Abstract
Anxiety impairs the ability to think and concentrate, suggesting that the interaction between emotion and cognition may elucidate the debilitating nature of pathological anxiety. Using a verbal n-back task that parametrically modulated cognitive load, we explored the effect of experimentally-induced anxiety on task performance and the startle reflex. Findings suggest there is a crucial inflection point between moderate and high cognitive load, where resources shift from anxious apprehension to focus on task demands. Specifically, we demonstrate that anxiety impairs performance under low-load, but is reduced when subjects engage in a difficult task that occupies executive resources. We propose a two-component model of anxiety that describes a cognitive mechanism behind performance impairment and an automatic response that supports sustained anxiety-potentiated startle. Implications for therapeutic interventions and emotional pathology are discussed.
Although anxiety is adaptive, it can be debilitating when it interferes with our daily life and our goal-directed behaviors. Some of the prominent cognitive problems of anxiety are mediated by impaired attentional mechanisms. Anxious individuals complain of being easily distracted and of having difficulty concentrating, and population-based studies report impairments in executive functioning and episodic memory across anxiety disorders (Airaksinen, Larsson, & Forsell, 2005). However, despite the prevalence and symptom severity of pathological anxiety, the deleterious impact of anxiety, whether clinical or sub-clinical (e.g., state or trait), on cognitive performance has not been demonstrated consistently or robustly in the laboratory (e.g., Castaneda, Suvisaari, Marttunen, Perälä, Saarni, Aalto-Setälä, et al. 2011; Bishop, 2008; Eysenck, Derakshan, Santos, & Calvo, 2007; King & Schaefer, 2010; Shackman, Sarinopoulos, Maxwell et al., 2006). Nevertheless, there is ample evidence to suggest that cognition and emotion interact in observable ways at the behavioral, physiological, and neural level (Pessoa, 2008; 2009; Shackman, Maxwell, McMenamin, Greischar, & Davidson, 2011; Shackman, Salomons, Slagter, Fox, Winter, & Davidson, 2011). Understanding this interaction is a first step toward identifying pathological mechanisms.
The current state of the literature leaves two important questions unanswered: 1) How does anxiety impair cognitive processing? 2) Can engaging in a cognitive task alleviate anxiety? The aim of this study was to investigate the influence of anxiety on task performance, by taking an exploratory approach across varying levels of task difficulty to determine the precise point of greatest impairment.
1) How does anxiety impair cognitive processing?
The overwhelming basis of most theories that explain the interaction between emotion and cognition is competition for resources. Whether those critical resources apply to executive processes (Eysenck & Calvo, 1992), phonological processes (Morris, Davis, & Hutchings, 1981), working memory (King et al., 2010), perceptual processes (Bishop, 2008; For a review see Lavie, 2005), or all of the above remains unclear. To further complicate the interpretation of this interaction, data and claims support opposing effects of resource demands on susceptibility to emotion-related disruption (low-load or high-load: Bishop, 2008, and Eysenck & Calvo, 1992; Lavie, 2000), and on the type of information that is compromised by such disruption (spatial or verbal: Shackman et al., 2006, and King et al., 2010, perceptual or cognitive: Lavie, 2000, and King et al., 2010). For example, Lavie (2005) emphasized differences in the impact of external distractors on perceptual load versus cognitive load; distractors have a greater impact on low-load perceptual tasks, and a greater impact on high-load cognitive tasks (e.g., difficult working memory tasks). These findings suggest that high-load cognitive tasks may be more susceptible to anxiety-related disruption, a claim supported by the proposal that anxious worrying competes with, and subsequently overcomes high-load task performance (Eysenck et al., 2007). This proposal, known as the processing efficiency theory (Eysenck & Calvo, 1992), is based on the claim that the deleterious effects of anxiety on cognition are greatest when task demands are high because the executive processing resources that subserve working memory are also engaged by worrisome thoughts. In contrast to Eysenck et al., (2007)’s cognitive theory, Bishop (2008) has argued that anxiety1 has a greater impact on perceptual tasks that place a lower demand on processing resources, while tasks that place a higher demand on resources reduce the deleterious impact of anxiety (an extension of the selective attention proposal put forth by Lavie (2005)). Although Bishop (2008) focuses on the impact of anxiety on perception (where top-down attentional control mechanisms compete for resources with bottom-up sensory mechanisms), studies demonstrating top-down cognitive control of emotion suggest that higher-demand tasks and cognitive strategies reallocate resources towards task demands, thereby reducing anxiety (King et al., 2010). It follows that task performance (whether the task is perceptual or cognitive in nature) may be more vulnerable to disruption when demands are low and anxiety is high, and less vulnerable when the task is difficult and competes for resources via top-down control mechanisms.
Thus far, experimental manipulations of negative emotional states have demonstrated partial support for Eysenck et al.’s (1992) theory, but only in the context of spatial working memory paradigms (Lavric, Rippon, & Gray, 2003; Shackman et al., 2006), and without support for Eysenck et al.’s mechanism of interference (i.e., “worrisome thoughts”). Research in verbal working memory has failed to lend clarity to this interaction by presenting findings that demonstrate negative emotional context impairs (Schaefer, Braver, Reynolds, Burgess, Yarkoni, & Gray, 2006), facilitates (Gray, Braver, & Raichle, 2002), or has no impact on verbal working memory (Lavric, Rippon, & Gray, 2003; Shackman et al., 2006; Qin, Hermans, van Marle, Luo, & Fernández, 2009), reinforcing the problem of reproducing the clinical impairment seen in anxiety patients outside of the laboratory. Given the above inconsistencies in verbal working memory findings, the present study sought to better comprehend the interaction between this type of memory and anxiety. Our worked was guided by three main assumptions. First, anxiety involves several distinct emotional, cognitive, and perceptual components, the cognitive component being particularly central to the current study. This component consists of anxious apprehension (Heller, Nitschke, Etienne, & Miller, 1997; Barlow 1991), also referred to as worry (Borkovec, Robinson, Pruzinsky, & DePree, 1983) or worrisome thoughts (Eysenck et al., 1992). Second, we advocate Eysenck et al.’s (1992) proposal that verbal working memory is impaired by worrisome thoughts and that disruption results from shared executive resources between these two processes (e.g., anterior cingulate cortex; working memory: Bunge Klinberg, Jacobson, & Gabrieli, [2000], Raichle [1993], worrisome thoughts: Paulesu, Sambugaro, Torti, Danelli, Ferri, Scialfa, et al. [2009]). Finally, in contrast to defensive priming mechanisms, which are reflexive and may be pervasively active under threat (Cornwell, Alvarez, Lissek, Kaplan, Ernst, & Grillon, 2011), we argue that anxious apprehension mechanisms are more malleable, as evidenced by studies supporting cognitive control of emotion (Kalisch, Wiech, Herrmann, & Dolan, 2006; Pessoa, Padmala, & Morland, 2005, for a review see Ochsner, & Gross, 2005). As a consequence, the impact of anxious apprehension on verbal working memory may be somewhat transient and more readily inhibited by a resource shift toward challenging goal-directed verbal tasks.
Although methodological limitations and inconsistencies undoubtedly play a role in these discrepant findings (see Erk, Kleczar, & Walter, 2007 for an in-depth review, and Shackman et al., 2006, for proposed methodological desiderata), the elusive nature of anxiety’s impact on verbal working memory may be the result of a more complex interaction between emotion and cognition (e.g., a differential impact of anxious apprehension on tasks of varying cognitive load), as suggested by the work of Eysenck et al. (2007), Lavie (2005), and Bishop (2008), rather than a global effect of anxiety on performance. Specifically, the complexity of this interaction may be manifested where anxiety-related cognition has a greater impact on low-load versus high-load verbal tasks, in support of evidence that indicates high demand tasks can successfully reduce anxiety and its impact on performance. Indeed, Erk et al. (2007) note that the current corpus of work in this area lacks experimental investigations that explore the effects of anxiety on tasks of varying difficulty, by explicitly manipulating working memory load. In order to lend clarity to this body of findings, we sought to identify the effect of anxiety on working memory performance under different levels of cognitive load (no load, 1-back, 2-back, and 3-back tasks), and to explore the effects of engaging in different levels of cognitive load on anxiety.
2) Can engaging in a cognitive task alleviate anxiety?
Several studies have shown that anxiety and threat-related activity in the brain and body can be down-regulated by the use of different cognitive strategies such as self-distraction, reappraisal, or working memory load (Dvorak-Bertsch, Curtin, Rubinstein, & Newman 2007; Kalisch et al., 2006; King et al., 2010; Pessoa et al., 2005). Specifically, this effect appears to have the greatest impact when the task involves high cognitive (King et al., 2010) or perceptual load (Doallo, Holguin, & Cadaveira, 2006). Moreover, there is evidence to suggest that therapeutic techniques based on this assumption (e.g., Cognitive Behavioral Therapy (CBT)) are highly effective in reducing anxiety in patient populations (Chambless & Gillis, 1993). However, it is important to note that despite reductions some aspects of an anxiety-related response, amygdala activity has been shown to subsist even under high perceptual load, suggesting that a preparatory component of anxiety like defensive priming may still be active (Cornwell et al., 2011). Taken together, these findings suggest that when attentional demands are high, task performance takes precedence over anxiety-related cognitive processing. In parallel, preparatory mechanisms like increased vigilance may remain active to facilitate a rapid response to potential threat. Here, we sought to determine whether engaging in a working memory task can successfully relieve induced-anxiety, and if so, what level of cognitive load is most effective in reducing anxious responding (measured by performance impairment and startle magnitude).
Current Study
We used a well-established threat of shock paradigm to induce anxiety (Grillon, Baas, Lissek, Smith, & Milstein, 2004; Robinson, Letkiewicz, Overstreet, Ernst, & Grillon, 2011) while subjects were engaged in a working memory task. Subjects were told that they were at risk for receiving unpleasant shocks during threat but not safe conditions. Anticipatory anxiety was assessed using the acoustic startle reflex (eyeblink), a physiological measure that reflects defensive preparations in humans and non-human animals (Davis, 1998; Grillon, 2002), and a self-report measure (subjective anxiety rating) that reflects awareness of an internal emotional state. The startle reflex is robustly potentiated under threat versus safe conditions and this measure provides a well-validated index of anxiety (Davis, 1998; Grillon, 2002). Working memory load was parametrically modulated in order to explore the impact of anxiety on performance at varying levels of task difficulty. Based on evidence suggesting that high-load tasks reduce anxiety (Dvorak-Bertsch et al., 2007; King et al., 2010) and low-demand tasks may be more susceptible to anxiety-related disruption (Bishop, 2008), we predicted that low working memory load performance (e.g., 1-back and 2-back) would be impaired under threat compared to safe conditions. In contrast, we predicted that high-load working memory (3-back) performance would not differ between threat and safe, and that anxiety (as indexed by anxiety-potentiated startle) would be reduced by high working memory load. These predictions were based on the findings that high-load verbal working memory is not disrupted by induced anxiety/stress (Shackman et al., 2006; Qin et al., 2009) and high-load working memory attenuates anxious responding (Dvorak-Bertsch et al., 2007; King et al., 2010). In addition, we expected that performance deficits would be accompanied by a sustained anticipatory anxiety response (indexed by anxiety-potentiated startle and anxiety ratings), reinforcing the claim that this emotional state is impairing performance. In sum, we predicted that there would be a reciprocal interaction between anxiety and cognitive load on working memory performance, such that anxiety would lead to impaired performance on low-demands tasks, and that engaging in high-demand tasks would reduce anxiety.
Methods
Participants
Thirty-one healthy individuals (16 females) received monetary compensation for their participation in the study. Participants were recruited for the study via flyers and advertisements placed in local newspapers. Upon arrival, participants completed an intake evaluation consisting of a physical exam, urine screen, and a Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Williams, & Gibbon, 1995). Exclusion was based on the following criteria: 1) contraindicated medical condition, 2) past or current psychiatric disorders, and 3) use of psychoactive medications or illicit drugs. Five participants were excluded because of equipment failure. The final group of participants consisted of 26 adults (13 females; mean age 29.2 years; age range: 19–45 years). Subjects provided written informed consent that was approved by the Combined Neuroscience Institutional Review Board of the National Institutes of Health.
Stimuli and Apparatus
Presentation® software (Version 0.70, www.neurobs.com) was used to control all stimuli and present visual stimuli, and a commercial system (Contact Precision Instruments, London, United Kingdom) was used to present shocks and startle probes. Acoustic startle probes (40ms, 103 dB(A), near instantaneous rise/fall times) were presented binaurally through headphones. The eyeblink reflex was measured using two 6mm Ag/AgCl electrodes (impedances below 15 kΩ) placed below the right eye over the orbicularis oculi muscle. Electromyographic (EMG) data were recorded by Psylab 7 software (Contact Precision Instruments, London, United Kingdom). The shocks were produced by a constant current stimulator and administered to the median nerve of the left wrist using two 6mm Ag/AgCl electrodes. Electric shocks up to 5mA and 200ms duration were administered. Shock level was determined individually by a shock workup procedure where the shock level began at 3.5mA and was increased by increments of .2mA until the subject rated the shock as moderately painful, but still tolerable (M = 6.2; SD = 1.5) based on a 1–9 scale (1, not at all painful, to 9, extremely painful).
Procedure
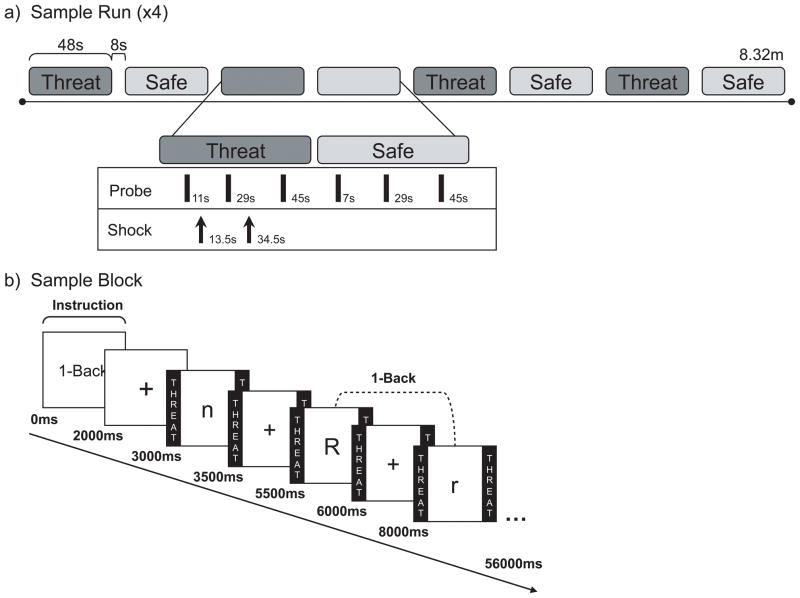
To reduce learning-related increases in performance across time, participants practiced each level of the n-back task once prior to the experiment. Above chance performance and verbal confirmation of task comprehension were used to determine adequate familiarity. All participants were comfortable with the task after one round of practice. Participants indicated “same” or “different” with a keyboard button press based on the letter 1-back, 2-back, or 3-back from the current letter, or simply attended to the letters (“view” task) without making a response (see Figure 1b for a sample trial). Following practice, participants were presented with nine startle probes every 17–20 s during a rest period in order to habituate initial startle reactivity (results not shown).
Figure 1.
Four experimental runs, each consisting of eight alternating threat and safe n-back blocks, followed the practice trials and startle habituation (see Figure 1a for a sample run). Approximately 5 minutes separated the practice trials from the experimental runs. N-back blocks were randomly ordered in the first experimental run so that no level of the n-back task was ever presented sequentially, and subsequent runs were counterbalanced using a Latin square technique. All task levels were presented twice in each run. A second counterbalanced series of runs was used for half the subjects. In the second counterbalancing, late and early blocks switched positions, and all threat and safe stimuli were exchanged. Participants were reminded of the condition they were in (Threat [under threat of shock] or Safe [no shocks were delivered]) by colored borders with the word THREAT or SAFE written inside (see Figure 1b for a sample block). Each run began with three habituation probes, followed by a 2-second instruction screen and a 1000 ms fixation cross. Upper and lowercase letters (18 in each block) were presented for 500 ms each, separated by 2000 ms (+/− 250 ms) fixation inter-trial intervals (ITIs). Both upper and lowercase letters were used to reduce reliance on perceptual information; letter case varied randomly for both targets and distractors such that some targets were perceptually identical and others were not. Participants responded to each letter by pressing one button if the letter was the same as the letter 1, 2, or 3-letters back, and another button if the letter was different. Approximately 35% of trials were targets (i.e., “same” responses), in keeping with ratios used in previous n-back research (Braver, Cohen, Nystrom, Jonides, Smith, & Noll, 1997; Carlson, Martinkauppi, Rämä, Salli, Korvenoja, & Aronen, 1998; Ragland, Turetsky, Gur, Gunning-Dixon, Turner, Schroeder, et al., 2002). A low target-to-distractor ratio is advantageous in a short block of stimuli because it allows for equal numbers of unique response patterns across levels of load, thereby reducing pattern recognition, while preserving the number of unique targets, thereby reducing potential target-related response biases.
During the n-back tasks, participants were presented with nine startle probes, one every 17–20 s, and a total of twelve shocks (3 per run; 0–2 per threat block) during the ITI. To minimize sensitization effects of the shocks on startle, shocks preceded probes by at least 16 s, and followed probes with a mean latency of approximately 2 s. Shocks were not delivered during fifty-percent of the threat blocks in order to reduce potential effects of the shock on performance and startle, and in order to prevent shock desensitization. Blocks were separated by an 8 s inter-block interval. After each run, subjects made several retrospective ratings: 1) anxiety during threat, 2) anxiety during safe, 3) distraction from shock during view, 4) distraction from shock during 1-back, 5) distraction from shock during 2-back, and 6) distraction from shock during 3-back. All ratings were made on a scale from 1–9 (with 1, being no anxiety or no distraction, and 9, being extreme anxiety or extreme distraction).
Data Reduction and Analysis
EMG data were sampled at 1000 Hz and filtered (30–500Hz). Data were rectified and smoothed with a 20-ms time constant. Startle responses were defined by the peak magnitude of the blink reflex that occurred 20–100 ms after stimulus onset, relative to a 50-ms average baseline that immediately preceded the probe onset. Exclusion of trials based on large baseline artifacts resulted in the removal of less than one percent of trials. For each subject, peak eyeblink magnitudes were T-scored (based on all conditions) and subsequently averaged within each condition. T-score transformation was used to attenuate large inter-individual differences in reflex magnitude. Retrospective subjective ratings were averaged across runs. Performance on trials that preceded or followed shocks, and those that preceded or followed probes were analyzed separately from those that did not contain a probe or shock. Accuracy did not differ as a result of shock or probe administration, and thus all trials were included in the final analysis. Trials where participants did not respond before the next letter appeared on the screen (i.e., within 2500 ms) were omitted. However, omissions were very infrequent and they did not occur systematically across conditions. Performance, startle magnitude, and subjective ratings were analyzed with repeated measures analyses of variance (ANOVA), paired-sample t-tests, and Pearson product-moment correlation coefficients (two-tailed tests) in order to assess within-subjects effects. Alpha was set at 0.05 for all statistical tests. Greenhouse-Geisser corrections (GG-ε) were used in all repeated-measures ANOVAs involving factors with more than two levels; uncorrected degrees of freedom, corrected p-values, and ε values are reported in these cases.
Psychometrics
In order to make more decisive inferences about the differential effect of anxiety on low-load versus high-load in the absence of a double dissociation (where two or more experimental manipulations have opposing effects on two or more dependent variables), it is important to demonstrate that the tasks are psychometrically equivalent (Shackman et al., 2006). To address the issue of psychometric equivalency we calculated discriminating power (Chapman & Chapman, 2001), which quantifies the sensitivity of a test to detect an experimental manipulation (or group differences). Discriminating power was computed by calculating reliability (Cronbach’s coefficient alpha) in accuracy across baseline (safe) runs and multiplying that value by the accuracy variance. Comparison of low-load to high-load discriminating power demonstrated that sensitivity was greater in the high-load task than in the low-load task (t(24) = 2.07, p < .05; M = 22.05 [high-load], M = 15.48 [low-load]). This finding indicates that low-load is less sensitive than high-load, and that potential performance differences between threat and safe under high-load may be attributable to differences in discriminating power, whereas potential performance differences observed under low-load cannot be attributed to differences in discriminating power.
Results
Task Performance
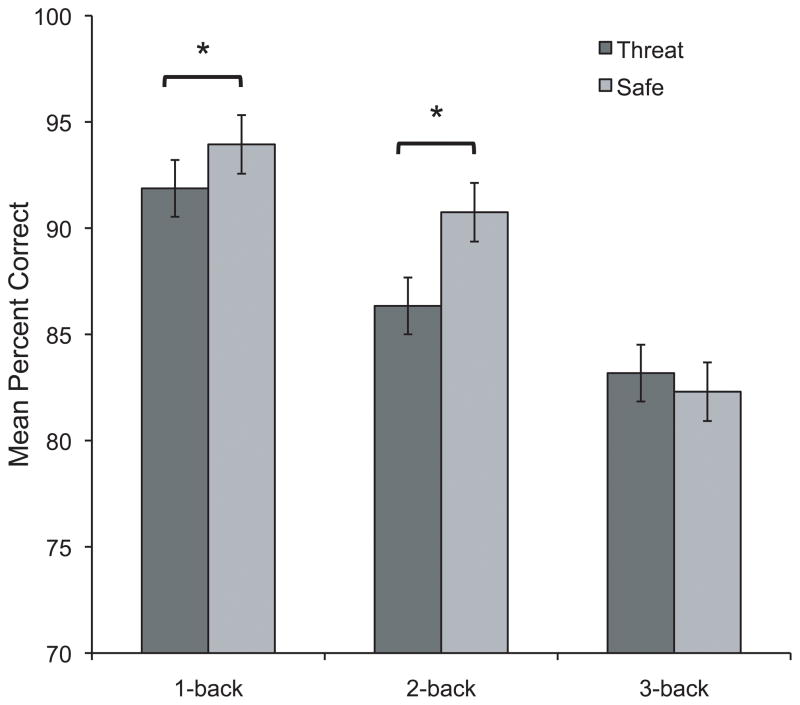
A series of binomial tests at the individual level confirmed that participants performed above chance in each condition. Reaction time (RT) was analyzed using 2 (Condition: threat, safe) × 3 (Load: 1-back, 2-back, 3-back) repeated-measures ANOVA, and results of the interaction indicated that RT did not differ between threat and safe across Load, F(2,50) = 1.1, p = .328. To examine the effect of induced anxiety on performance across different levels of cognitive load, n-back accuracy was entered into a 2 (Condition: threat, safe) × 3 (Load: 1-back, 2-back, 3-back) repeated measures ANOVA. There were significant main effects of Condition, F(1,25) = 7.2, p < .02, η2 = .22, and Load, F(2,50) = 48.0, p < .0001, η2 = .66, ε = .990, on performance, indicating that performance was significantly impaired during threat as compared to safe, and that overall performance differed across varying levels of cognitive load (planned comparisons demonstrated that participants performed progressively worse as task difficulty increased: 2-back performance was lower than 1-back, t(25) = −4.3, p < .001; and 3-back performance was lower than 2-back, t(25) = −5.7, p < .001). Importantly, there was a significant Condition × Load interaction, F(2,50) = 6.3, p < .006, ε = .910, η2 = .20, which demonstrated that participants’ 1-back and 2-back performance was impaired during threat as compared to safe (t(25) = −3.1, p < .006, and t(25) = −2.9, p < .009, respectively), but their 3-back performance did not differ between conditions (t(25) = 1.1, p = .262) (see Figure 2). This finding indicates that under low cognitive load, an anxiogenic context impaired working memory, whereas under high cognitive load, the same anxiogenic context did not disrupt working memory. The differential effect of threat of shock on low-load versus high-load cannot be attributed to differences in psychometric properties between the n-back tasks because these differences favored the low versus high-load tasks. As such, the performance data indicate that low-load verbal working memory was more susceptible to disruption by threat of shock than high-load working memory.
Figure 2.
Startle Reflex
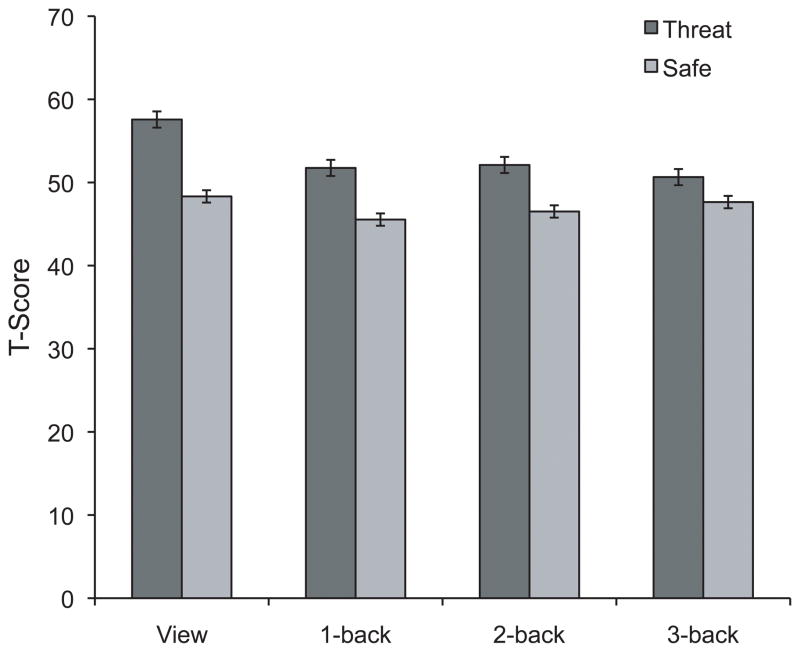
To examine the effect of induced-anxiety on the startle reflex across different levels of cognitive load, startle magnitude scores were entered into a 2 (Condition: threat, safe) × 4 (Load: view, 1-back, 2-back, 3-back) repeated measures ANOVA, similar to the performance data. There were significant main effects of Condition, F(1,25) = 47.7, p < .0001, η2 = .66, and Load, F(3,75) = 18.2, p < .0001, ε = .757, η2 = .40, on startle, indicating that startle was potentiated by threat as compared to safe, and that overall startle differed across varying levels of cognitive load. Startle magnitude was confirmed to be greater in threat than safe across all levels of cognitive load (view, t(25) = 7.5, p < .001; 1-back, t(25) = 6.3, p < .001; 2-back, t(25) = 5.0, p < .001; and 3-back, t(25) = 2.7, p < .02). Planned comparisons demonstrated that startle magnitude was higher during view than all other conditions: vs. 1-back, t(25) = 6.1, p < .001; 2-back, t(25) = 4.6, p < .001; and 3-back, t(25) = 4.7, p < .001. In addition, there was a significant Condition × Load interaction, F(3,75) = 10.1, p < .001, ε = .930, η2 = .30 (see Figure 3). This interaction is best described by a linear trend difference in startle magnitude between threat and safe across Load: in the threat condition, there was a significant linear decrease in startle magnitude as Load increased (linear trend: F(1,25) = 27.8 p < .001, η2 = .527), in the safe condition, there was no linear relationship between startle magnitude and Load (linear trend: F(1,25) = .174, p = .681, η2 = .007). Importantly, this means that startle magnitude during safe was not reduced under high-load (further, startle between low-load (view) and high (3-back) do not differ t(25) = .81, p = .426).
Figure 3.
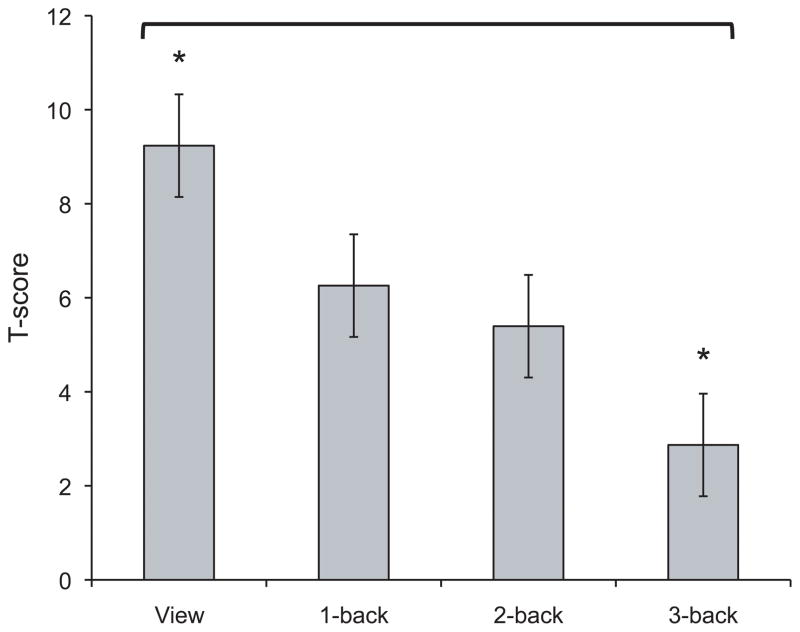
To simplify the interpretation of this interaction, we collapsed the Condition factor and examined the effect of Load on anxiety-potentiated startle (defined as the difference in startle magnitude between threat and safe; i.e., threat - safe). Planned comparisons demonstrated that anxiety-potentiated startle magnitude during view was significantly higher than anxiety-potentiated startle at all other levels of cognitive load: vs. 1-back, t(25) = 2.7, p < .02, 2-back, t(25) = 3.1, p < .005, and 3-back, t(25) = 5.5, p < .001 (see Figure 4). Anxiety-potentiated startle during 3-back was significantly lower than all other levels of cognitive load: vs. 1-back, t(25) = −3.1, p < .006, and 2-back, t(25) = −2.3, p < .04. Anxiety-potentiated startle during 1-back and 2-back did not differ, t(25) = 0.9, p = .331. These findings indicate that anxiety had the greatest impact on the startle reflex when subjects were not engaged in any task, and the least impact when subjects were engaged in a demanding cognitive task. Although 3-back anxiety-potentiated startle was significantly lower than all other conditions, it was significantly different from zero, t(25) = 2.6, p < .05, demonstrating that startle was still potentiated under high-load. This is in contrast with the finding that anxiety-related performance impairment was eliminated under high-load (i.e., performance did not differ between threat and safe).
Figure 4.
Ratings
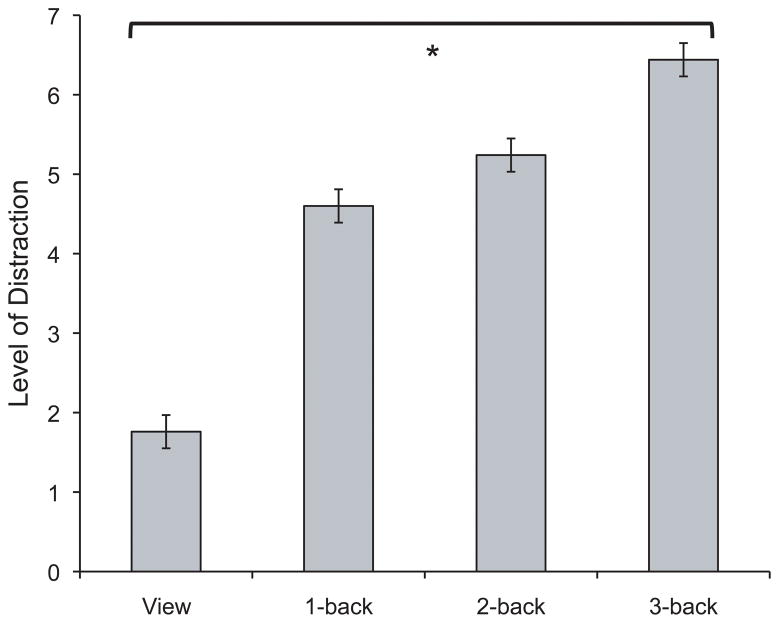
A t-test using subjective anxiety scores confirmed greater anxiety during threat than during safe (t(25) = 10.0, p < .0001; M=5.5, SD= 1.6; M=2.1, SD=1.2, respectively). Task-related distraction from shock was analyzed using a repeated-measures ANOVA across different levels of Load. As predicted, participants were progressively more distracted as task difficulty increased (linear trend: F(1,24) = 101.4, p < .001, η2 = .81; see Figure 5). The omnibus test revealed differences in the level of distraction across all levels of Load (F(3,72) = 61.9, p < .001,η2 = .72), with planned contrasts showing significantly more distraction from shock between view and 1-back, 1-back and 2-back, 2-back and 3-back (t(25) = 7.8, p < .0001; t(25) = 2.8, p < .02; t(25) = 4.2, p < .0001, respectively).
Figure 5.
Correlations
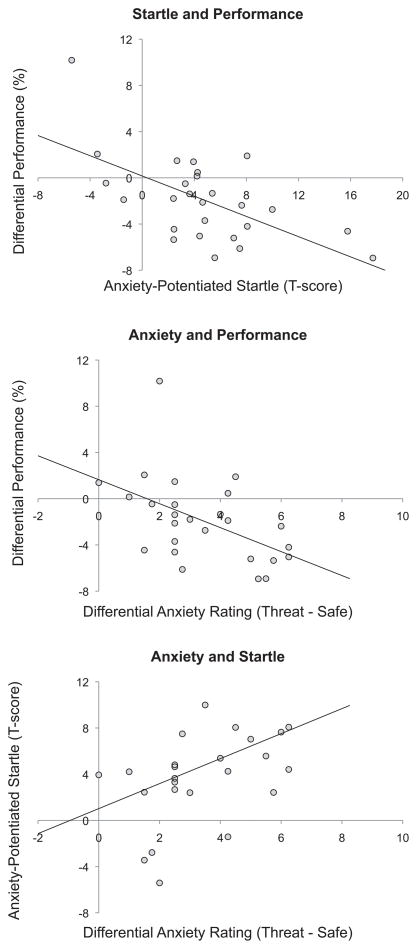
A series of Pearson product-moment correlation coefficients were calculated to examine the strength of the relationship between anxiety (as indexed by anxiety-potentiated startle and subjective ratings) and performance. We found a strong negative correlation between anxiety-potentiated startle and differential performance (threat - safe) (r = −.62, p < .01) meaning that increased startle potentiation was associated with a decrease in performance (see Figure 6). In parallel, we found a strong negative correlation between anxiety ratings and differential performance (r = −.49, p < .02), suggesting that greater startle potentiation and high anxiety ratings were both associated with greater performance impairment. In addition, there was a moderate positive correlation between anxiety ratings and anxiety-potentiated startle (r = .37, p < .05), suggesting that subjective anxiety was associated with an increase in startle. These findings indicate that anxiety (as indexed by anxiety-potentiated startle and subjective evaluation) is a strong predictor of threat-related working memory impairment.
Figure 6.
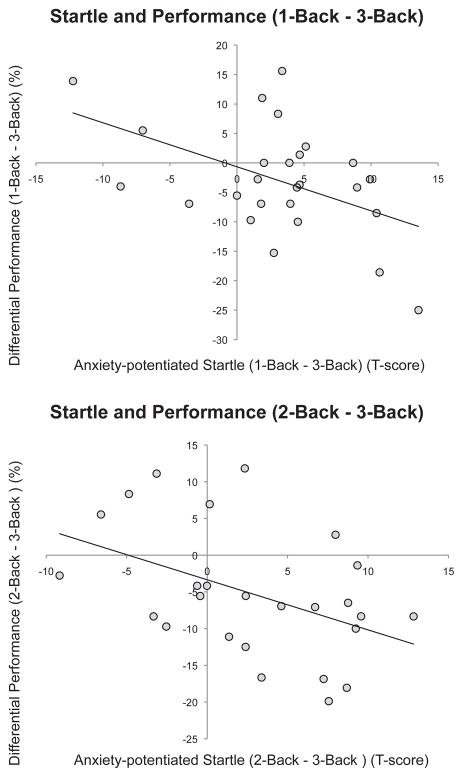
To further assess the relationship between anxiety and performance impairment, we calculated a second set of Pearson product-moment correlation coefficients that quantified the strength of the association between the reduction in anxiety potentiated startle from low-load to high-load and the reduction in threat-induced performance impairment from low-load to high-load. First, we examined the relationship between anxiety-potentiated startle reduction from 1-back to 3-back and the performance difference between threat and safe from 1-back to 3-back. We found that reduction in startle potentiation from low to high-load exhibited a strong negative correlation with a reduction in threat-related performance impairments (r = −.48, p < .02) (see Figure 7). To further map out the relationship between startle and performance as load increases, we examined the relationship between anxiety-potentiated startle reduction from 2-back to 3-back and the performance difference between threat and safe from 2-back to 3-back. This correlation demonstrated that reduction in startle potentiation from moderate to high-load exhibited a strong negative correlation with a reduction in threat-related performance impairments (r = −.45, p < .03). Taken together, these correlations indicate that anxiety (as indexed by anxiety-potentiated startle and subjective evaluation) is strongly associated with threat-related performance disruption, and that the reduction in anxiety from low to high-load (as indexed by anxiety-potentiated startle) predicts a reduction in threat-related performance disruption from low to high-load. In other words, subjects who showed the greatest reduction in anxiety-potentiated startle as load increased (1 to 3-back and 2 to 3-back) also showed the greatest reduction in performance impairment as load increased (1 to 3-back and 2 to 3-back).
Figure 7.
Discussion
General
The aim of this study was to examine the interplay between cognition and anxiety as cognitive load increases. Consistent with predictions we demonstrated that 1) anxiety impaired cognition under low but not high-load, and 2) increasing cognitive load linearly reduced anxiety. Specifically, we found that anxiety disrupted low-load working memory processes, and at least one component of anxiety (i.e., anxious apprehension [Heller, et al., 1997; Barlow 1991]), see below) was alleviated when subjects were engaged in a demanding task. This latter effect was demonstrated by comparable levels of 3-back performance during threat and safe, a significant increase in subjective reports of task-related distraction from low-load to high-load tasks, and a reduction in startle from low-load to high-load tasks. Moreover, we find that the reduction in startle potentiation from low to high-load predicts the reduction in threat-related performance impairments from low to high-load. This indicates that performance disruption under low-load, and the absence of disruption under high-load can be attributed to a high level and a low level of anxiety, respectively. Our findings suggest that there is a crucial inflection point between moderate and high cognitive load, where cognitive resources shift from divided attention between anxiety and the task to a predominant focus on task demand. This effect was indexed by a decrease in performance impairment and anxiety-potentiated startle between 2-back and 3-back. All of these findings were further underscored by the correlations observed between anxiety and performance, startle and performance, and anxiety and startle potentiation, suggesting that anxiety is linked to working memory performance impairment. We integrate these findings in a Two-Component Theory of Anxiety described below.
1) Anxiety can impair cognition
Although the impact of anxiety on performance was predicted, it may seem at odds with studies that fail to find an effect of anxiety on working memory performance or those that find an effect in the opposite direction. However, these apparent contradictions do not hold up when the effects are examined more closely. For example, Lavie (2005) described findings that suggest high-load cognitive tasks as more susceptible to outside influence. Yet, it is important to note that 1) the distractors they used were benign visual stimuli (not emotionally evocative stimuli like threat of shock) and 2) their high-load trials were likely more susceptible to distractor influence because the task was also visual, and higher load (longer letter strings) required more scanning and involved more visual input than low-load. Another potential contradiction arises from Shackman et al.’s (2006) finding that spatial but not verbal working memory was impaired by anxiety. However, Shackman et al. only examined the effects of anxiety on a high-load verbal task, a level of cognitive engagement where we also did not find an effect. In contrast, our findings demonstrate that when the effects of anxiety on verbal working memory are examined more thoroughly by varying cognitive load, processing deficits are observed under low-load, suggesting the need for a more comprehensive explanation of this interaction.
2) Cognitive load reduces anxiety
The fact that psychophysiological concomitants of anxiety can be reduced by simply engaging in a demanding task holds implications for understanding emotion regulation and for refining therapeutic interventions such as CBT, which rely in-part on distraction-based cognitive strategies. However, the strong association between anxiety and performance impairment suggests additional clinical implications. It is possible that patients with an anxiety disorder may be unable overcome their anxiety to engage fully in the high demand task. In particular, patients with social anxiety disorder and post-traumatic stress disorder have shown marked deficits in executive function and verbal working memory (Airaksinen, et al., 2005), suggesting that these populations may be more susceptible to anxiety-related cognitive disruption. With an increased tendency toward anxious apprehension, patients’ executive resources may be subsumed by anxiety, and performance may continue to suffer in comparison to healthy controls. If this were the case, we would expect that threat-related impairment would subsist even under high cognitive load. By characterizing the mechanisms behind both anxiety-related impairment and cognitive-based anxiety reduction, we can address questions about pathology (e.g., excessive worry, cognitive disruption) and treatment (e.g., distraction-based cognitive techniques).
3) Two-Component Theory of Anxiety
The reciprocal effects of anxiety and cognitive load on performance, coupled with evidence suggesting that anxiety-potentiated startle is sustained even when anxiety-related performance impairment is eliminated, indicate that there is more than one mechanism supporting an anxious response. We propose that one component (automatic preparatory response, here indexed by startle potentiation) primes defensive mechanisms (Lang, Bradley, & Cuthbert, 1998), and may be accompanied by an increase in perceptual sensitivity (Cornwell et al., 2011) and autonomic arousal (e.g., increase in heart rate and respiration). We claim that a second component (anxious apprehension [Heller et al., 1997], here indexed by performance impairment and startle potentiation, see below), which engages executive resources, is comprised of anxiety-related cognitive processes. When these resources are depleted by high-load verbal working memory tasks, anxious apprehension has no workspace with which to operate, and the effect of this anticipation on performance is eliminated. As Eysenck et al. (1992) proposed, phonologically-dependent “worry” negatively impacts performance, but contrary to their proposal that high demand tasks are most vulnerable, we demonstrate that anxious apprehension disrupts verbal cognition only when task demands are low, at least in healthy participants. Although our findings suggest that the competition for executive resources drives anxiety-related impairment, in contrast to the processing efficiency theory, we find that easy verbal tasks leave resources free for worry-related disruption to operate, whereas difficult verbal tasks are viable competition for such resources. The verbal nature of this cognitive component of anxiety explains why anxiety-related impairment during threat is abolished in high-load verbal working memory tasks, but not high-load spatial working memory (Shackman et al., 2006).
It is important to note, however, that the automatic component indexed by startle is not entirely divorced from influencing cognition. In fact, startle potentiation appears to be indexing several anxiety-related processes including physiological arousal (priming of defensive mechanisms) and subjective anxiety (as demonstrated by the correlations). When presented in the context of pathological symptoms, this relationship is acutely evident (e.g., worrying about a social exchange can increase eccrine gland production in an individual with social phobia; a racing heart beat can trigger a full-blown panic attack in an individual with panic disorder). Yet, these two components are demonstrably separable, as evidenced by their dissociation under high cognitive load: startle reactivity is sustained (albeit with a reduction in magnitude) and performance impairment is eliminated. The protracted nature of startle potentiation suggests that high cognitive load does not eliminate all aspects of anxiety, and that some processes vital for survival may be preserved. This finding is consistent with evidence suggesting high-load tasks block amygdala activation to fearful faces under safety, but not under threat (Cornwell, et al., 2011). Together, these findings suggest that conscious appraisal of short-duration threat (fearful faces) and long-duration threat (unpredictable shock) operates under low-load, but is strongly reduced or abolished under high-load. In contrast, defense mechanisms continue to be primed under threatening conditions regardless of load level, possibly to enable fast responding to imminent danger.
The apparent dissociation between the cognitive component and automatic preparatory component of anxiety is in line with the proposal that the differential effect of anxiety on spatial versus verbal cognition is a result of inherent processing differences between the two types of memory. In particular, if spatial working memory impairment is primarily a product of resource competition with defensive priming mechanisms (which are consistently active when a threat is present), and verbal working memory impairment is primarily a product of anxious apprehension (which is active when cognitive resources are not otherwise engaged) then we would expect a differential effect of anxiety on spatial versus verbal working memory as load increases. As a consequence, spatial working memory would be consistently disrupted by the automatic preparatory component of anxiety that is pervasive under all levels of load. In support of this, previous research has demonstrated anxiety-related disruption of spatial working memory under high-load (i.e., 3-back; Lavric et al., 2003; Shackman et al., 2006). Defensive priming may selectively disrupt spatial tasks because it engages processes that facilitate affective biases (e.g., preparatory actions, increased vigilance), which share neural resources (e.g., right prefrontal cortex) with spatial working memory but not verbal working memory (Shackman et al., 2006). In contrast, verbal working memory would be disrupted by the cognitive component of anxiety, which is also verbal in nature and engages different mechanisms than defensive priming. As a result, verbal working memory disruption would occur only under low-load, where anxiety can initiate and maintain a strong grasp on cognitive resources. This prediction is supported by the performance impairment we observed in under 1- and 2-back. We propose that the shared neural real estate between working memory and the cognitive component of anxiety (e.g., ACC; working memory: Bunge et al., [2000], Raichle [1993], worrisome thoughts: Paulesu et al., [2009]) may serve as the mechanism by which anxiety disrupts verbal cognition. Yet under high-load, anxious apprehension may fail to fully engage and may consequently be preempted by a shift in resource allocation to verbal working memory. In line with previous research (Lavric et al., 2003; Shackman et al., 2006) and with the findings of the current study, we found that verbal working memory is not impaired under high working verbal memory load.
Strengths, Limitations and Future Directions
By varying the levels of cognitive load placed on our subjects, we captured the interplay between anxiety and cognition as well as pinpointed the level of load where resources shift from engaging in anxious apprehension to goal-directed task requirements. Coupled with the use of a well-validated method to induce anxiety (shock), this paradigm afforded us a novel approach to investigating the reciprocal interaction between anxiety and cognition. Previous research has primarily focused on perceptual load and short-duration distractors/threat (e.g., Bishop, 2008; Lavie 1995; Lavie & Cox, 1997), with the exception of Shackman et al. (2006) and Lavric et al. (2003), reinforcing the impact our findings have on understanding the influence of anxiety on cognitive processes.
Among the limitations, we make the claim that anxiety is reduced by high cognitive load; however, our measures of anxiety across different levels of task demand were limited to anxiety-potentiated startle and task-related distraction ratings. One alternative interpretation to startle inhibition as an index of anxiety inhibition, is that the reduction in startle potentiation under high-load may be due to modality-specific shifts in attention from acoustic (startle) to visual (n-back). However, two observations contradict this interpretation: 1) because subjects could not rely on visual perceptual similarity to make their responses, they reported engaging in subvocal rehearsal, which would increasingly allocate attentional resources to the acoustic domain, not away from it, and 2) cognitive load reduces startle under threat but not safe, demonstrating that cognitive load does not have a global impact on startle. Nevertheless, it is unclear if subvocal rehearsal facilitates or inhibits startle by shifting attention to higher-order acoustic processes. We found that engaging in a verbal working memory task in the absence of shock does not consistently decrease or increase startle magnitude, suggesting the effect, if present, is small. The influence of covert verbal processes on startle should be explored in greater detail. Future studies should also consider including additional measures of anxiety such as measures of autonomic arousal (e.g., heart rate variation) and in particular, measures that are sensitive to valence (e.g., corrugator supercilli EMG). In addition, the use of a more direct measure of anxious apprehension (e.g., Penn State Worry Questionnaire [Molina & Borkovec, 1994], or questions that directly probe awareness of the presence of worrisome thoughts) may shed light on the proposal that worrisome thoughts are related to the observed disruption. We also propose that induced anxiety has a different effect on verbal versus spatial working memory, when taking into account our findings and the findings of Shackman et al. (2006). Additional research should parametrically modulate spatial load and investigate the impairment associated with induced anxiety. It is also important to note that our sample consisted solely of healthy individuals, and therefore any conclusions drawn regarding pathological anxiety should be interpreted with caution. The mechanisms we investigated may reflect some of the same mechanisms that have gone awry in anxiety disorders, but these effects need to be explored in patient samples to determine if these claims hold true. Future research with patient populations should be used to better understand the impact of induced anxiety on impairment in individuals with emotional pathology.
In summary, the findings suggest that anxiety is comprised of two components: an anxious apprehension component and an automatic preparatory component. These components dissociate at the point of inflection where the effect of anxiety on cognition is overcome by the effect of cognitive load on anxiety, thereby demonstrating that 1) anxiety can impair cognition but also that 2) cognitive performance can reduce anxiety. These effects are further underscored by the strong negative relationship exhibited between anxiety and performance, suggesting that individual differences in anxiety drive differences in performance impairment. Our findings have important implications for understanding the deleterious impact of anxiety on cognition, and as a corollary, they have the potential to impact the characterization and treatment of individuals with anxiety disorders.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Mental Health.
Footnotes
The bulk of the studies that Bishop (2008) reviewed focus primarily on trait anxiety (Spielberger State-Trait Anxiety Inventory (STAI), Spielberger [1983]), although a couple address the impact of state anxiety on attention. However, Bishop (2008) acknowledges that the state and trait measures are highly correlated, which allows for cautious inferences to be made when only one of the two is reported.
Conflict of Interest
The author(s) declare that, except for income received from the primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Airaksinen E, Larsson M, Forsell Y. Neuropsychological functions in anxiety disorders in population-based samples: evidence of episodic memory dysfunction. Journal of Psychiatric Research. 2005;39(2):207–214. doi: 10.1016/j.jpsychires.2004.06.001.. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Disorders of emotion. Psychological Inquiry. 1991;2:58–71. doi: 10.1207/s15327965pli0201_15.. [DOI] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2008;12(1):92–98. doi: 10.1038/nn.2242.. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Robinson E, Pruzinsky T, DePree JA. Preliminary exploration of worry: Some characteristics and processes. Behavior Research and Therapy. 1983;21:9–16. doi: 10.1016/0005-7967(83)90121-3.. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A Parametric Study of Prefrontal Cortex Involvement in Human Working Memory. NeuroImage. 1997;5(1):49–62. doi: 10.1006/nimg.1996.0247.. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Klinberg T, Jacobson RB, Gabrieli DE. A resource model of the neural basis of executive working memory. Proceedings of the National Academy of Sciences USA. 2000;97:3573–3578. doi: 10.1073/pnas.97.7.3573.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S, Martinkauppi S, Rämä P, Salli E, Korvenoja A, Aronen HJ. Distribution of cortical activation during visuospatial n-back tasks as revealed by functional magnetic resonance imaging. Cerebral Cortex. 1998;8(8):743–752. doi: 10.1093/cercor/8.8.743.. [DOI] [PubMed] [Google Scholar]
- Chambless DL, Gillis MM. Cognitive therapy of anxiety disorders. Journal of Consulting and Clinical Psychology. 1993;61(2):248–260. doi: 10.1037/0022-006X.61.2.248.. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Commentary on two articles concerning generalized and specific cognitive deficits. Journal of Abnormal Psychology. 2001;110:31–39. doi: 10.1037//0021-843X.110.1.31.. [DOI] [PubMed] [Google Scholar]
- Castaneda AE, Suvisaari J, Marttunen M, Perälä J, Saarni SI, Aalto-Setälä, et al. Cognitive functioning in a population-based sample of young adults with anxiety disorders. European Psychiatry. 2011;26(6):346–353. doi: 10.1016/j.eurpsy.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Alvarez RP, Lissek S, Kaplan R, Ernst M, Grillon C. Anxiety overrides the blocking effects of high perceptual load on amygdala reactivity to threat-related distractors. Neuropsychologia. 2011;49(5):1363–1368. doi: 10.1016/j.neuropsychologia.2011.02.049.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Asymmetric brain function, affective style, and psychopathology: The role of early experience and plasticity. Development and Psychopathology. 1994;6:741–758. doi: 10.1017/S0954579400004764. [DOI] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148.. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry. 1998;44:1239–1247. doi: 10.1016/S0006-3223(98)00288-1.. [DOI] [PubMed] [Google Scholar]
- Doallo S, Holguin SR, Cadaveira F. Attentional load affects automatic emotional processing: Evidence from event-related potentials. NeuroReport. 2006;17:5. doi: 10.1002/hbm.20636.. [DOI] [PubMed] [Google Scholar]
- Dvorak-Bertsch JD, Curtin JJ, Rubinstein TJ, Newman JP. Anxiety Moderates the Interplay Between Cognitive and Affective Processing. Psychological Science. 2007;18(8):699–705. doi: 10.1111/j.1467-9280.2007.01963.x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S, Kleczar A, Walter H. Valence-specific regulation effects in a working memory task with emotional context. NeuroImage. 2007;37:623–632. doi: 10.1016/j.neuroimage.2007.05.006.. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Calvo MG. Anxiety and performance: The processing efficiency theory. Cognition and Emotion. 1992;6(6):409–434. doi: 10.1080/02699939208409696. [DOI] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7(2):336–353. doi: 10.1037/1528-3542.7.2.336.. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RI, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) Washington, DC: American Psychiatric Association; 1995. [Google Scholar]
- Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences. 2002;99(6):4115–4120. doi: 10.1073/pnas.062381899.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biological Psychiatry. 2002;52:958–97. doi: 10.1016/S0006-3223(02)01665-7.. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious Responses to Predictable and Unpredictable Aversive Events. Behavioral Neuroscience. 2004;118(5):916–924. doi: 10.1037/0735-7044.118.5.916.. [DOI] [PubMed] [Google Scholar]
- Heller W, Nitschke JB, Etienne MA, Miller GA. Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology. 1997;106:376–385. doi: 10.1037/0021-843X.106.3.376.. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Herrmann K, Dolan RJ. Neural correlates of self-distraction from anxiety and a process model of cognitive emotion regulation. Journal of Cognitive Neuroscience. 2006;18(8):1266–1276. doi: 10.1162/jocn.2006.18.8.1266.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Wang PS. The Descriptive Epidemiology of Commonly Occurring Mental Disorders in the United States. Annual Review of Public Health. 2008;29(1):115–129. doi: 10.1146/annurev.publhealth.29.020907.090847.. [DOI] [PubMed] [Google Scholar]
- King R, Schaefer A. The emotional startle effect is disrupted by a concurrent working memory task. Psychophysiology. 2010;48(2):269–272. doi: 10.1111/j.1469-8986.2010.01062.x.. [DOI] [PubMed] [Google Scholar]
- Landis C, Hunt WA. The startle pattern. Farrar and Rinehart; New York: 1939. p. 168. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biological Psychiatry. 1998;44(12):1248–1263. doi: 10.1016/S0006-3223(98)00275-3.. [DOI] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused?: Selective attention under load. Trends in Cognitive Sciences. 2005;9(2):75–82. doi: 10.1016/j.tics.2004.12.004.. [DOI] [PubMed] [Google Scholar]
- Lavie N. Selective attention and cognitive control: Dissociating attentional functions through different types of load. In: Monsell S, Driver J, editors. Attention and performance XVIII. Cambridge, Massachusetts: MIT Press; 2000. pp. 175–194. [Google Scholar]
- Lavie N. Perceptual load as a necessary condition for selective attention. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:451–4684. doi: 10.1037/0096-1523.21.3.451.. [DOI] [PubMed] [Google Scholar]
- Lavie N, Cox S. On the efficiency of attentional selection: Efficient visual search results in inefficient rejection of distraction. Psychological Science. 1997;8:395–398. doi: 10.1111/j.1467-9280.1997.tb00432.x.. [DOI] [Google Scholar]
- Lavric A, Rippon G, Gray JR. Threat-evoked anxiety disrupts spatial working memory performance: An attentional account. Cognitive Therapy and Research. 2003;27:489–504. doi: 10.1023/A:1026300619569.. [DOI] [Google Scholar]
- Masson MEJ, Loftus GR. Using confidence for graphically based data interpretation. Canadian Journal of Experimental Psychology. 2003;57:203–220. doi: 10.1037/h0087426.. [DOI] [PubMed] [Google Scholar]
- Molina S, Borkovec TD. The Penn State Worry Questionnaire: Psychometric properties and associated characteristics. In: Davey GCL, Tallis F, editors. Worrying: Perspectives on theory, assessment and treatment. Wiley series in clinical psychology. Chinchester, England UK: Wiley; 1994. pp. 265–283. [Google Scholar]
- Morris LW, Davis MA, Hutchings CH. Cognitive and emotional components of anxiety: Literature review and a revised worry-emotionality scale. Journal of Educational Psychology. 1981;73(4):541–555. doi: 10.1037/0022-0663.73.4.541.. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010.. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Sambugaro E, Torti T, Danelli L, Ferri F, Scialfa G, Sberna M, Ruggiero GM, Bottini G, Sassaroli S. Neural correlates of worry in generalized anxiety disorder and in normal controls: A functional MRI study. Psychological Medicine. 2009;40:117–124. doi: 10.1017/S0033291709005649.. [DOI] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends in Cognitive Sciences. 2009;13(4):160–166. doi: 10.1016/j.tics.2009.01.006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9(2):148–158. doi: 10.1038/nrn2317.. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. NeuroImage. 2005;28(1):249–255. doi: 10.1016/j.neuroimage.2005.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Hermans EJ, van Marle HJF, Luo J, Fernández G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biological Psychiatry. 2009;66(1):25–32. doi: 10.1016/j.biopsych.2009.03.006.. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, et al. Working memory for complex figures: An fMRI comparison of letter and fractal n-back tasks. Neuropsychology. 2002;16(3):370–379. doi: 10.1037/0894-4105.16.3.370.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The scratchpad of the minds. Nature. 1993;363:583–584. doi: 10.1038/363583a0.. [DOI] [PubMed] [Google Scholar]
- Robinson O, Letkiewicz A, Overstreet C, Ernst M, Grillon C. The effect of induced anxiety on cognition: threat of shock enhances aversive processing in healthy individuals. Cognitive, Affective, & Behavioral Neuroscience. 2011;11(2):217–227. doi: 10.3758/s13415-011-0030-5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Maxwell JS, McMenamin BW, Greischar LL, Davidson RJ. Stress potentiates early and attenuates late stages of visual processing. Journal of Neuroscience. 2011;31(3):1156–1161. doi: 10.1523/JNEUROSCI.3384-10.2011.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain, and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12:154–167. doi: 10.1038/nrn299.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Sarinopoulos I, Maxwell JS, Pizzagalli DA, Lavric A, Davidson RJ. Anxiety selectively disrupts visuospatial working memory. Emotion. 2006;6(1):40–61. doi: 10.1037/1528-3542.6.1.40.. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Braver TS, Reynolds JR, Burgess GC, Yarkoni T, Gray JR. Individual Differences in Amygdala Activity Predict Response Speed during Working Memory. The Journal of Neuroscience. 2006;26(40):10120–10128. doi: 10.1523/JNEUROSCI.2567-06.2006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State–Trait Anxiety Inventory STAI (Form Y) Palo Alto, CA: Mind Garden; 1983. [Google Scholar]