ribonucleic Acid. Science. 1965;147:1462–5. doi: 10.1126/science.147.3664.1462. [DOI] [PubMed] [Google Scholar]
- [4].Holley RW, Everett GA, Madison JT, Zamir A. Nucleotide Sequences in the Yeast Alanine Transfer Ribonucleic Acid. J Biol Chem. 1965;240:2122–8. [PubMed] [Google Scholar]
- [5].Crick FH. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966;19:548–55. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- [6].Lonergan KM, Gray MW. Predicted editing of additional transfer RNAs in Acanthamoeba castellanii mitochondria. Nucleic Acids Res. 1993;21:4402. doi: 10.1093/nar/21.18.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lonergan KM, Gray MW. Editing of transfer RNAs in Acanthamoeba castellanii mitochondria. Science. 1993;259:812–6. doi: 10.1126/science.8430334. [DOI] [PubMed] [Google Scholar]
- [8].Benne R, Van den Burg J, Brakenhoff JP, Sloof P, Van Boom JH, Tromp MC. Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell. 1986;46:819–26. doi: 10.1016/0092-8674(86)90063-2. [DOI] [PubMed] [Google Scholar]
- [9].Tomita K, Ueda T, Watanabe K. RNA editing in the acceptor stem of squid mitochondrial tRNA(Tyr) Nucleic Acids Res. 1996;24:4987–91. doi: 10.1093/nar/24.24.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yokobori S, Paabo S. Transfer RNA editing in land snail mitochondria. Proc Natl Acad Sci U S A. 1995;92:10432–5. doi: 10.1073/pnas.92.22.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Antes T, Costandy H, Mahendran R, Spottswood M, Miller D. Insertional editing of mitochondrial tRNAs of Physarum polycephalum and Didymium nigripes. Mol Cell Biol. 1998;18:7521–7. doi: 10.1128/mcb.18.12.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gott JM, Somerlot BH, Gray MW. Two forms of RNA editing are required for tRNA maturation in Physarum mitochondria. Rna. 16:482–8. doi: 10.1261/rna.1958810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bullerwell CE, Gray MW. In vitro characterization of a tRNA editing activity in the mitochondria of Spizellomyces punctatus, a Chytridiomycete fungus. J Biol Chem. 2005;280:2463–70. doi: 10.1074/jbc.M411273200. [DOI] [PubMed] [Google Scholar]
- [14].Price DH, Gray MW. A novel nucleotide incorporation activity implicated in the editing of mitochondrial transfer RNAs in Acanthamoeba castellanii. Rna. 1999;5:302–17. doi: 10.1017/s1355838299981840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jackman JE, Phizicky EM. tRNAHis guanylyltransferase adds G-1 to the 5′ end of tRNAHis by recognition of the anticodon, one of several features unexpectedly shared with tRNA synthetases. Rna. 2006;12:1007–14. doi: 10.1261/rna.54706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cooley L, Appel B, Soll D. Post-transcriptional nucleotide addition is responsible for the formation of the 5′ terminus of histidine tRNA. Proc Natl Acad Sci U S A. 1982;79:6475–9. doi: 10.1073/pnas.79.21.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jackman JE, Phizicky EM. tRNAHis guanylyltransferase catalyzes a 3′-5′ polymerization reaction that is distinct from G-1 addition. Proc Natl Acad Sci U S A. 2006;103:8640–5. doi: 10.1073/pnas.0603068103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lavrov DV, Brown WM, Boore JL. A novel type of RNA editing occurs in the mitochondrial tRNAs of the centipede Lithobius forficatus. Proc Natl Acad Sci U S A. 2000;97:13738–42. doi: 10.1073/pnas.250402997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Leigh J, Lang BF. Mitochondrial 3′ tRNA editing in the jakobid Seculamonas ecuadoriensis: a novel mechanism and implications for tRNA processing. Rna. 2004;10:615–21. doi: 10.1261/rna.5195504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Grosjean H, Constantinesco F, Foiret D, Benachenhou N. A novel enzymatic pathway leading to 1-methylinosine modification in Haloferax volcanii tRNA. Nucleic Acids Res. 1995;23:4312–9. doi: 10.1093/nar/23.21.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Maas S, Gerber AP, Rich A. Identification and characterization of a human tRNA-specific adenosine deaminase related to the ADAR family of pre-mRNA editing enzymes. Proc Natl Acad Sci U S A. 1999;96:8895–900. doi: 10.1073/pnas.96.16.8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gerber A, Grosjean H, Melcher T, Keller W. Tad1p, a yeast tRNA-specific adenosine deaminase, is related to the mammalian pre-mRNA editing enzymes ADAR1 and ADAR2. Embo J. 1998;17:4780–9. doi: 10.1093/emboj/17.16.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gerber AP, Keller W. An adenosine deaminase that generates inosine at the wobble position of tRNAs. Science. 1999;286:1146–9. doi: 10.1126/science.286.5442.1146. [DOI] [PubMed] [Google Scholar]
- [24].Losey HC, Ruthenburg AJ, Verdine GL. Crystal structure of Staphylococcus aureus tRNA adenosine deaminase TadA in complex with RNA. Nat Struct Mol Biol. 2006;13:153–9. doi: 10.1038/nsmb1047. [DOI] [PubMed] [Google Scholar]
- [25].Droogmans L, Grosjean H. 2′-O-methylation and inosine formation in the wobble position of anticodon-substituted tRNA-Phe in a homologous yeast in vitro system. Biochimie. 1991;73:1021–5. doi: 10.1016/0300-9084(91)90143-o. [DOI] [PubMed] [Google Scholar]
- [26].Sprinzl M, Horn C, Brown M, Ioudovitch A, Steinberg S. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1998;26:148–53. doi: 10.1093/nar/26.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Grosjean H, Auxilien S, Constantinesco F, Simon C, Corda Y, Becker HF, et al. Enzymatic conversion of adenosine to inosine and to N1-methylinosine in transfer RNAs: a review. Biochimie. 1996;78:488–501. doi: 10.1016/0300-9084(96)84755-9. [DOI] [PubMed] [Google Scholar]
- [28].Senger B, Auxilien S, Englisch U, Cramer F, Fasiolo F. The modified wobble base inosine in yeast tRNAIle is a positive determinant for aminoacylation by isoleucyl-tRNA synthetase. Biochemistry. 1997;36:8269–75. doi: 10.1021/bi970206l. [DOI] [PubMed] [Google Scholar]
- [29].Spears JL, Rubio MA, Gaston KW, Wywial E, Strikoudis A, Bujnicki JM, et al. A single zinc ion is sufficient for an active Trypanosoma brucei tRNA editing deaminase. J Biol Chem. 2011;286:20366–74. doi: 10.1074/jbc.M111.243568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rubio MA, Pastar I, Gaston KW, Ragone FL, Janzen CJ, Cross GA, et al. An adenosine-to-inosine tRNA-editing enzyme that can perform C-to-U deamination of DNA. Proc Natl Acad Sci U S A. 2007;104:7821–6. doi: 10.1073/pnas.0702394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wolf J, Gerber AP, Keller W. tadA, an essential tRNA-specific adenosine deaminase from Escherichia coli. Embo J. 2002;21:3841–51. doi: 10.1093/emboj/cdf362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Auxilien S, Crain PF, Trewyn RW, Grosjean H. Mechanism, specificity and general properties of the yeast enzyme catalysing the formation of inosine 34 in the anticodon of transfer RNA. J Mol Biol. 1996;262:437–58. doi: 10.1006/jmbi.1996.0527. [DOI] [PubMed] [Google Scholar]
- [33].Kuratani M, Ishii R, Bessho Y, Fukunaga R, Sengoku T, Shirouzu M, et al. Crystal structure of tRNA adenosine deaminase (TadA) from Aquifex aeolicus. J Biol Chem. 2005;280:16002–8. doi: 10.1074/jbc.M414541200. [DOI] [PubMed] [Google Scholar]
- [34].Kim J, Malashkevich V, Roday S, Lisbin M, Schramm VL, Almo SC. Structural and kinetic characterization of Escherichia coli TadA, the wobble-specific tRNA deaminase. Biochemistry. 2006;45:6407–16. doi: 10.1021/bi0522394. [DOI] [PubMed] [Google Scholar]
- [35].Elias Y, Huang RH. Biochemical and structural studies of A-to-I editing by tRNA:A34 deaminases at the wobble position of transfer RNA. Biochemistry. 2005;44:12057–65. doi: 10.1021/bi050499f. [DOI] [PubMed] [Google Scholar]
- [36].Ragone FL, Spears JL, Wohlgamuth-Benedum JM, Kreel N, Papavasiliou FN, Alfonzo JD. The C-terminal end of the Trypanosoma brucei editing deaminase plays a critical role in tRNA binding. Rna. 2011;7:1296–306. doi: 10.1261/rna.2748211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Karcher D, Bock R. Identification of the chloroplast adenosine-to-inosine tRNA editing enzyme. Rna. 2009;15:1251–7. doi: 10.1261/rna.1600609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Delannoy E, Le Ret M, Faivre-Nitschke E, Estavillo GM, Bergdoll M, Taylor NL, et al. Arabidopsis tRNA adenosine deaminase arginine edits the wobble nucleotide of chloroplast tRNAArg(ACG) and is essential for efficient chloroplast translation. Plant Cell. 2009;21:2058–71. doi: 10.1105/tpc.109.066654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Alfonzo JD, Blanc V, Estevez AM, Rubio MA, Simpson L. C to U editing of the anticodon of imported mitochondrial tRNA(Trp) allows decoding of the UGA stop codon in Leishmania tarentolae. Embo J. 1999;18:7056–62. doi: 10.1093/emboj/18.24.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Janke A, Paabo S. Editing of a tRNA anticodon in marsupial mitochondria changes its codon recognition. Nucleic Acids Res. 1993;21:1523–5. doi: 10.1093/nar/21.7.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Grewe F, Herres S, Viehover P, Polsakiewicz M, Weisshaar B, Knoop V. A unique transcriptome: 1782 positions of RNA editing alter 1406 codon identities in mitochondrial mRNAs of the lycophyte Isoetes engelmannii. Nucleic Acids Res. 2011;39:2890–902. doi: 10.1093/nar/gkq1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Marchfelder A, Brennicke A, Binder S. RNA editing is required for efficient excision of tRNA(Phe) from precursors in plant mitochondria. J Biol Chem. 1996;271:1898–903. doi: 10.1074/jbc.271.4.1898. [DOI] [PubMed] [Google Scholar]
- [43].Randau L, Stanley BJ, Kohlway A, Mechta S, Xiong Y, Soll D. A cytidine deaminase edits C to U in transfer RNAs in Archaea. Science. 2009;324:657–9. doi: 10.1126/science.1170123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fey J, Weil JH, Tomita K, Cosset A, Dietrich A, Small I, et al. Role of editing in plant mitochondrial transfer RNAs. Gene. 2002;286:21–4. doi: 10.1016/s0378-1119(01)00817-4. [DOI] [PubMed] [Google Scholar]
- [45].Tomita K, Ueda T, Watanabe K. Two nucleotides 5′-adjacent to the anticodon of rat cytoplasmic tRNA(Asp) are not edited. Biochimie. 1996;78:1001–6. doi: 10.1016/s0300-9084(97)86723-5. [DOI] [PubMed] [Google Scholar]
- [46].Beier H, Lee MC, Sekiya T, Kuchino Y, Nishimura S. Two nucleotides next to the anticodon of cytoplasmic rat tRNA(Asp) are likely generated by RNA editing. Nucleic Acids Res. 1992;20:2679–83. doi: 10.1093/nar/20.11.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Romby P, Moras D, Bergdoll M, Dumas P, Vlassov VV, Westhof E, et al. Yeast tRNAAsp tertiary structure in solution and areas of interaction of the tRNA with aspartyl-tRNA synthetase. A comparative study of the yeast phenylalanine system by phosphate alkylation experiments with ethylnitrosourea. J Mol Biol. 1985;184:455–71. doi: 10.1016/0022-2836(85)90294-3. [DOI] [PubMed] [Google Scholar]
- [48].Borner GV, Morl M, Janke A, Paabo S. RNA editing changes the identity of a mitochondrial tRNA in marsupials. Embo J. 1996;15:5949–57. [PMC free article] [PubMed] [Google Scholar]
- [49].Charriere F, Helgadottir S, Horn EK, Soll D, Schneider A. Dual targeting of a single tRNA(Trp) requires two different tryptophanyl-tRNA synthetases in Trypanosoma brucei. Proc Natl Acad Sci U S A. 2006;103:6847–52. doi: 10.1073/pnas.0602362103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Alfonzo JD, Soll D. Mitochondrial tRNA import--the challenge to understand has just begun. Biol Chem. 2009;390:717–22. doi: 10.1515/BC.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Marechal-Drouard L, Cosset A, Remacle C, Ramamonjisoa D, Dietrich A. A single editing event is a prerequisite for efficient processing of potato mitochondrial phenylalanine tRNA. Mol Cell Biol. 1996;16:3504–10. doi: 10.1128/mcb.16.7.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Binder S, Marchfelder A, Brennicke A. RNA editing of tRNA(Phe) and tRNA(Cys) in mitochondria of Oenothera berteriana is initiated in precursor molecules. Mol Gen Genet. 1994;244:67–74. doi: 10.1007/BF00280188. [DOI] [PubMed] [Google Scholar]
- [53].Kunzmann A, Brennicke A, Marchfelder A. 5′ end maturation and RNA editing have to precede tRNA 3′ processing in plant mitochondria. Proc Natl Acad Sci U S A. 1998;95:108–13. doi: 10.1073/pnas.95.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rubio MA, Ragone FL, Gaston KW, Ibba M, Alfonzo JD. C to U Editing Stimulates A to I Editing in the Anticodon Loop of a Cytoplasmic Threonyl tRNA in Trypanosoma brucei. J Biol Chem. 2006;281:115–20. doi: 10.1074/jbc.M510136200. [DOI] [PubMed] [Google Scholar]
- [55].MATaA J.D. Rubio. Editing and modification in trypanosomatids: the reshaping of non-coding RNAs. In: Grosjean H, editor. Topics in Current Genetics. Springer-Verlag; 2005. pp. 71–86. [Google Scholar]
- [56].Crain PF, Alfonzo JD, Rozenski J, Kapushoc ST, McCloskey JA, Simpson L. Modification of the universally unmodified uridine-33 in a mitochondria-imported edited tRNA and the role of the anticodon arm structure on editing efficiency. Rna. 2002;8:752–61. doi: 10.1017/s1355838202022045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bruske EI, Sendfeld F, Schneider A. Thiolated tRNAs of Trypanosoma brucei are imported into mitochondria and dethiolated after import. J Biol Chem. 2009;284:36491–9. doi: 10.1074/jbc.M109.064527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wohlgamuth-Benedum JM, Rubio MA, Paris Z, Long S, Poliak P, Lukes J, et al. Thiolation controls cytoplasmic tRNA stability and acts as a negative determinant for tRNA editing in mitochondria. J Biol Chem. 2009;284:23947–53. doi: 10.1074/jbc.M109.029421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Alfonzo JD, Lukes J. Assembling Fe/S-clusters and modifying tRNAs: ancient co-factors meet ancient adaptors. Trends Parasitol. 2011;27:235–8. doi: 10.1016/j.pt.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Morl M, Dorner M, Paabo S. C to U editing and modifications during the maturation of the mitochondrial tRNA(Asp) in marsupials. Nucleic Acids Res. 1995;23:3380–4. doi: 10.1093/nar/23.17.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Vinayak M, Pathak C. Queuosine modification of tRNA: its divergent role in cellular machinery. Biosci Rep. 2009;30:135–48. doi: 10.1042/BSR20090057. [DOI] [PubMed] [Google Scholar]