Abstract
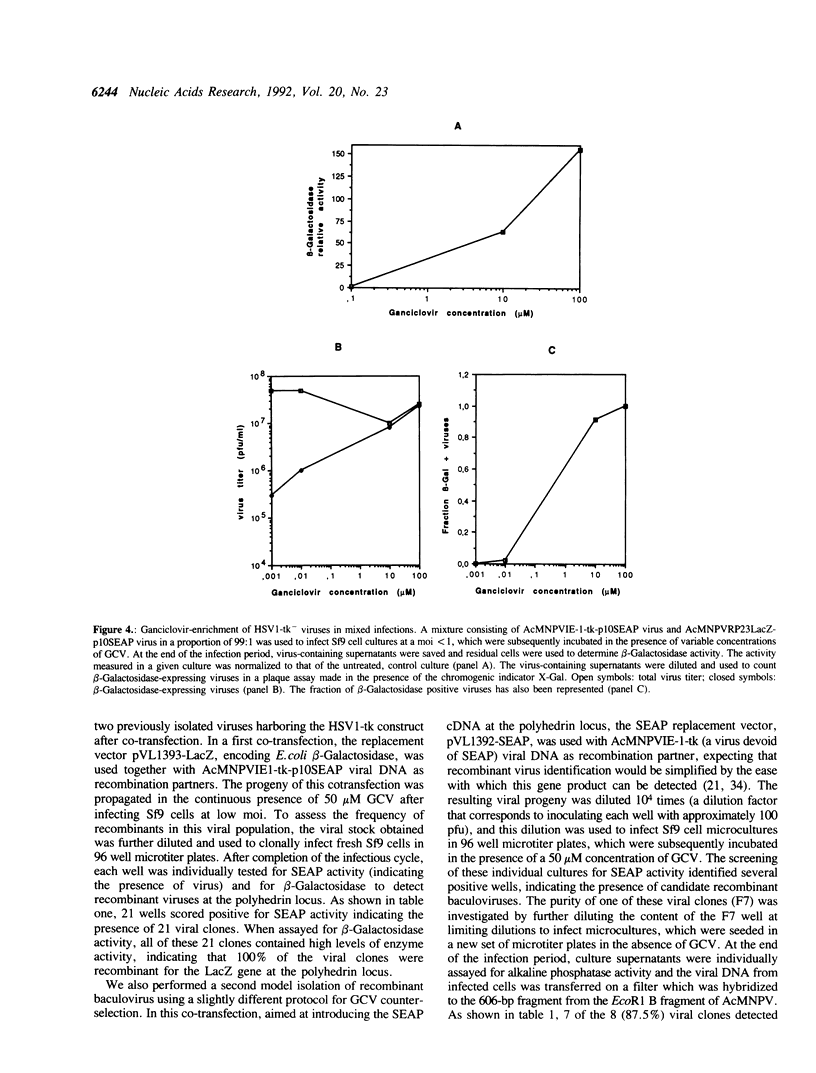
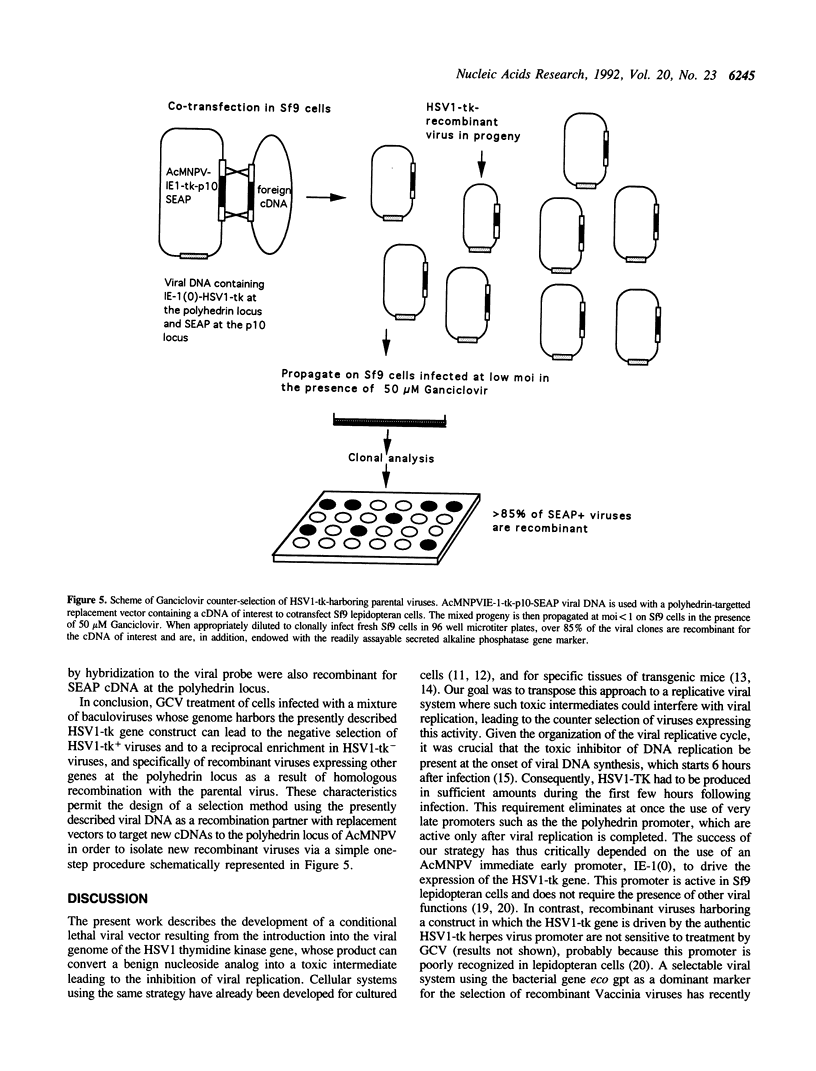
The expression of the thymidine-thymidylate kinase (HSV1-TK), (ATP: thymidine 5'-phosphotransferase; EC 2.7.1.21) of herpes simplex virus type 1 endows the host cell with a conditional lethal phenotype which depends on the presence of nucleoside analogues metabolized by this enzyme into toxic inhibitors of DNA replication. To generate a recombinant baculovirus that could be selected against by nucleoside analogs, the HSV1-tk coding sequence was placed under the control of the Autographa californica multicapsid nuclear polyhedrosis virus (AcMNPV) immediate early promoterm IE-1(0), and this construction was introduced via homologous recombination into the polyhedrin locus of AcMNPV. Two recombinant baculoviruses harboring this gene construct at the polyhedrin locus were isolated and tested for their ability to replicate in the presence of various concentrations of the nucleoside analog 9-(1,3-Dihydroxy-2-propoxymethyl)guanine (Ganciclovir). Neither Sf9 lepidopteran cell viability nor replication of wild type or beta-Galactosidase-expressing recombinant AcMNPVs were affected by concentrations of Ganciclovir up to 100 microM. In contrast, replication of the recombinant AcMNPV virus harboring the HSV1-tk gene was inhibited by Ganciclovir in a dose-dependent manner. The inhibition was detectable at 2 microM and complete at 100 microM. This property was exploited in model isolations aimed at purifying new recombinant viruses having lost this counter-selectable gene marker as a result of homologous recombination at the polyhedrin locus after cotransfection of the viral DNA with a replacement vector. After being propagated in the presence of Ganciclovir, the progeny of such co-transfections contained over 85% recombinant viruses, demonstrating that counter-selection of parental HSV1-tk-containing viruses by Ganciclovir constitutes a novel approach for recombinant baculovirus isolation.
Full text
PDF

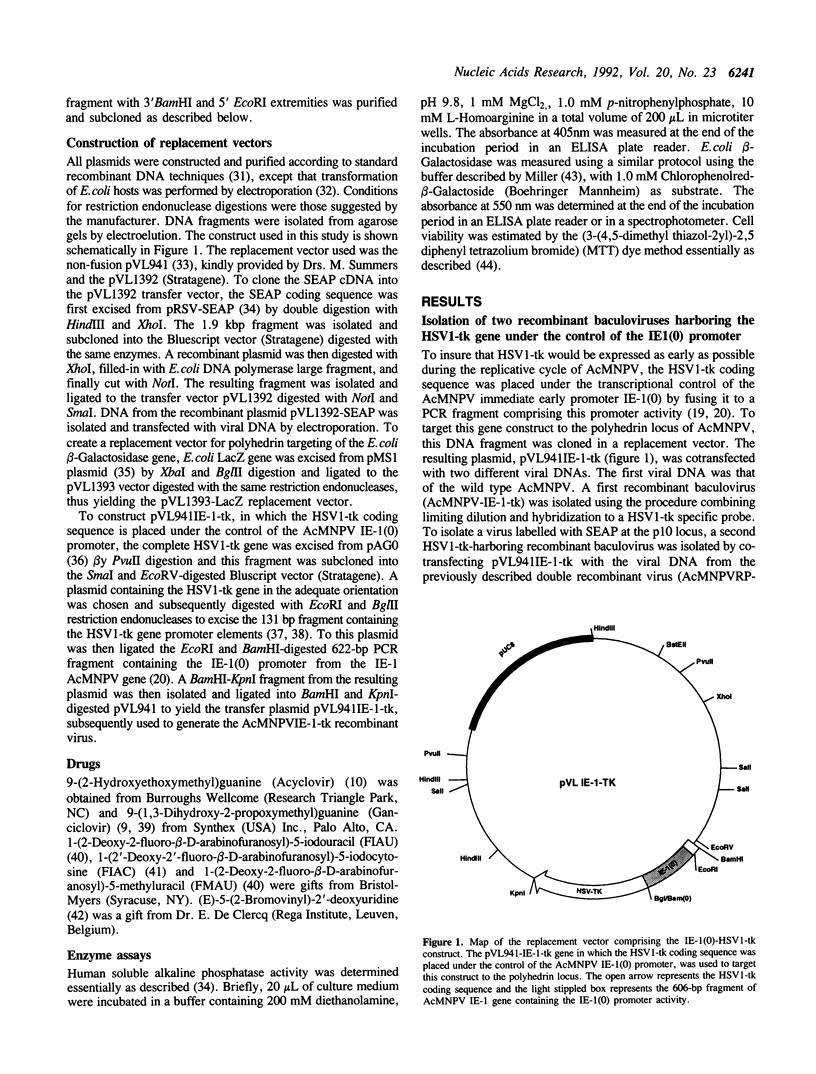

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allaudeen H. S., Descamps J., Sehgal R. K., Fox J. J. Selective inhibition of DNA replication in herpes simplex virus infected cells by 1-(2'-deoxy-2'-fluoro-beta-D-arabinofuranosyl)-5-iodocytosine. J Biol Chem. 1982 Oct 25;257(20):11879–11882. [PubMed] [Google Scholar]
- Berger J., Hauber J., Hauber R., Geiger R., Cullen B. R. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene. 1988 Jun 15;66(1):1–10. doi: 10.1016/0378-1119(88)90219-3. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Heyman R. A., Arias C., Sawchenko P. E., Evans R. M. Transgenic mice with inducible dwarfism. Nature. 1989 Jun 15;339(6225):538–541. doi: 10.1038/339538a0. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Heyman R., Hsi M., Evans R. M. Targeting of an inducible toxic phenotype in animal cells. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7572–7576. doi: 10.1073/pnas.85.20.7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Grill S. P., Dutschman G. E., Nakayama K., Bastow K. F. Metabolism of 9-(1,3-dihydroxy-2-propoxymethyl)guanine, a new anti-herpes virus compound, in herpes simplex virus-infected cells. J Biol Chem. 1983 Oct 25;258(20):12460–12464. [PubMed] [Google Scholar]
- Cheng Y. C., Huang E. S., Lin J. C., Mar E. C., Pagano J. S., Dutschman G. E., Grill S. P. Unique spectrum of activity of 9-[(1,3-dihydroxy-2-propoxy)methyl]-guanine against herpesviruses in vitro and its mode of action against herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1983 May;80(9):2767–2770. doi: 10.1073/pnas.80.9.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm G. E., Henner D. J. Multiple early transcripts and splicing of the Autographa californica nuclear polyhedrosis virus IE-1 gene. J Virol. 1988 Sep;62(9):3193–3200. doi: 10.1128/jvi.62.9.3193-3200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Schaffer P. A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbere-Garapin F., Chousterman S., Horodniceanu F., Kourilsky P., Garapin A. C. Cloning of the active thymidine kinase gene of herpes simplex virus type 1 in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3755–3759. doi: 10.1073/pnas.76.8.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Descamps J., De Somer P., Barr P. J., Jones A. S., Walker R. T. (E)-5-(2-Bromovinyl)-2'-deoxyuridine: a potent and selective anti-herpes agent. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2947–2951. doi: 10.1073/pnas.76.6.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner F. G., Moss B. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J Virol. 1988 Jun;62(6):1849–1854. doi: 10.1128/jvi.62.6.1849-1854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Field A. K., Davies M. E., DeWitt C., Perry H. C., Liou R., Germershausen J., Karkas J. D., Ashton W. T., Johnston D. B., Tolman R. L. 9-([2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine: a selective inhibitor of herpes group virus replication. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4139–4143. doi: 10.1073/pnas.80.13.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- Godeau F., Casanova J. L., Fairchild K. D., Saucier C., Delarbre C., Gachelin G., Kourilsky P. Expression and characterization of recombinant mouse beta 2-microglobulin type a in insect cells infected with recombinant baculoviruses. Res Immunol. 1991 Jun-Aug;142(5-6):409–416. doi: 10.1016/0923-2494(91)90039-l. [DOI] [PubMed] [Google Scholar]
- Godeau F., Casanova J. L., Luescher I. F., Fairchild K. D., Delarbre C., Saucier C., Gachelin G., Kourilsky P. Binding of low concentration of peptide to H-2Kd produced in insect cells requires mouse beta 2-microglobulin co-expression. Int Immunol. 1992 Feb;4(2):265–275. doi: 10.1093/intimm/4.2.265. [DOI] [PubMed] [Google Scholar]
- Guarino L. A., Summers M. D. Functional mapping of a trans-activating gene required for expression of a baculovirus delayed-early gene. J Virol. 1986 Feb;57(2):563–571. doi: 10.1128/jvi.57.2.563-571.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino L. A., Summers M. D. Nucleotide sequence and temporal expression of a baculovirus regulatory gene. J Virol. 1987 Jul;61(7):2091–2099. doi: 10.1128/jvi.61.7.2091-2099.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman R. A., Borrelli E., Lesley J., Anderson D., Richman D. D., Baird S. M., Hyman R., Evans R. M. Thymidine kinase obliteration: creation of transgenic mice with controlled immune deficiency. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2698–2702. doi: 10.1073/pnas.86.8.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitts P. A., Ayres M. D., Possee R. D. Linearization of baculovirus DNA enhances the recovery of recombinant virus expression vectors. Nucleic Acids Res. 1990 Oct 11;18(19):5667–5672. doi: 10.1093/nar/18.19.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G. R., Guarino L. A., Summers M. D. Novel regulatory properties of the IE1 and IE0 transactivators encoded by the baculovirus Autographa californica multicapsid nuclear polyhedrosis virus. J Virol. 1991 Oct;65(10):5281–5288. doi: 10.1128/jvi.65.10.5281-5288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz A., Spencer R. W., Tam T. F., Thomas E., Copp L. J. Design of alternate substrate inhibitors of serine proteases. Synergistic use of alkyl substitution to impede enzyme-catalyzed deacylation. J Med Chem. 1987 Apr;30(4):489–491. doi: 10.1021/jm00387a001. [DOI] [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. High level expression of nonfused foreign genes with Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1989 May;170(1):31–39. doi: 10.1016/0042-6822(89)90348-6. [DOI] [PubMed] [Google Scholar]
- Maeda S. Expression of foreign genes in insects using baculovirus vectors. Annu Rev Entomol. 1989;34:351–372. doi: 10.1146/annurev.en.34.010189.002031. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R. Expression of the herpes thymidine kinase gene in Xenopus laevis oocytes: an assay for the study of deletion mutants constructed in vitro. Nucleic Acids Res. 1980 Dec 20;8(24):5931–5948. doi: 10.1093/nar/8.24.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acids Res. 1980 Dec 20;8(24):5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medin J. A., Hunt L., Gathy K., Evans R. K., Coleman M. S. Efficient, low-cost protein factories: expression of human adenosine deaminase in baculovirus-infected insect larvae. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2760–2764. doi: 10.1073/pnas.87.7.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. K. Baculoviruses as gene expression vectors. Annu Rev Microbiol. 1988;42:177–199. doi: 10.1146/annurev.mi.42.100188.001141. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf A., Mattei D., Schreiber M. Parasite antigens expressed in Escherichia coli. A refined approach for epidemiological analysis. J Immunol Methods. 1990 Mar 27;128(1):81–87. doi: 10.1016/0022-1759(90)90466-9. [DOI] [PubMed] [Google Scholar]
- Vialard J., Lalumière M., Vernet T., Briedis D., Alkhatib G., Henning D., Levin D., Richardson C. Synthesis of the membrane fusion and hemagglutinin proteins of measles virus, using a novel baculovirus vector containing the beta-galactosidase gene. J Virol. 1990 Jan;64(1):37–50. doi: 10.1128/jvi.64.1.37-50.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. V., Rohel D. Z., Kuzio J., Faulkner P. A cytopathological investigation of Autographa californica nuclear polyhedrosis virus p10 gene function using insertion/deletion mutants. J Gen Virol. 1989 Jan;70(Pt 1):187–202. doi: 10.1099/0022-1317-70-1-187. [DOI] [PubMed] [Google Scholar]