SUMMARY
Vertebrate pigment cells in the eye and skin are useful models for cell types that use specialized endosomal trafficking pathways to partition cargo proteins to unique lysosome-related organelles such as melanosomes. This review describes current models of protein trafficking required for melanosome biogenesis in mammalian melanocytes.
Introduction
The secretory and endocytic systems of all eukaryotic cells are subdivided into unique membranous organelles, each with its own set of functions and molecular components. Cells must maintain organelle identity despite constant membrane flux and transfer of materials among organelles. Eukaryotes have evolved elaborate vesicular trafficking mechanisms that accomplish this feat by ensuring (i) proper macromolecule sorting and delivery to, or retention within, the appropriate destination, and (ii) proper maturation of secretory and endocytic organelles (15). These basic mechanisms are conserved among all eukaryotic cell types.
Besides ubiquitous secretory and endocytic organelles, certain cell types in multicellular organisms require additional unique organelles to perform specific physiological functions. One class of such organelles are the lysosome-related organelles (LROs, some of which are also referred to as secretory lysosomes; ref. 11), a group of tissue-specific membranous structures that share some features with conventional lysosomes, such as derivation from the endocytic system, acidic lumenal pH, and a cohort of lysosomal resident proteins (38, 148). While some LROs such as cytolytic granules of cytotoxic T cells and natural killer cells appear to be modified lysosomes, other LROs are distinct and coexist with lysosomes within their respective cell type (148). Examples of such LROs with important physiological functions are dense granules in platelets, lamellar bodies in lung epithelial type II cells, signaling endosomes in plasmacytoid dendritic cells (10, 157), and melanosomes in pigment cells. Cells that harbor these LROs must exploit specific mechanisms to divert cargoes from conventional endocytic organelles and deliver them to nascent LROs. Failure of these mechanisms can lead to LRO deficiencies and consequent disease. The best-studied example of such an LRO is the mammalian melanosome – an organelle found in melanocytes of the eye and skin and pigmented epithelial cells of the retina, iris and ciliary body of the eye, in which melanin pigments are synthesized and stored (120, 139). Because the same cellular machinery is used to sort cargoes to LROs in diverse cell types, insights gained from studying trafficking in pigment cells such as mammalian melanocytes apply to multiple physiological systems and inform us regarding disease etiology. This review describes current models of melanosomal protein trafficking in melanocytes.
Melanin Synthesis and Melanosome Biogenesis
Melanin is a heterogeneous, insoluble non-protein polymer composed of subunits that are products of enzymatically modified tyrosine (97). Mammals produce two types of melanins, black/brown eumelanins and red/yellow pheomelanins. The rate-limiting initial steps in all melanin synthesis – the hydroxylation of tyrosine to L-DOPA and oxidation of L-DOPA to DOPAquinone – are catalyzed by the pigment-cell-specific enzyme tyrosinase (TYR; 108). Subsequent spontaneous oxidation and isomerization steps yield a mixture of eumelanin intermediates. Two other enzymes – DOPAchrome tautomerase (DCT) and tyrosinase-related protein 1 (TYRP1) – influence the nature of the intermediates and properties of the eumelanins (166). Mutations in the genes encoding these and other melanosomal constituents result in oculocutaneous albinism (OCA; Table 1), characterized by partial or complete loss of eumelanin synthesis. If sufficient cysteine is present, DOPAquinone is modified to cysteinyl L-DOPA, and downstream steps yield pheomelanins (166). Melanosomes that make predominantly pheomelanins have distinct properties from those that make predominantly eumelanins (53, 100, 126) and will not be discussed further.
Table 1.
Hypopigmentation genes and their products
| Complex | Gene | Human disease | Gene product | Mouse model | Drosophil a model | Other models* |
|---|---|---|---|---|---|---|
| TYR | OCA1 | TYR | albino | several | ||
| OCA2 | OCA2 | OCA2/P | pink-eyed dilution | i-3(k) | ||
| TYRP1 | OCA3 | TYRP1/TRP-1 | brown | several | ||
| SLC45A2 | OCA4 | SLC45A2/ MATP | underwhite | b series (k) | ||
| DCT | DCT/ TYRP2/ TRP-2 | slaty | ||||
| PMEL | PMEL/Pmel17/ Silver/ gp100 | silver |
Dominant white (ch) fading vision (z) Merle (d) Silver (h) |
|||
| GPR143 | OA1 | GPR143/ OA1 | Oa1 | |||
| MLANA | MART1/melan-a | melan-a | ||||
| BLOC-1 | MUTED | Muted | muted | |||
| CNO | Cappuccino | cappuccino | ||||
| DTNBP1 | HPS7 | Dysbbindin | sandy | |||
| BLOC1S3 | HPS8 | BLOS3 | reduced pigmentation | |||
| PLDN | HPS9 | Pallidin | pallid | |||
| BLOC1S1 | BLOS1 | |||||
| BLOC1S2 | BLOS2 | |||||
| SNAPIN | Snapin | |||||
| BLOC-2 | HPS3 | HPS3 | HPS3 | cocoa | ||
| HPS5 | HPS5 | HPS5 | ruby-eye 2 | pink | ||
| HPS6 | HPS6 | HPS6 | ruby-eye | |||
| BLOC-3 | HPS1 | HPS1 | HPS1 | pale ear | ||
| HPS4 | HPS4 | HPS4 | light ear | |||
| Class C Vps | VPS 11 | VPS 11 |
vps-11(ok1664) (n) pale gray eyes (k) |
|||
| VPS 16 | VPS 16 | vps-16(ok719) (n) | ||||
| VPS 18 | VPS 18 | deep orange |
vps18(hi2499A) (z) vps-18(tm1125) (n) |
|||
| VPS33A | VPS33A | buff | carnation | vps-33(tm327) (n) | ||
| VPS39 | VPS39/Vam6 |
leberknodel (z) vps-39(tm327) (n) |
||||
| VPS41 | VPS41 | light | vps-41(ep402) (n) | |||
| VPS33B | ARCS1 | VPS33B | ||||
| C14orf133 | ARCS2 | VIPAR/VPS16B | full-of-bacteria | spe-39 (n) | ||
| RAB38 | Rab38 | chocolate | lightoid |
Ruby (rat) glo-1(zu437) (n) |
||
| RABGGTA | α subunit of Rab geranylgeranyl transferase II | gunmetal | ||||
| RPGR? | RPGR? | claret | glo-4(ok623) (n) | |||
| LYST | CHS | LYST | beige | |||
| MYO5A | GS1 | Myosin VA | dilute | |||
| RAB27A | GS2 | RAB27A | ashen | |||
| MLPH | GS3 | Melanophilin | leaden | |||
| STX17 | Syntaxin 17 | Gray (h) | ||||
| TRAPP | TRAPPC6A | TRAPPC6A | mhyp | |||
| FIG4 | CMT4J | Fig4/ SAC3 | pale tremor | |||
| VAC14 | VAC14/ ARPIKFYVE | Ingls (Infantile gliosis) | ||||
Abbreviations: ARCS, arthrogryposis-renal dysfunction-cholestasis syndrome; CHS, Chediak-Higashi syndrome; CMT4J, Charcot-Marie Tooth syndrome type 4J; GS, Griscelli syndrome; HPS, Hermansky-Pudlak syndrome; OA, ocular albinism; OCA, oculocutaneous albinism.
Key: (n) nematode, (z) zebrafish, (k) medaka fish, (h) horse, (ch) chicken, (d) dog
To sequester oxidative melanin intermediates, melanin synthesis is limited to the lumen of melanosomes. Melanosomes mature through four morphologically distinct stages (Figure 1), such that melanins synthesized in the last two stages are deposited on a “melanosome matrix” formed within the first two stages (161). Stage I melanosomes are vacuolar domains of early endosomes that harbor intralumenal vesicles (ILVs; 149); they are distinguished from early endosomes in other cells by irregular arrays of amyloid fibrils, composed of the pigment cell-specific protein, PMEL (8, 9, 102, 149), that emanate from the ILVs and eventually form the melanosome matrix (87, 161; Figure 1). Stage II melanosomes are functionally segregated from the endocytic pathway and are characterized by a fully formed melanosome matrix in which the fibrils are organized into arrays of parallel sheets (87, 106, 149). Melanin synthesis and deposition are initiated by the delivery to Stage II melanosomes of enzymes such as TYR and TYRP1 and other proteins such as the copper transporter ATP7A and the putative transporter OCA2. This causes darkening and thickening of the fibers in Stage III melanosomes until all internal structure is obscured in Stage IV melanosomes (161; Figure 1). In mammals, mature melanosomes are transferred from skin melanocytes to neighboring keratinocytes, while melanosomes generated in eye pigment cells are retained.
Figure 1. Melanosome maturation and segregation from endocytic organelles.
Represented are maturation stages of endosomes (left) and melanosomes (right) from early endosomes (top) and their putative derivation by maturation from earlier compartments (red arrows). Protein contents of melanosomes as described in the text are indicated in italics to the right. Early endosomes (top) form by coalescence of membranes derived by internalization from the plasma membrane and by input from the secretory pathway. They consist of tubular domains and vacuolar domains; within the vacuolar domains, internal vesicles begin to form by invaginationof the limiting membrane in regions rich in bilayered clathrin-and Hrs-containing coats (black arrows). The vacuolar domains mature into late endosomal multivesicularbodies (MVB) as in other cell types by exchange with other compartments via tubular connections. Lumenal contents of late endosomes are degraded upon fusion with lysosomes. In pigment cells, the vacuolar domains of early endosomes also correspond to StageI melanosomes, but are distinguished from vacuolar early endosomes of other cells by the presence of fibrils emanating from the internal vesicles (arrowheads). These fibrils elongate and assemble into sheets in Stage II melanosomes. Delivery of contents to Stage II melanosomes by vesicular traffic (not shown) or by tubular intermediates from early endosomes (as shown) results in the deposition of melanins on the fibrils in Stage III melanosomes. Continued melanin deposition masks internal structure in Stage IV melanosomes.
Melanosome resident proteins transit the early secretory pathway
Most known protein constituents of stage II–IV melanosomes are integral membrane glycoproteins. Like all such glycoproteins, melanosomal proteins are synthesized by ribosomes associated with the endoplasmic reticulum (ER), become integrated in the ER membrane and translocate their lumenal portions across the ER membrane via the ER translocation channel (translocon), are N-glycosylated by the translocon-associated oligosaccharyltransferase complex, and are folded and assembled by ER resident chaperone proteins and enzymes (47). TYR, TYRP1 and DCT are heavily glycosylated and modified by numerous disulfide bonds; their proper folding is thus highly reliant on ER redox conditions and calnexin – an integral membrane chaperone that binds to monoglucosylated N-linked oligosaccharides (67, 129, 130). TYR in particular is modified by six or seven N-linked oligosaccharides, and its folding and assembly are under tight control; we refer readers to an excellent review by Wang and Hebert for details (191). Failure to fold properly, as occurs in a number of albinism-associated forms of TYR, TYRP1 and OA1, results in retrotranslocation from the ER and degradation by the proteasome (19, 33, 66, 67, 182, 183). Some evidence suggests that TYRP1, OCA2, and the putative transporter SLC45A2 regulate the folding and ER exit of TYR in ways that are poorly understood (27, 31, 99, 184). Mutations in the genes encoding these proteins thus result in various isoforms of OCA (Table 1).
Melanosomal proteins that are properly folded in the ER transit through the Golgi complex en route to melanosomes, where their N-linked oligosaccharides are modified by resident glycosyltransferases, and in some cases – as for PMEL – O-linked oligosaccharides are added and modified. TYR first acquires enzymatic activity in the trans Golgi network (136), likely because this is where it first encounters copper (162), which is a critical cofactor (109). How melanosomal proteins are targeted from the Golgi to endosomal intermediates en route to melanosomes (see below) is not known, but Gaip C-terminus interacting protein (GIPC) – a PDZ domain-containing protein of unknown function – might facilitate TYRP1 trafficking through or from the Golgi. GIPC binds to the C-terminus of newly-synthesized TYRP1 within the Golgi or closely associated compartments (112). Knockdown of GIPC or of the associated APPL adaptor protein in melanocytes results in impaired melanogenesis and decreased TYR activity (95), suggesting that this interaction is functionally critical for trafficking of TYRP1 and perhaps other melanogenic enzymes. Where or how GIPC and APPL exert their functions, however, is not yet clear.
There has been some debate regarding whether PMEL traverses the Golgi en route to stage II melanosomes. The debate was sparked by the cofractionation of stage II melanosome-enriched subcellular fractions of a human pigmented melanoma cell line with PMEL isoforms containing N-linked glycans that lacked Golgi modifications (102). A number of subsequent publications purported to identify similarly unprocessed forms of PMEL at the plasma membrane or in endosomal compartments (186, 187). However, it is now clear that only PMEL forms with Golgi modifications, such as complex O-linked and sialylated N- and O-linked oligosaccharides, are detected in post-Golgi compartments and mature PMEL fibrils (68, 149); indeed, complex O-linked glycans appear to be required for formation of mature PMEL fibrils (68, 187). The earlier results likely reflected contamination of melanosome-enriched subcellular fractions with ER membranes (178), poorly characterized antibody epitopes, and low resolution techniques.
Generation of Early Stage Melanosomes
To generate functional melanosomes, melanosome contents must be diverted from classical endocytic compartments within pigment cells. PMEL is diverted to early stage melanosomes by mechanisms that are incompletely understood. PMEL is synthesized as an integral membrane protein with a large lumenal domain that has amyloidogenic properties, a single membrane spanning domain and a short cytoplasmic domain (178, 193). The latter bears a C-terminal ER exit signal that initiates transport through the secretory pathway and a conventional dileucine-based endocytic signal (see below) that facilitates internalization from the plasma membrane; a cytoplasmic domain truncation that disrupts these signals underlies the mild hypopigmentation observed in the silver mouse (121, 180) and essentially phenocopies a PMEL null mutation (72), indicating that these are essential steps in PMEL maturation. Following endocytosis, PMEL is delivered via the early endosomal system to Stage I melanosomes, where the lumenal domain becomes incorporated into ILVs that invaginate from the limiting membrane (8, 149). Two concomitant proteolytic cleavages within the PMEL lumenal domain liberate the fibrillogenic peptide (8, 9, 101, 179), although some of these cleavages might also occur in the TGN (107). PMEL sorting to ILVs depends on a lumenal determinant (78, 179) that overlaps with the fibrillogenic determinant (194) and is required for amyloid formation (179, 188). Unlike most other proteins that are sorted to ILVs, PMEL does not require ubiquitylation or the activity of the ESCRT machinery for its sorting (179), but rather requires the tetraspanin CD63 (188). PMEL fibril formation initiates from the ILVs, and the fibrils ultimately assemble into sheets that characterize Stage II melanosomes (87). While ILV formation and fibrillogenesis are initiated in Stage I melanosomes that are accessible to endocytic tracers, it is not known how these compartments mature into separate late endosomal and Stage II melanosomal compartments (Figure 1).
Two other pigment cell-specific proteins are found in early stage melanosomes but also in other compartments. GPR143, also known as OA1, is a G-protein coupled receptor that is mutated in ocular albinism type 1 (5, 158; Table 1). GPR143 is detected in late endosomes, lysosomes, and melanosomes, including especially early-stage melanosomes (61, 143), and some evidence suggests that intralumenal L-DOPA can serve as its ligand (118). Two sorting signals in the cytoplasmic C-terminal domain – an unusual dileucine-based signal and a tryptophan-glutamate doublet – are together necessary and sufficient for lysosomal and melanosomal localization (143), but the effectors that recognize these signals and direct GPR143 delivery are not known. GPR143 is down-regulated by ubiquitylation and ESCRT-dependent sorting to ILVs of late endosomes, and inhibition of this process leads to enhanced localization to mature melanosomes (60), indicating that the ubiquitylation state regulates GPR143 distribution between melanosomes and lysosomal degradation. MART1 (also called melan-a) is an unusual small integral membrane protein with no signal sequence (94). Like GPR143, MART1 localizes to multiple compartments, including late endosomes and lysosomes (34), to which it is targeted by ubiquitylation and ESCRT-dependent partitioning to lysosome-bound ILVs (110, 179). MART1 can be coimmunoprecipitated with GPR143 (61) and with PMEL (61, 77), but the functional consequences of these interactions are not clear. Gene targeting of the Mart1 gene in the mouse results in modest hypopigmentation and abnormalities in melanosome morphology (3).
Protein Sorting to Mature Melanosomes
The maturation of Stage II melanosomes to pigmented Stage III and IV requires the delivery of melanogenic enzymes and transporters, such as TYR, TYRP1 and OCA2, from early endosomes/stage I melanosomes. These integral membrane proteins are recognized as melanosome cargoes in part by conventional cytoplasmically-exposed endosomal sorting signals (7, 19, 165, 168, 181, 189), likely functioning in concert with lumenal determinants (63, 168). The sorting signals in TYR, TYRP1 and OCA2 conform to the acidic dileucine-based consensus sequence of [D/E]XXXL[L/I] (14). Similar signals found in the cytoplasmic domains of many endosomal an lysosomal integral membrane proteins serve as independent, transferable sorting signals for endocytosis and/or for subsequent endosomal sorting. Likewise, the acidic dileucine motifs in TYR, TYRP1 and OCA2 confer protein localization to late endosomes and lysosomes in non-melanocytes (19, 165, 168, 189) and are necessary for melanosome localization in melanocytes (7, 168, 189).
Acidic dileucine signals effect protein sorting by binding to conserved sites on heterotetrameric Adaptor Protein (AP) complexes. APs are a family of five related complexes that function as soluble sorting adaptors. They are recruited to membranes at least in part by binding to phosphoinositides (54) and/or Arf GTPases (45, 205), and then bind integral membrane cargo proteins that contain AP binding sites and cluster them into coated membrane domains. Membrane-bound AP-1, AP-2 and AP-3 also recruit clathrin, and thus facilitate cargo incorporation into clathrin-coated vesicles that bud from these domains at the trans-Golgi network, the cell surface or endosomes, respectively (12, 14, 153). These vesicles are then directed to target membranes. AP-4 does not engage clathrin and sorts distinct sets of cargoes at the TGN toward endosomes (17). AP-5 was recently described and likely functions in receptor recycling out of endosomes (75).
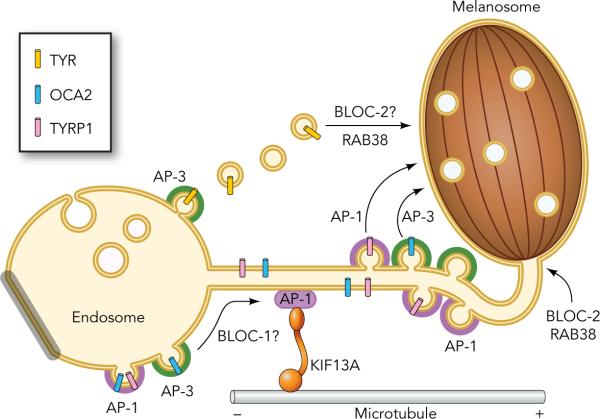
In melanocytes, AP-1, AP-2 and AP-3 have been implicated in cargo trafficking to melanosomes. Signals in the OCA2 and TYR cytoplasmic domains interact with AP-1, AP-2, and AP-3 in vitro (23, 80, 168, 181). TYR can be localized to endosomal buds and tubules that are decorated with AP-1 or AP-3 (141, 181), and significant cohorts of OCA2 and TYR are mislocalized in melanocytes that are genetically deficient in AP-3 (84, 151, 181; A. Sitaram, unpublished). By contrast, the TYRP1 sorting signal interacts with AP-1 but not AP-3 in vitro (181) and TYRP1 can be crosslinked to and co-immunoprecipitated with AP-1 in melanoma cells (35). Consistently, TYRP1 is correctly localized to melanosomes in AP-3-deficient melanocytes (84, 163), but is mislocalized to vacuolar early endosomal domains in a human melanoma cell line in which AP-1 is depleted by siRNA (35). Besides its adaptor function, AP-1 is also required to position endosomes in the melanocyte periphery near maturing melanosomes by recruiting the microtubule motor KIF13A; this likely facilitates the formation of transient connections between endosomes and melanosomes for cargo transfer (35; Figure 2). AP-2 is required for efficient PMEL delivery to endosomes and for melanosomal accumulation in melanocytes (152), likely by interacting with the acidic dileucine signal in the PMEL cytoplasmic domain (180) (note this signal is not necessary for post-endocytic sorting to early stage melanosomes; 179, 180). From these results it is clear that dileucine motifs and AP complexes play major roles in melanosomal trafficking. Interestingly, the melanin biosynthetic enzyme DCT lacks a cytoplasmic dileucine-based signal, but harbors a sequence conforming to AP-binding tyrosine-based motifs (14) that likely serves a similar function.
Figure 2. Model of delivery of selected cargoes to late-stage melanosomes.
Adaptor proteins AP-1 and AP-3 concentrate specific melanosomal integral membrane cargo proteins such as OCA2 (blue bars), TYRP1 (pink bars) and tyrosinase (gold bars) within early endosomes. AP-1 also engages the microtubule plus-end directed kinesin KIF13A to establish tubules that emanate from recycling endosomal domains in the cell periphery and fuse with the membrane of nearby melanosomes. AP-1 and perhaps AP-3 direct cargoes such as OCA2 and TYRP1 into this pathway. BLOC-1 also functions on this pathway during cargo entry into the tubules. BLOC-2 and RAB38 appear to act downstream, perhaps to specify the targeting of these tubules to melanosomes. Engagement ofcargo proteins on the tubules with AP-1 or AP-3 might be required for efficient delivery of cargoes from the tubules to the melanosome. AP-3 also functions from a distinct domain of early endosomes to direct BLOC-1-independent vesicular trafficking of TYR to the melanosome. RAB38 and perhaps BLOC-2 facilitate melanosomal targeting in this pathway. This figure is modified from Figure 8 of Delevoye et al., 2009, originally published in Journal of Cell Biology, vol. 187, pp. 247–264 (35).
Diseases of melanosome biogenesis and protein trafficking
A number of components of the sorting machinery required for the proper targeting of melanosomal proteins have been identified through the discovery of human patients in whom LRO function is disrupted, resulting in oculocutaneous albinism and other functional disorders (83, 147; Table 1). Griscelli Syndrome is one well-characterized genetic disease of LROs, but it affects organelle motility and not maturation and will be briefly discussed later. Genetic diseases of LRO biogenesis include Chediak-Higashi Syndrome and Hermansky-Pudlak Syndrome (HPS; 83). Chediak-Higashi syndrome is a lethal multisystem disorder that results from defects in the gene encoding a large protein called CHS1 or Lyst (93). Lyst deficiency results in decreased fission of lysosomes and presumably of LROs (46), but how Lyst functions in this process is not known. HPS is characterized minimally by oculocutaneous albinism and prolonged bleeding due respectively to the malformation of melanosomes and platelet dense granules; most patients additionally suffer from lung fibroses that most likely result from defective lamellar body function, leading to death in midlife (195), and some patients also exhibit immune deficiencies because of defects in LRO biogenesis or specific protein sorting defects in cells of the adaptive and innate immune system (10, 30, 52, 157, 171). There are at least nine subtypes of HPS, each caused by defects in a different gene (32, 41, 83, 195). Mouse models exist for each HPS subtype and at least six additional mouse models show similar symptoms (41, 111; Table 1).
Although the clinical phenotypes of HPS arise specifically within tissues that harbor LROs, each of the affected genes is ubiquitously expressed. The proteins encoded by the nine human and 13 of the 15 mouse HPS-associated genes are subunits of five distinct multisubunit complexes: the Class C Vps complex (a subcomplex of the homotypic fusion and vacuole sorting, or HOPS, complex), AP-3, and the Biogenesis of Lysosome-Related Organelles Complex-1, -2, and -3 (BLOC-1, -2, and -3; Table 1). Each of these complexes has been implicated in membrane trafficking events that are required for melanosome biogenesis as discussed above (for AP-3) and below.
HPS-associated multisubunit complexes
Class C Vps complex
A point mutation in the gene encoding the VPS33A subunit of the foursubunit Class C Vps complex underlies the HPS phenotype of the buff mouse (173). The Class C Vps complex has been best described in the yeast, Saccharomyces cerevisiae, in which it is a core component of HOPS (160) and CORVET (142). HOPS and CORVET are effectors of endosomal Rab GTPases and function as “tethering” complexes that promote SNARE complex assembly and selectivity (reviewed in ref. 196). The mammalian HOPS complex also functions in endosomal and lysosomal fusion events (20, 96, 144, 150). Mutations in genes encoding several Class C Vps subunits cause LRO defects in mouse, fly, and nematode models (73, 113, 145, 164, 169, 173). How this complex functions during melanosome biogenesis is not clear. The yeast HOPS complex facilitates docking of AP-3-coated vesicles with the vacuole (2), suggesting that the VPS33A-containing HOPS complex in mammals might regulate fusion of AP-3-dependent transport intermediates with nascent melanosomes in melanocytes. Interestingly, a mutation in the human gene encoding a homologous Vps-C subunit, VPS33B, causes arthrogryposis-renal dysfunction-cholestasis syndrome, which is associated with defects in neuronal, kidney and bile duct dysfunction as well as bleeding diathesis, but not albinism (62). This suggests that distinct mammalian Vps-C complexes function in different membrane trafficking steps.
AP-3
The ubiquitously-expressed heterotetrameric AP-3 complex consists of β3A, μ3A, σ3, and δ subunits, whereas β3B and μ3B subunits replace the respective ubiquitous subunits in a neuron-specific AP-3B complex (37). The AP3B1 gene encoding the β3A subunit is mutated in HPS2 patients and the pearl mouse (51, 201, 204). The Ap3d gene encoding the δ subunit is mutated in the mocha mouse (92), which suffers from neurological disorders in addition to HPS (103, 135, 175) due to the loss of both the ubiquitous and neuron-specific complexes. Nematode and fly models of AP-3 deficiency also have LRO defects (73, 127, 128, 138, 167). In melanocytes, AP-3 is required for the efficient delivery of TYR (84, 181) and OCA2 (81 and A. Sitaram, unpublished data) to nascent melanosomes. AP-3 localizes predominantly to clathrincoated buds on early endosomal tubules in melanocytic cells, and is thought to function by recruiting cargoes such as TYR into buds and eventually vesicles that are destined for melanosomes (181). AP-3 might also function from recycling endosomal tubules for some cargoes (141).
BLOCs
The majority of the HPS-affected genes encode subunits of BLOC-1, -2 and -3, which are well-conserved in metazoans; distant homologues of BLOC-1 and BLOC-3 can be found in Dictyostelium and S. cerevisiae (24, 70). Each complex is an obligate oligomer, such that most subunits of the corresponding BLOC are destabilized by known HPS-associated mutations in one BLOC subunit. BLOC subunits lack homology to proteins of known function, and the molecular functions of the BLOCs are not yet known. However, clues to their functions have been deduced from the phenotypes of cells lacking BLOC subunits and from genetic and biochemical interactions of BLOC subunits with other effectors.
BLOC-1
BLOC-1 comprises at least eight subunits, and mutations in the genes encoding three and five of them, respectively, underlie human and mouse HPS models (32, 41, 124). Among the mouse HPS models, BLOC-1-deficient mice are the most severely hypopigmented (36). This is due at least in part to a requirement for BLOC-1 in the delivery of the copper transporter, ATP7A, to melanosomes; copper is an obligate cofactor for TYR, and BLOC-1-deficient melanocytes thus lack TYR activity within melanosomes despite only a modest deficiency in TYR localization to melanosomes (162). Analyses of TYRP1 trafficking have shown that BLOC-1 facilitates the exit of selected melanosomal cargoes from recycling endosomal domains toward melanosomes (32, 163), consistent with genetic interactions between BLOC-1 and the recycling endosomal Rab11 GTPase in Drosophilay (25) and in a bioinformatics screen (154). Similarly, in neurons BLOC-1 functions in effecting cargo transport from early endosomes to synaptic vesicles (131, 156). In neurons, this BLOC-1 activity functions in concert with AP-3 (131, 156), and consistently a cohort of BLOC-1 physically associates with AP-3 in several cell types (42). Nevertheless, in melanocytes AP-3 and BLOC-1 localize at steady state to nonoverlapping domains of early endosomes (42) and regulate melanosome localization of distinct cargoes – TYR delivery is highly dependent on AP-3 but less so on BLOC-1, whereas TYRP1 and ATP7A delivery is highly dependent on BLOC-1 but independent of AP-3 (32, 84, 162, 163, 181). Although some cargoes might require both (AS, unpublished data), this suggests that AP-3 is not an obligate BLOC-1 partner.
How BLOC-1 functions at the molecular level is unclear. Physical interactions between BLOC-1 subunits or intact BLOC-1 with the endosomal t-SNARE component syntaxin 13 (59, 82, 125) and the exocytic tSNARE components SNAP23 and SNAP25 (59, 89, 190) suggest that BLOC-1 might influence either endosomal membrane fusion events or sorting of endosomal SNAREs, consistent with observations of SNARE missorting in BLOC-1-deficient neurons (156). Intriguingly, the phenotype of melanocytes from BLOC-1-deficient animals or patients resembles that of a pigmented human melanoma line in which AP-1 was knocked down by short interfering RNAs, in that cells are highly hypopigmented, TYRP1 is mislocalized, and endosomal vacuoles accumulate (35, 162, 163). To date, however, interactions between BLOC-1 and AP-1 have not been reported.
BLOC-2
BLOC-2 comprises three large subunits, each the product of human and mouse HPS-associated genes (41), and an HPS5 orthologue is defective in a Drosophila eye color mutant (49). BLOC-2-deficient mice have a more modest coat color dilution than BLOC-1 mutants, but melanosomes in choroidal melanocytes uniquely clump into multi-melanosomal structures (58, 203). In primary melanocytes from BLOC-2-deficient HPS patients, TYR and TYRP1 are mislocalized (13, 71, 85, 151); similarly, TYRP1 in BLOC-2-deficient mouse melanocytes is mislocalized to endosomal structures but does not accumulate in vacuolar endosomal domains, suggesting that BLOC-2 might function downstream of BLOC-1 (163). Consistent with a function in the BLOC-1 pathway, BLOC-2 localizes to endosomal tubules in melanocytes and physically interacts with a cohort of BLOC-1 (42, 156). Skin melanocytes from BLOC-2- deficient patients accumulate TYR-containing vesicular structures in the cytoplasm (13, 71), suggesting that BLOC-2 might play a role in fusion of BLOC-1-dependent transport intermediates with maturing melanosomes.
BLOC-3
BLOC-3 is composed of two subunits that are defective in HPS patients and mouse models, including the most prevalent HPS, type 1 (39). BLOC-3-deficient patients have the most dramatic lung fibrosis (1, 57, 64), making this subset of patients the most clinically important, yet among the BLOCs we understand the least about BLOC-3. Loss of BLOC-3 in mouse models has a mild effect on coat and eye color but a more dramatic effect on the hairless skin of the ears and tail – indicating differences in melanosome biogenesis and development in follicular versus interfollicular melanocytes (57, 133) – and results in melanosome enlargement in choroidal melanocytes (50, 56, 174). The heightened severity of LRO phenotypes in double mutants lacking both BLOC-3 and either BLOC-2 or AP-3 suggest that BLOC-3 works in a separate pathway from those complexes and perhaps from BLOC-1 as well (42, 50, 57). The molecular function of BLOC-3, however, is not clear. Knockdown of BLOC-3 subunit expression in fibroblasts results in altered distribution of late endosomes/ lysosomes, suggesting a potential primary role for BLOC-3 in organelle motility (48). This is consistent with defects in melanosome transfer observed in hair follicles of BLOC-3-deficient mice (132, 134). Intriguingly, BLOC-3 in insect cells appears to be required for optimal gene silencing mediated by small RNAs (69, 105), suggesting that some of the effects of BLOC-3 deficiency might reflect ineffective gene silencing rather than a direct activity of BLOC-3 on membrane trafficking.
Other Effectors of Melanosome Biogenesis
Rab proteins
Among additional known effectors of melanosome biogenesis are several members of the Rab family of small GTPases. Rabs function as molecular switches; in their GTP-bound state they bind to membranes and recruit effector proteins, whereas in their GDP-bound state they generally fail to bind to effectors (170). Among known effectors are tethering complexes, fusion proteins, cytoskeletal motors, phosphoinositide kinases and phosphatases, and GTPase activating proteins or guanine nucleotide exchange factors for other Rabs. The HPS model gunmetal mouse is deficient in the α subunit of the ubiquitously-expressed Rab geranylgeranyltransferase II – needed to modify target Rab proteins with a C-terminal prenyl group to allow for membrane-anchoring (40, 202) – but displays phenotypes primarily in platelets and melanocytes (159). One of the targets of RABGGTA is RAB27A, a tissue-restricted Rab family member that is expressed in melanocytes and a number of other cell types (26, 146).
In melanocytes, activated RAB27A associates with mature melanosomes and, together with its effector protein melanophilin, recruits the actin motor myosin VA (86, 198, 199). This enables transfer of melanosomes from microtubules onto the peripheral actin network of skin melanocytes, allowing for subsequent transfer to keratinocytes. Mutations in the genes encoding either RAB27A, melanophilin or myosin VA lead to abnormal perinuclear clu stering of melanosomes (4) and give rise respectively to the hypopigmentation phenotype of ashen, leaden, and dilute mice, models of three subtypes of Griscelli Syndrome (122, 123, 140, 197; Table 1). A similar RAB27A-containing tripartite complex, with a distinct adaptor (MyRIP) and myosin motor (MyoVIIa), maintains peripheral accumulation of melanosomes in the apical domain of retinal pigment epithelia (117). While RAB27A deficiency affects primarily melanosome positioning, the reduction in melanosome number seen in gunmetal mice and other data implicate additional Rabs in melanosome biogenesis, including potentially Rab11 as discussed earlier (25, 35, 154, 202). For the sake of brevity, only a few examples are discussed here.
Mutations in the Rab38 gene in mice are associated with modest diminution in coat color (114) but a dramatic defect in melanosome maturation in the retinal pigment epithelia (116), and a mutation in rat Rab38 is associated with an HPS-like phenotype (137). The modest effect on coat color in the chocolate Rab38 mutant mouse appears to reflect functional redundancy in skin melanocytes between RAB38 and the closely related RAB32 (192). A RAB38/32 orthologue in Drosophila is also required for pigment granule formation (119). RAB38 localizes to melanosomes and to transport intermediates en route to melanosomes, and in RAB38/RAB32-deficient melanocytes both TYR and TYRP1 are mislocalized to the TGN and/ or degraded in lysosomes (192). Similar functional outcomes result from the depletion of VARP, a RAB38/RAB32 effector (177), and this defect can be rescued by wild-type VARP but not by a mutant that fails to interact with RAB38/32 (176). This suggests that RAB38 (and RAB32 in melanocytes), at least in part via recruiting VARP, functions in specifying the delivery of transport intermediates from either the Golgi or endosomes to melanosomes, and that in its absence at least TYRP1 is largely degraded in lysosomes. Interestingly, VARP also binds to VAMP7 (also called TI-VAMP; 16), a vSNARE involved in late endosomal maturation and late endosome: lysosome fusion in other cell types (22). Interference with the VARP-VAMP7 interaction also disrupts TYRP1 trafficking (176), suggesting that the RAB38-VARP complex might regulate the formation of trans-SNARE complexes required for cargo delivery to melanosomes.
Other Rab GTPases likely play additional roles in melanosome biogenesis, but have been less well characterized. For example, the ubiquitous RAB7A appears to regulate motility of early stage melanosomes through its ability to recruit cytoplasmic dynein, a minus-end-directed microtubule motor (91), with potential downstream effects on recruitment of melanosome markers (74). The ubiquitous RAB9 physically interacts with BLOC3 when in its active GTP-bound state, suggesting that BLOC3 might be an effector of RAB9 (98), although functional data supporting this contention are lacking. RAB17 is coregulated with melanosomal proteins in melanocytes, and together with RAB8 (21) influences peripheral actin dynamics and melanosome secretion (6). A number of less well characterized RAB proteins, including RAB31, RAB32 and RAB33A in addition to RAB27A and RAB38, are down-regulated during melanocyte transformation to melanoma (79), and PMEL and TYRP1 levels are reduced in cells expressing a putative dominant active RAB33A variant (28). Elucidating how these Rab proteins function with other trafficking machinery will be important to understand the regulation of melanosome biogenesis.
ESCRTs
ESCRTs are a series of highly conserved multisubunit complexes that have well-defined roles in the formation of and cargo entry to the internal vesicles of endosomes in all eukaryotes, as well as in viral budding, exosome formation, and cytokinesis (reviewed in 88). The ESCRT-0, ESCRT-I and ESCRT-II complexes concentrate cargoes that are ubiquitylated or recognized by other means into clathrin-coated membrane patches on maturing early endosomal vacuoles, and drive these cargoes into invaginating membranes; ESCRT-III and the AAA ATPase VPS4 then functions to release these vesicles into the endosomal lumen. In pigment cells, ESCRTs function as expected in the lysosomal sorting and degradation of MART-1 and GPR143 (60, 110, 179, 185), but they also appear to play additional roles in melanosome maturation. For example, in cells that are depleted of the ESCRT-I component Tsg101 or in which ESCRT function is inhibited by expression of a dominant negative form of VPS4B, TYRP1 is missorted into autophagosome-like structures (185). Whether this reflects a direct role for ESCRT-I in sorting of melanosomal cargoes or an indirect role in regulating endosomal morphology required for melanosome maturation is not yet clear, but points to a non-classical function for ESCRT-I and perhaps other ESCRTs in pigment cells. Interestingly, Stage III and IV melanosomes, like late endosomes, harbor intralumenal vesicles on which TYR accumulates (181). Whether these vesicles form in an ESCRT-dependent fashion or whether ubiquitylation is required for cargo sorting into these vesicles is not yet known.
Syntaxin 17
The distinctive age-related coat depigmentation and high incidence of melanoma in the widely-bred Gray horse was found to correlate with a duplication of an intronic region with in the gene for syntaxin 17 (STX17; 155). Within melanoma tissue, the mutation causes increased expression of STX17 and the neighboring nuclear receptor protein NR4A3, due to the duplication of a binding site for the pigment cell-specific transcription factor MITF (172). STX17 is poorly characterized within the syntaxin family of SNAREs, and STX17 protein levels and function were not investigated; thus it is unclear whether altered trafficking to melanosomes plays a role in the phenotype. Alternatively, age-related depigmentation might be caused by reduced hair bulb melanocyte survival, as in the autoimmune disease vitiligo (104) and potentially PMEL-deficient mice (72).
TRAPP
TRAPPI and TRAPPII are cytosolic multisubunit complexes in yeast that act as both Rab GEFs and vesicle tethers in ER-Golgi and intra-Golgi membrane trafficking, respectively (18). Both TRAPPs share a common core of seven subunits, and TRAPPII contains an additional three. TRAPP subunits are conserved in mammals and are expressed in many tissues (115), and mammalian TRAPP also acts as a Rab GEF and a tether in the secretory pathway (200). Importantly, TRAPP has been implicated in melanosome biogenesis. Disruption of murine Trappc6a – encoding the homologue of the Trs33p subunit of the yeast core TRAPP complex-in the mhyp mouse is associated with mosaic coat hypopigmentation and a reduction in RPE melanosomes (65). However, the structure of mammalian TRAPP is not identical to the yeast complex (206), and the precise mechanism of TRAPP involvement in melanosome biogenesis remains to be elucidated.
Phosphoinositides and autophagy regulators
Recent reports have revealed two unexpected sets of effectors of melanosome biogenesis. One is phosphatidylinositol(3,5)bisphosphate [PtdIns(3,5)P2], a rare lipid found nearly exclusively in late endosomal organelles (43). The gene encoding FIG4, a phosphatase that removes the 5' phosphate from PtdIns(3,5) P2, is mutated in the pale tremor mouse and patients with Charcot-Marie Tooth syndrome type 4J (29). Both the pale tremor mouse and mutants in Vac14, a gene encoding a scaffolding protein in the FIG4 complex, are severely hypopigmented (29, 90). This suggests that PtdIns(3,5)P2 plays a unique and important role in melanosome biogenesis. The second unsuspected pathway is autophagy. Several components of the autophagy pathway were identified in a siRNA silencing screen for genes required for pigmentation in a human melanoma cell line, and targeted knockdown of these and other components impaired melanogenesis (55). This appears to reflect a negative impact of mTOR kinase signaling in melanosome biogenesis (76). Intriguingly, both the PtdIns(3,5)P2 and autophagy pathways might be linked by a single protein, WIPI1, which engages both the autophagy pathway (76) and is an effector of PtdIns(3,5)P2 (44). Whether these effects are solely mediated at the transcriptional level or more directly influence melanosome biogenesis is not yet clear.
Perspectives and a Model for Membrane Trafficking in Melanosome Maturation
As described in this review, the routes through which cargo proteins access nascent melanosomes are numerous and complex and involve many regulators, but it remains unclear how these regulators are integrated to effect proper melanosome maturation. Based on the analyses of HPS models and several additional effectors, we propose a model whereby early stage melanosomes are matured to stage III and IV by a dual pathway of cargo delivery, one that is BLOC-1-dependent and one that is BLOC-1-independent (Figure 2). Based on observations of tubular connections between endosomes and melanosomes by both three-dimensional reconstruction of electron tomograms and live cell imaging (35), we propose that the BLOC-1-dependent cargoes are delivered via tubular intermediates between recycling endosomal domains and maturing melanosomes (Figures 1 and 2). The integrity of this pathway requires AP-1 and its associated KIF13A. A second, BLOC-1-independent pathway is likely mediated by vesicles that bud from sorting endosomal domains. How other effectors fit into this pathway remains unclear, but we speculate that RAB38, BLOC-2, and perhaps RAB33A and HOPS function during fusion of these transport intermediates with maturing melanosomes. Analyses of additional cargoes will be needed to better characterize these pathways and to test this model. The function of the BLOCs and the mechanism underlying their cargo selectivity remain an intriguing mystery and should be a major focus of future investigation. Finally, comparative studies of trafficking in other LRO-containing cells such as platelets and immune cells are yielding insights that may lead to a more unified and general model of trafficking specialization within the endosomal system.
Acknowledgments
We are grateful to past and present members of the Marks and Raposo laboratories for helpful discussions and valuable insights, as well as with other members of the trafficking community, and are particularly grateful to Cédric Delevoye for critical comments on the manuscript. We apologize to the authors of relevant works that were not mentioned in this review. This work was supported by National Institutes of Health grants R01 EY015625 from the National Eye Institute and R01 AR048155 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and by National Institutes of Health training grants 2T32 HL007971 from the National Heart Lung and Blood Institute and 5T32 GM007229 from the National Institute of General Medical Sciences.
References
- 1.Anderson PD, Huizing M, Claassen DA, White J, Gahl WA. Hermansky-Pudlak syndrome type 4 (HPS-4): clinical and molecular characteristics. Hum. Genet. 2003;113(1):10–17. doi: 10.1007/s00439-003-0933-5. 10–7. [DOI] [PubMed] [Google Scholar]
- 2.Angers CG, Merz AJ. HOPS interacts with Apl5 at the vacuole membrane and is required for consumption of AP-3 transport vesicles. Mol. Biol. Cell. 2009;20:4563–4574. doi: 10.1091/mbc.E09-04-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aydin IT, Hummler E, Smit NP, Beermann F. Coat color dilution in mice because of inactivation of the melanoma antigen MART-1. Pigment Cell Melanoma Res. 2012;25:37–46. doi: 10.1111/j.1755-148X.2011.00910.x. [DOI] [PubMed] [Google Scholar]
- 4.Barral DC, Seabra MC. The melanosome as a model to study organelle motility in mammals. Pigment Cell Melanoma Res. 2004;17:111–118. doi: 10.1111/j.1600-0749.2004.00138.x. [DOI] [PubMed] [Google Scholar]
- 5.Bassi MT, Schiaffino MV, Renieri A, De Nigris F, Galli L, Bruttini M, Gebbia M, Bergen AA, Lewis RA, Ballabio A. Cloning of the gene for ocular albinism type 1 from the distal short arm of the X chromosome. Nature Genet. 1995;10:13–19. doi: 10.1038/ng0595-13. [DOI] [PubMed] [Google Scholar]
- 6.Beaumont KA, Hamilton NA, Moores MT, Brown DL, Ohbayashi N, Cairncross O, Cook AL, Smith AG, Misaki R, Fukuda M, Taguchi T, Sturm RA, Stow JL. The recycling endosome protein Rab17 regulates melanocytic filopodia formation and melanosome trafficking. Traffic. 2011 doi: 10.1111/j.1600-0854.2011.01172.x. In press: 10.1111/j.1600-0854.2011.01172.x. [DOI] [PubMed] [Google Scholar]
- 7.Beermann F, Orlow SJ, Boissy RE, Schmidt A, Boissy YL, Lamoreux ML. Misrouting of tyrosinase with a truncated cytoplasmic tail as a result of the murine platinum (C(p)) mutation. Exp. Eye Res. 1995;61:599–607. doi: 10.1016/s0014-4835(05)80053-3. [DOI] [PubMed] [Google Scholar]
- 8.Berson JF, Harper D, Tenza D, Raposo G, Marks MS. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol. Biol. Cell. 2001;12:3451–3464. doi: 10.1091/mbc.12.11.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berson JF, Theos AC, Harper DC, Tenza D, Raposo G, Marks MS. Proprotein convertase cleavage liberates a fibrillogenic fragment of a resident glycoprotein to initiate melanosome biogenesis. J. Cell Biol. 2003;161:521–533. doi: 10.1083/jcb.200302072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blasius AL, Arnold CN, Georgel P, Rutschmann S, Xia Y, Lin P, Ross C, Li X, Smart NG, Beutler B. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 2010;107:19973–19978. doi: 10.1073/pnas.1014051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blott EJ, Griffiths GM. Secretory lysosomes. Nature Rev. Mol. Cell Biol. 2002;3:122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- 12.Boehm M, Bonifacino JS. Genetic analyses of adaptin function from yeast to mammals. Gene. 2002;286:175–186. doi: 10.1016/s0378-1119(02)00422-5. [DOI] [PubMed] [Google Scholar]
- 13.Boissy RE, Richmond B, Huizing M, Helip-Wooley A, Zhao Y, Koshoffer A, Gahl WA. Melanocyte-specific proteins are aberrantly trafficked in melanocytes of Hermansky-Pudlak syndrome-type 3. Am. J. Pathol. 2005;166:231–240. doi: 10.1016/S0002-9440(10)62247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 15.Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- 16.Burgo A, Sotirakis E, Simmler MC, Verraes A, Chamot C, Simpson JC, Lanzetti L, Proux-Gillardeaux V, Galli T. Role of Varp, a Rab21 exchange factor and TI-VAMP/VAMP7 partner, in neurite growth. EMBO Rep. 2009;10:1117–1124. doi: 10.1038/embor.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgos PV, Mardones GA, Rojas AL, daSilva LLP, Prabhu Y, Hurley JH, Bonifacino JS. Sorting of the Alzheimer's disease amyloid precursor protein mediated by the AP-4 complex. Dev. Cell. 2010;18:425–436. doi: 10.1016/j.devcel.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev. Cell. 2007;12:671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Calvo PA, Frank DW, Bieler BM, Berson JF, Marks MS. A cytoplasmic sequence in human tyrosinase defines a second class of di-leucine-based sorting signals for late endosomal and lysosomal delivery. J. Biol. Chem. 1999;274:12780–12789. doi: 10.1074/jbc.274.18.12780. [DOI] [PubMed] [Google Scholar]
- 20.Caplan S, Hartnell LM, Aguilar RC, Naslavsky N, Bonifacino JS. Human Vam6p promotes lysosome clustering and fusion in vivo. J. Cell Biol. 2001;154:109–121. doi: 10.1083/jcb.200102142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chabrillat ML, Wilhelm C, Wasmeier C, Sviderskaya EV, Louvard D, Coudrier E. Rab8 regulates the actin-based movement of melanosomes. Mol. Biol. Cell. 2005;16:1640–1650. doi: 10.1091/mbc.E04-09-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaineau M, Danglot L, Galli T. Multiple roles of the vesicular-SNARE TI-VAMP in post-Golgi and endosomal trafficking. FEBS Lett. 2009;583:3817–3826. doi: 10.1016/j.febslet.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Chaudhuri R, Mattera R, Lindwasser OW, Robinson MS, Bonifacino JS. A basic patch on alpha-adaptin is required for binding of human immunodeficiency virus type 1 Nef and cooperative assembly of a CD4-Nef-AP-2 complex. J. Virol. 2009;83:2518–2530. doi: 10.1128/JVI.02227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheli VT, Dell'angelica EC. Early origin of genes encoding subunits of biogenesis of lysosome-related organelles complex-1, -2 and -3. Traffic. 2010;11:579–586. doi: 10.1111/j.1600-0854.2010.01044.x. [DOI] [PubMed] [Google Scholar]
- 25.Cheli VT, Daniels RW, Godoy R, Hoyle DJ, Kandachar V, Starcevic M, Martinez-Agosto JA, Poole S, Diantonio A, Lloyd VK, Chang HC, Krantz DE, Dell'angelica EC. Genetic modifiers of abnormal organelle biogenesis in a Drosophila model of BLOC-1 deficiency. Hum. Mol. Genet. 2010;19:861–878. doi: 10.1093/hmg/ddp555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen D, Guo J, Miki T, Tachibana M, Gahl WA. Molecular cloning and characterization of rab27a and rab27b, novel human rab proteins shared by melanocytes and platelets. Biochem. Mol. Med. 1997;60:27–37. doi: 10.1006/bmme.1996.2559. [DOI] [PubMed] [Google Scholar]
- 27.Chen K, Manga P, Orlow SJ. Pink-eyed dilution protein controls the processing of tyrosinase. Mol. Biol. Cell. 2002;13:1953–1964. doi: 10.1091/mbc.02-02-0022.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng E, Trombetta SE, Kovacs D, Beech RD, Ariyan S, Reyes-Mugica M, McNiff JM, Narayan D, Kluger HM, Picardo M, Halaban R. Rab33A: characterization, expression, and suppression by epigenetic modification. J. Invest. Dermatol. 2006;126:2257–2271. doi: 10.1038/sj.jid.5700386. [DOI] [PubMed] [Google Scholar]
- 29.Chow CY, Zhang Y, Dowling JJ, Jin N, Adamska M, Shiga K, Szigeti K, Shy ME, Li J, Zhang X, Lupski JR, Weisman LS, Meisler MH. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448:68–72. doi: 10.1038/nature05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark RH, Stinchcombe JC, Day A, Blott E, Booth S, Bossi G, Hamblin T, Davies EG, Griffiths GM. Adaptor protein 3–dependent microtubule-mediated movement of lytic granules to the immunological synapse. Nature Immunol. 2003;4:1111–1120. doi: 10.1038/ni1000. [DOI] [PubMed] [Google Scholar]
- 31.Costin G-E, Valencia JC, Vieira WD, Lamoreux ML, Hearing VJ. Tyrosinase processing and intracellular trafficking is disrupted in mouse primary melanocytes carrying the underwhite (uw) mutation. A model for oculocutaneous albinism (OCA) type 4. J. Cell Sci. 2003;116:3203–3212. doi: 10.1242/jcs.00598. [DOI] [PubMed] [Google Scholar]
- 32.Cullinane AR, Curry JA, Carmona-Rivera C, Summers CG, Ciccone C, Cardillo ND, Dorward H, Hess RA, White JG, Adams D, Huizing M, Gahl WA. A BLOC-1 mutations screen reveals that PLDN is mutated in Hermansky-Pudlak syndrome type 9. Am. J. Human Genet. 2011;88:778–787. doi: 10.1016/j.ajhg.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.d'Addio M, Pizzigoni A, Bassi MT, Baschirotto C, Valetti C, Incerti B, Clementi M, De Luca M, Ballabio A, Schiaffino MV. Defective intracellular transport and processing of OA1 is a major cause of ocular albinism type 1. Hum. Mol. Genet. 2000;9:3011–3018. doi: 10.1093/hmg/9.20.3011. [DOI] [PubMed] [Google Scholar]
- 34.de Mazière AM, Muehlethaler K, van Donselaar E, Salvi S, Davoust J, Cerottini J-C, Lévy F, Slot JW, Rimoldi D. The melanocytic protein melan-A/MART-1 has a subcellular localization distinct from typical melanosomal proteins. Traffic. 2002;3:678–693. doi: 10.1034/j.1600-0854.2002.30909.x. [DOI] [PubMed] [Google Scholar]
- 35.Delevoye C, Hurbain I, Tenza D, Sibarita J-B, Uzan-Gafsou S, Ohno H, Geerts WJC, Verkleij AJ, Salamero J, Marks MS, Raposo G. AP-1 and KIF13A coordinate endosomal sorting and positioning during melanosome biogenesis. J. Cell Biol. 2009;187:247–264. doi: 10.1083/jcb.200907122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dell'Angelica EC. The building BLOC(k)s of lysosomes and related organelles. Curr. Opin. Cell Biol. 2004;16:458–464. doi: 10.1016/j.ceb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Dell'Angelica EC. AP-3-dependent trafficking and disease: the first decade. Curr. Opin. Cell Biol. 2009;21:552–559. doi: 10.1016/j.ceb.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Dell'Angelica EC, Mullins C, Caplan S, Bonifacino JS. Lysosome-related organelles. FASEB J. 2000;14:1265–1278. doi: 10.1096/fj.14.10.1265. [DOI] [PubMed] [Google Scholar]
- 39.Dell'Angelica EC, Aguilar RC, Wolins N, Hazelwood S, Gahl WA, Bonifacino JS. Molecular characterization of the protein encoded by the Hermansky-Pudlak Syndrome type 1 gene. J. Biol. Chem. 2000;275:1300–1306. doi: 10.1074/jbc.275.2.1300. [DOI] [PubMed] [Google Scholar]
- 40.Detter JC, Zhang Q, Mules EH, Novak EK, Mishra VS, Li W, McMurtrie EB, Tchernev VT, Wallace MR, Seabra MC, Swank RT, Kingsmore SF. Rab geranylgeranyl transferase alpha mutation in the gunmetal mouse reduces Rab prenylation and platelet synthesis. Proc Natl Acad Sci U S A. 2000;97:4144–9. doi: 10.1073/pnas.080517697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Pietro SM, Dell'Angelica EC. The cell biology of Hermansky-Pudlak syndrome: recent advances. Traffic. 2005;6:525–533. doi: 10.1111/j.1600-0854.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 42.Di Pietro SM, Falcon-Perez JM, Tenza D, Setty SRG, Marks MS, Raposo G, Dell'Angelica EC. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol. Biol. Cell. 2006;17:4027–4038. doi: 10.1091/mbc.E06-05-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dove SK, Dong K, Kobayashi T, Williams FK, Michell RH. Phosphatidylinositol 3,5-bisphosphate and Fab1p/PIKfyve underPPIn endo-lysosome function. Biochem J. 2009;419:1–13. doi: 10.1042/BJ20081950. [DOI] [PubMed] [Google Scholar]
- 44.Dove SK, Piper RC, McEwen RK, Yu JW, King MC, Hughe sDC, Thuring J, Holmes AB, Cooke FT, Michell RH, Parker PJ, Lemmon MA. Svp1p defines a family of phosphatidylinositol 3,5-bisphosphate effectors. EMBO J. 2004;23:1922–1933. doi: 10.1038/sj.emboj.7600203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drake MT, Zhu Y, Kornfeld S. The assembly of AP-3 adaptor complex-containing clathrin-coated vesicles on synthetic liposomes. Mol. Biol. Cell. 2000;11:3723–3736. doi: 10.1091/mbc.11.11.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Durchfort N, Verhoef S, Vaughn MB, Shrestha R, Adam D, Kaplan J, Ward DM. The enlarged lysosomes in beigej cells result from decreased lysosome fission and not increased lysosome fusion. Traffic. 2012 doi: 10.1111/j.1600-0854.2011.01300.x. in press: 10.1111/j.1600-0854.2011.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 48.Falcón-Pérez JM, Nazarian R, Sabatti C, Dell'Angelica EC. Distribution and dynamics of Lamp1-containing endocytic organelles in fibroblasts deficient in BLOC-3. J. Cell Sci. 2005;118:5243–5255. doi: 10.1242/jcs.02633. [DOI] [PubMed] [Google Scholar]
- 49.Falcón-Pérez JM, Romero-Calderón R, Brooks ES, Krantz DE, Dell'Angelica EC. The drosophila pigmentation gene pink (p) encodes a homologue of human Hermansky-Pudlak Syndrome 5 (HPS5) Traffic. 2007;8:154–168. doi: 10.1111/j.1600-0854.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- 50.Feng L, Novak EK, Hartnell LM, Bonifacino JS, Collinson LM, Swank RT. The Hermansky-Pudlak syndrome 1 (HPS1) and HPS2 genes independently contribute to the production and function of platelet dense granules, melanosomes, and lysosomes. Blood. 2002;99:1651–1658. [PubMed] [Google Scholar]
- 51.Feng L, Seymour AB, Jiang S, To A, Peden AA, Novak EK, Zhen L, Rusiniak ME, Eicher EM, Robinson MS, Gorin MB, Swank RT. The β3A subunit gene (Ap3b1) of the AP-3 adaptor complex is altered in the mouse hypopigmentation mutant pearl, a model for Hermansky-Pudlak syndrome and night blindness. Hum. Mol. Genet. 1999;8:323–330. doi: 10.1093/hmg/8.2.323. [DOI] [PubMed] [Google Scholar]
- 52.Fontana S, Parolini S, Vermi W, Booth S, Gallo F, Donini M, Benassi M, Gentili F, Ferrari D, Notarangelo LD, Cavadini P, Marcenaro E, Dusi S, Cassatella M, Facchetti F, Griffiths GM, Moretta A, Notarangelo LD, Badolato R. Innate immunity defects in Hermansky-Pudlak type 2 syndrome. Blood. 2006;107:4857–4864. doi: 10.1182/blood-2005-11-4398. [DOI] [PubMed] [Google Scholar]
- 53.Furumura M, Sakai C, Potterf SB, Vieira WD, Barsh GS, Hearing VJ. Characterization of genes modulated during pheomelanogenesis using differential display. Proc. Natl. Acad. Sci. USA. 1998;95:7374–7378. doi: 10.1073/pnas.95.13.7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaidarov I, Keen JH. Phosphoinositide-AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J. Cell Biol. 1999;146:755–764. doi: 10.1083/jcb.146.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ganesan AK, Ho H, Bodemann B, Petersen S, Aruri J, Koshy S, Richardson Z, Le LQ, Krasieva T, Roth MG, Farmer P, White MA. Genome-wide siRNA-based functional genomics of pigmentation identifies novel genes and pathways that impact melanogenesis in human cells. PLoS Genet. 2008;4:e1000298. doi: 10.1371/journal.pgen.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gardner JM, Wildenberg SC, Keiper NM, Novak EK, Rusiniak ME, Swank RT, Puri N, Finger JN, Hagiwara N, Lehman AL, Gales TL, Bayer ME, King RA, Brilliant MH. The mouse pale ear (ep) mutation is the homologue of human Hermansky-Pudlak syndrome. Proc. Natl. Acad. Sci. USA. 1997;94:9238–9243. doi: 10.1073/pnas.94.17.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gautam R, Novak EK, Tan J, Wakamatsu K, Ito S, Swank RT. Interaction of Hermansky-Pudlak syndrome genes in the regulation of lysosome-related organelles. Traffic. 2006;7:779–792. doi: 10.1111/j.1600-0854.2006.00431.x. [DOI] [PubMed] [Google Scholar]
- 58.Gautam R, Chintala S, Li W, Zhang Q, Tan J, Novak EK, Di Pietro SM, Dell'Angelica EC, Swank RT. The Hermansky-Pudlak syndrome 3 (cocoa) protein is a component of the biogenesis of lysosome-related organelles complex-2 (BLOC-2) J. Biol. Chem. 2004;279:12935–12942. doi: 10.1074/jbc.M311311200. [DOI] [PubMed] [Google Scholar]
- 59.Ghiani CA, Starcevic M, Rodriguez-Fernandez IA, Nazarian R, Cheli VT, Chan LN, Malvar JS, de Vellis J, Sabatti C, Dell'angelica EC. The dysbindin-containing complex (BLOC-1) in brain: developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Mol. Psychiatry. 2010;15:204–215. doi: 10.1038/mp.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giordano F, Simoes S, Raposo G. The ocular albinism type 1 (OA1) GPCR is ubiquitinated and its traffic requires endosomal sorting complex responsible for transport (ESCRT) function. Proc. Natl. Acad. Sci. U.S.A. 2011;108:11906–11911. doi: 10.1073/pnas.1103381108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giordano F, Bonetti C, Surace EM, Marigo V, Raposo G. The Ocular Albinism type 1 (OA1) G-protein coupled receptor functions with MART-1 at early stages of melanogenesis to control melanosome identity and composition. Hum. Mol. Genet. 2009;18:4530–4545. doi: 10.1093/hmg/ddp415. [DOI] [PubMed] [Google Scholar]
- 62.Gissen P, Johnson CA, Morgan NV, Stapelbroek JM, Forshew T, Cooper WN, McKiernan PJ, Klomp LW, Morris AA, Wraith JE, McClean P, Lynch SA, Thompson RJ, Lo B, Quarrell OW, Di Rocco M, Trembath RC, Mandel H, Wali S, Karet FE, Knisely AS, Houwen RH, Kelly DA, Maher ER. Mutations in VPS33B, encoding a regulator of SNARE-dependent membrane fusion, cause arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome. Nat. Genet. 2004;363:400–404. doi: 10.1038/ng1325. [DOI] [PubMed] [Google Scholar]
- 63.Groux-Degroote S, van Dijk SM, Wolthoorn J, Neumann S, Theos AC, De Mazière AM, Klumperman J, van Meer G, Sprong H. Glycolipid-dependent sorting of melanosomal from lysosomal membrane proteins by lumenal determinants. Traffic. 2008;9:951–963. doi: 10.1111/j.1600-0854.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- 64.Guttentag SH, Akhtar A, Tao JQ, Atochina E, Rusiniak ME, Swank RT, Bates SR. Defective surfactant secretion in a mouse model of Hermansky-Pudlak syndrome. Am. J. Respir. Cell Mol. Biol. 2005;33:14–21. doi: 10.1165/rcmb.2004-0293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gwynn B, Smith RS, Rowe LB, Taylor BA, Peters LL. A mouse TRAPP-related protein is involved in pigmentation. Genomics. 2006;88:196–203. doi: 10.1016/j.ygeno.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 66.Halaban R, Svedine S, Cheng E, Smicun Y, Aron R, Hebert DN. Endoplasmic reticulum retention is a common defect associated with tyrosinase-negative albinism. Proc. Natl. Acad. Sci. USA. 2000;97:5889–5894. doi: 10.1073/pnas.97.11.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Halaban R, Cheng E, Zhang Y, Moellmann G, Hanlon D, Michalak M, Setaluri V, Hebert DN. Aberrant retention of tyrosinase in the endoplasmic reticulum mediates accelerated degradation of the enzyme and contributes to the dedifferentiated phenotype of amelanotic melanoma cells. Proc. Natl. Acad. Sci. U.S.A. 1997;94:6210–6215. doi: 10.1073/pnas.94.12.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harper DC, Theos AC, Herman KE, Tenza D, Raposo G, Marks MS. Premelanosome amyloid-like fibrils are composed of only golgi-processed forms of pmel17 that have been proteolytically processed in endosomes. J. Biol. Chem. 2008;283:2307–2322. doi: 10.1074/jbc.M708007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harris DA, Kim K, Nakahara K, Vásquez-Doorman C, Carthew RW. Cargo sorting to lysosome-related organelles regulates siRNA-mediated gene silencing. J. Cell Biol. 2011;194:77–87. doi: 10.1083/jcb.201102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hayes MJ, Bryon K, Satkurunathan J, Levine TP. Yeast homologues of three BLOC-1 subunits highlight KxDL proteins as conserved interactors of BLOC-1. Traffic. 2011;12:260–268. doi: 10.1111/j.1600-0854.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Helip-Wooley A, Westbroek W, Dorward HM, Koshoffer A, Huizing M, Boissy RE, Gahl WA. Improper trafficking of melanocyte-specific proteins in Hermansky-Pudlak syndrome type-5. J. Invest. Dermatol. 2007;127:1471–1478. doi: 10.1038/sj.jid.5700737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hellström AR, Watt B, Fard SS, Tenza D, Mannström P, Narfström K, Ekesten B, Ito S, Wakamatsu K, Larsson J, Ulfendahl M, Kullander K, Raposo G, Kerje S, Hallböök F, Marks MS, Andersson L. Inactivation of the Pmel gene alters melanosome shape but has only a subtle effect on visible pigmentation. PLoS Genet. 2011 doi: 10.1371/journal.pgen.1002285. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hermann GJ, Schroeder LK, Hieb CA, Kershner AM, Rabbitts BM, Fonarev P, Grant BD, Priess JR. Genetic analysis of lysosomal trafficking in Caenorhabditis elegans. Mol. Biol. Cell. 2005;16:3273–3288. doi: 10.1091/mbc.E05-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirosaki K, Yamashita T, Wada I, Jin HY, Jimbow K. Tyrosinase and tyrosinase-related protein 1 require Rab7 for their intracellular transport. J. Invest. Dermatol. 2002;119:475–480. doi: 10.1046/j.1523-1747.2002.01832.x. [DOI] [PubMed] [Google Scholar]
- 75.Hirst J, Barlow LD, Francisco GC, Sahlender DA, Seaman MN, Dacks JB, Robinson MS. The fifth adaptor protein complex. PLoS Biol. 2011;9:e1001170. doi: 10.1371/journal.pbio.1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ho H, Kapadia R, Al-Tahan S, Ahmad S, Ganesan AK. WIPI1 coordinates melanogenic gene transcription and melanosome formation via TORC1 inhibition. J. Biol. Chem. 2011;286:12509–12523. doi: 10.1074/jbc.M110.200543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoashi T, Watabe H, Muller J, Yamaguchi Y, Vieira WD, Hearing VJ. MART-1 is required for the function of the melanosomal matrix protein PMEL17/GP100 and the maturation of melanosomes. J. Biol. Chem. 2005;280:14006–14016. doi: 10.1074/jbc.M413692200. [DOI] [PubMed] [Google Scholar]
- 78.Hoashi T, Muller J, Vieira WD, Rouzaud F, Kikuchi K, Tamaki K, Hearing VJ. The repeat domain of the melanosomal matrix protein Pmel17/gp100 is required for the formation of organellar fibers. J. Biol. Chem. 2006;281:21198–22208. doi: 10.1074/jbc.M601643200. [DOI] [PubMed] [Google Scholar]
- 79.Hoek K, Rimm DL, Williams KR, Zhao H, Ariyan S, Lin A, Kluger HM, Berger AJ, Cheng E, Trombetta ES, Wu T, Niinobe M, Yoshikawa K, Hannigan GE, Halaban R. Expression profiling reveals novel pathways in the transformation of melanocytes to melanomas. Cancer Res. 2004;64:5270–5282. doi: 10.1158/0008-5472.CAN-04-0731. [DOI] [PubMed] [Google Scholar]
- 80.Höning S, Sandoval IV, von Figura K. A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tyrosinase mediates selective binding of AP-3. EMBO J. 1998;17:1304–1314. doi: 10.1093/emboj/17.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoyle DJ, Rodriguez-Fernandez IA, Dell'Angelica EC. Functional interactions between OCA2 and the protein complexes BLOC-1, BLOC-2 and AP-3 inferred from epistatic analyses of mouse coat pigmentation. Pigment Cell Melanoma Res. 2010 doi: 10.1111/j.1755-148X.2010.00815.x. In press: 10.1111/j.1755-148X.2010.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang L, Kuo YM, Gitschier J. The pallid gene encodes a novel, syntaxin 13-interacting protein involved in platelet storage pool deficiency. Nature Genet. 1999;23:329–332. doi: 10.1038/15507. [DOI] [PubMed] [Google Scholar]
- 83.Huizing M, Helip-Wooley A, Westbroek W, Gunay-Aygun M, Gahl WA. Disorders of lysosome-related organelle biogenesis: clinical and molecular genetics. Annu. Rev. Genomics Hum. Genet. 2008;9:359–386. doi: 10.1146/annurev.genom.9.081307.164303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huizing M, Sarangarajan R, Strovel E, Zho Y, Gahl WA, Boissy RE. AP-3 mediates tyrosinase but not TRP-1 trafficking in human melanocytes. Mol. Biol. Cell. 2001;12:2075–2085. doi: 10.1091/mbc.12.7.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huizing M, Pederson B, Hess RA, Griffin A, Helip-Wooley A, Westbroek W, Dorward H, O'Brien KJ, Golas G, Tsilou E, White JG, Gahl WA. Clinical and cellular characterisation of Hermansky-Pudlak syndrome type 6. J. Med. Genet. 2009;46:803–810. doi: 10.1136/jmg.2008.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hume AN, Collinson LM, Hopkins CR, Strom M, Barral DC, Bossi G, Griffiths GM, Seabra MC. The leaden gene product is required with rab27a to recruit myosin Va to melanosomes in melanocytes. Traffic. 2002;3:193–202. doi: 10.1034/j.1600-0854.2002.030305.x. [DOI] [PubMed] [Google Scholar]
- 87.Hurbain I, Geerts WJC, Boudier T, Marco S, Verkleij A, Marks MS, Raposo G. Electron tomography of early melanosomes: implications for melanogenesis and the generation of fibrillar amyloid sheets. Proc. Natl. Acad. Sci. U.S.A. 2008;105:19726–19731. doi: 10.1073/pnas.0803488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hurley JH. The ESCRT complexes. Crit. Rev. Biochem. Mol. Biol. 2010;45:463–487. doi: 10.3109/10409238.2010.502516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ilardi JM, Mochida S, Sheng ZH. Snapin: a SNARE-associated protein implicated in synaptic transmission. Nature Neurosci. 1999;2:119–124. doi: 10.1038/5673. [DOI] [PubMed] [Google Scholar]
- 90.Jin N, Chow CY, Liu L, Zolov SN, Bronson R, Davisson M, Petersen JL, Zhang Y, Park S, Duex JE, Goldowitz D, Meisler MH, Weisman LS. VAC14 nucleates a protein complex essential for the acute interconversion of PI3P and PI(3,5)P(2) in yeast and mouse. EMBO J. 2008;27:3221–3234. doi: 10.1038/emboj.2008.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jordens I, Westbroek W, Marsman M, Rocha N, Mommaas M, Huizing M, Lambert J, Naeyaert JM, Neefjes J. Rab7 and Rab27a control two motor protein activities involved in melanosomal transport. Pigment Cell Res. 2006;19:412–423. doi: 10.1111/j.1600-0749.2006.00329.x. [DOI] [PubMed] [Google Scholar]
- 92.Kantheti P, Qiao X, Diaz ME, Peden AA, Meyer GE, Carskadon SL, Kapfhamer D, Sufalko D, Robinson MS, Noebels JL, Burmeister M. Mutation in AP-3d in the mocha mouse links endosomal transport to storage deficiency in platelets, melanosomes, and synaptic vesicles. Neuron. 1998;21:111–122. doi: 10.1016/s0896-6273(00)80519-x. [DOI] [PubMed] [Google Scholar]
- 93.Kaplan J, De Domenico I, Ward DM. Chediak-Higashi syndrome. Curr. Opin. Hematol. 2008;15:22–29. doi: 10.1097/MOH.0b013e3282f2bcce. [DOI] [PubMed] [Google Scholar]
- 94.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Sakaguchi K, Appella E, Yannelli JR, Adema GJ, Miki T, Rosenberg SA. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc. Natl. Acad. Sci. U.S.A. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kedlaya R, Kandala G, Liu TF, Maddodi N, Devi S, Setaluri V. Interactions between GIPC-APPL and GIPC-TRP1 regulate melanosomal protein trafficking and melanogenesis in human melanocytes. Arch. Biochem. Biophys. 2011;508:227–233. doi: 10.1016/j.abb.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim BY, Kramer H, Yamamoto A, Kominami E. Molecular characterization of mammalian homologues of class C Vps proteins that interact with Syntaxin-7. J. Biol. Chem. 2001;276:29393–29402. doi: 10.1074/jbc.M101778200. [DOI] [PubMed] [Google Scholar]
- 97.King RA, Hearing VJ, Creel DJ, Oetting WS. Albinism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. McGraw-Hill, Inc.; New York: 1995. [Google Scholar]
- 98.Kloer DR, Rojas R, Ivan V, Moriyama K, van Vlijmen T, Murthy N, Ghirlando R, van der Sluijs P, Hurley JH, Bonifacino JS. Assembly of the biogenesis of lysosome-related organelles complex-3 (BLOC-3) and its interaction with Rab9. J. Biol. Chem. 2010;285:7794–7804. doi: 10.1074/jbc.M109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kobayashi T, Imokawa G, Bennett DC, Hearing VJ. Tyrosinase stabilization by Tyrp1 (the brown locus protein) J. Biol. Chem. 1998;273:31801–31805. doi: 10.1074/jbc.273.48.31801. [DOI] [PubMed] [Google Scholar]
- 100.Kobayashi T, Vieira WD, Potterf B, Sakai C, Imokawa G, Hearing VJ. Modulation of melanogenic protein expression during the switch from eu- to pheomelanogenesis. J. Cell Sci. 1995;108:2301–2309. doi: 10.1242/jcs.108.6.2301. [DOI] [PubMed] [Google Scholar]
- 101.Kummer MP, Maruyama H, Huelsmann C, Baches S, Weggen S, Koo EH. Formation of Pmel17 amyloid is regulated by juxtamembrane metalloproteinase cleavage, and the resulting C-terminal fragment is a substrate for gamma-secretase. J. Biol. Chem. 2009;284:2296–2306. doi: 10.1074/jbc.M808904200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kushimoto T, Basrur V, Valencia J, Matsunaga J, Vieira WD, Ferrans VJ, Muller J, Appella E, Hearing VJ. A model for melanosome biogenesis based on the purification and analysis of early melanosomes. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10698–10703. doi: 10.1073/pnas.191184798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lane PW, Deol MS. Mocha, a new coat color and behavior mutation on chromosome 10 of the mouse. J. Hered. 1974;65:362–364. doi: 10.1093/oxfordjournals.jhered.a108551. [DOI] [PubMed] [Google Scholar]
- 104.Le Poole IC, Luiten RM. Autoimmune etiology of generalized vitiligo. Curr. Dir. Autoimmun. 2008;10:227–243. doi: 10.1159/000131485. [DOI] [PubMed] [Google Scholar]
- 105.Lee YS, Pressman S, Andress AP, Kim K, White JL, Cassidy JJ, Li X, Lubell K, Lim DH, Cho IS, Nakahara K, Preall JB, Bellare P, Sontheimer EJ, Carthew RW. Silencing by small RNAs is linked to endosomal trafficking. Nat. Cell Biol. 2009;11:1150–1156. doi: 10.1038/ncb1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee ZH, Hou L, Moellmann G, Kuklinska E, Antol K, Fraser M, Halaban R, Kwon BS. Characterization and subcellular localization of human Pmel 17/silver, a 100-kDa (pre)melanosomal membrane protein associated with 5,6,-dihydroxyindole-2-carboxylic acid (DHICA) converting activity. J. Invest. Dermatol. 1996;106:605–610. doi: 10.1111/1523-1747.ep12345163. [DOI] [PubMed] [Google Scholar]
- 107.Leonhardt RM, Vigneron N, Rahner C, Cresswell P. Proprotein convertases process Pmel17 during secretion. J. Biol. Chem. 2011;286:9321–9337. doi: 10.1074/jbc.M110.168088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lerner AB, Fitzpatrick TB, Calkins E, Summerson WH. Mammalian tyrosinase: preparation and properties. J. Biol. Chem. 1949;178:185–195. [PubMed] [Google Scholar]
- 109.Lerner AB, Fitzpatrick TB, Calkins E, Summerson WH. Mammalian tyrosinase; the relationship of copper to enzymatic activity. J. Biol. Chem. 1950;187:793–802. [PubMed] [Google Scholar]
- 110.Lévy F, Muehlethaler K, Salvi S, Peitrequin A-L, Lindholm CK, Cerottini J-C, Rimoldi D. Ubiquitylation of a melanosomal protein by HECT-E3 ligases serves as sorting signal for lysosomal degradation. Mol. Biol. Cell. 2005;16:1777–1787. doi: 10.1091/mbc.E04-09-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li W, Rusiniak ME, Chintala S, Gautam R, Novak EK, Swank RT. Murine Hermansky-Pudlak syndrome genes: regulators of lysosome-related organelles. Bioessays. 2004;26:616–628. doi: 10.1002/bies.20042. [DOI] [PubMed] [Google Scholar]
- 112.Liu TF, Kandala G, Setaluri V. PDZ-domain protein GIPC interacts with the cytoplasmic tail of melanosomal membrane protein gp75 (tyrosinase related protein-1) J. Biol. Chem. 2001;276:35768–35777. doi: 10.1074/jbc.M103585200. [DOI] [PubMed] [Google Scholar]
- 113.Lloyd V, Ramaswami M, Krämer H. Not just pretty eyes: Drosophila eye-colour mutations and lysosomal delivery. Trends Cell Biol. 1998;8:257–259. doi: 10.1016/s0962-8924(98)01270-7. [DOI] [PubMed] [Google Scholar]
- 114.Loftus SK, Larson DM, Baxter LL, Antonellis A, Chen YA, Wu XS, Jiang Y, Bittner M, Hammer JA, III, Pavan WJ. Mutation of melanosome protein RAB38 in chocolate mice. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4471–4476. doi: 10.1073/pnas.072087599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Loh E, Peter F, Subramaniam VN, Hong W. Mammalian Bet3 functions as a cytosolic factor participating in transport from the ER to the Golgi apparatus. J. Cell Sci. 2005;118:1209–1222. doi: 10.1242/jcs.01723. [DOI] [PubMed] [Google Scholar]
- 116.Lopes VS, Wasmeier C, Seabra MC, Futter CE. Melanosome maturation defect in Rab38-deficient retinal pigment epithelium results in instability of immature melanosomes during transient melanogenesis. Mol. Biol. Cell. 2007;18:3914–3927. doi: 10.1091/mbc.E07-03-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lopes VS, Ramalho JS, Owen DM, Karl MO, Strauss O, Futter CE, Seabra MC. The ternary Rab27a-Myrip-myosin VIIa complex regulates melanosome motility in the retinal pigment epithelium. Traffic. 2007;8:486–499. doi: 10.1111/j.1600-0854.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lopez VM, Decatur CL, Stamer WD, Lynch RM, McKay BS. L-DOPA is an endogenous ligand for OA1. PLoS Biol. 2008;6:e236. doi: 10.1371/journal.pbio.0060236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ma J, Plesken H, Treisman JE, Edelman-Novemsky I, Ren M. Lightoid and Claret: a rab GTPase and its putative guanine nucleotide exchange factor in biogenesis of Drosophila eye pigment granules. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11652–11657. doi: 10.1073/pnas.0401926101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marks MS, Seabra MC. The melanosome: membrane dynamics in black and white. Nature Rev. Mol. Cell Biol. 2001;2:738–748. doi: 10.1038/35096009. [DOI] [PubMed] [Google Scholar]
- 121.Martĺnez-Esparza M, Jiménez-Cervantes C, Bennett DC, Lozano JA, Solano F, Garcĺa-Borrón JC. The mouse silver locus encodes a single transcript truncated by the silver mutation. Mammalian Genome. 1999;10:1168–1171. doi: 10.1007/s003359901184. [DOI] [PubMed] [Google Scholar]
- 122.Menasche G, Ho CH, Sanal O, Feldmann J, Tezcan I, Ersoy F, Houdusse A, Fischer A, de Saint Basile G. Griscelli syndrome restricted to hypopigmentation results from a melanophilin defect (GS3) or a MYO5A F-exon deletion (GS1) J Clin Invest. 2003;112:450–6. doi: 10.1172/JCI18264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Menasche G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, de Saint Basile G. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. 2000;25:173–6. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 124.Morgan NV, Pasha S, Johnson CA, Ainsworth JR, Eady RAJ, Dawood B, McKeown C, Trembath RC, Wilde J, Watson SP, Maher ER. A germline mutation in BLOC1S3/reduced pigmentation causes a novel variant of Hermansky-Pudlak Syndrome (HPS8) Am. J. Hum. Genet. 2006;78:160–166. doi: 10.1086/499338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moriyama K, Bonifacino JS. Pallidin is a component of a multi-protein complex involved in the biogenesis of lysosome-related organelles. Traffic. 2002;3:666–677. doi: 10.1034/j.1600-0854.2002.30908.x. [DOI] [PubMed] [Google Scholar]
- 126.Moyer FH. Genetic variations in the fine structure and ontogeny of mouse melanin granules. Am. Zoologist. 1966;6:43–66. doi: 10.1093/icb/6.1.43. [DOI] [PubMed] [Google Scholar]
- 127.Mullins C, Hartnell LM, Bonifacino JS. Distinct requirements for the AP-3 adaptor complex in pigment granule and synaptic vesicle biogenesis in Drosophila melanogaster. Mol. Gen. Genet. 2000;263:1003–1014. doi: 10.1007/pl00008688. [DOI] [PubMed] [Google Scholar]
- 128.Mullins C, Hartnell LM, Wassarman DA, Bonifacino JS. Defective expression of the mu3 subunit of the AP-3 adaptor complex in the Drosophila pigmentation mutant carmine. Mol. Gen. Genet. 1999;262:401–412. doi: 10.1007/s004380051099. [DOI] [PubMed] [Google Scholar]
- 129.Negroiu G, Dwek RA, Petrescu SM. Folding and maturation of Tyrosinase-related Protein-1 are regulated by the post-translational formation of disulfide bonds and by N-glycan processing. J. Biol. Chem. 2000;275:32200–32207. doi: 10.1074/jbc.M005186200. [DOI] [PubMed] [Google Scholar]
- 130.Negroiu G, Dwek RA, Petrescu SM. The inhibition of early N-glycan processing targets TRP-2 to degradation in B16 melanoma cells. J. Biol. Chem. 2003;278:27035–27042. doi: 10.1074/jbc.M303167200. [DOI] [PubMed] [Google Scholar]
- 131.Newell-Litwa K, Salazar G, Smith Y, Faundez V. Roles of BLOC-1 and adaptor protein-3 complexes in cargo sorting to synaptic vesicles. Mol. Biol. Cell. 2009;20:1441–1453. doi: 10.1091/mbc.E08-05-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nguyen T, Wei ML. Characterization of melanosomes in murine Hermansky-Pudlak syndrome: mechanisms of hypopigmentation. J. Invest. Dermatol. 2004;122:452–460. doi: 10.1046/j.0022-202X.2004.22117.x. [DOI] [PubMed] [Google Scholar]
- 133.Nguyen T, Wei ML. Hermansky-Pudlak HPS1/pale ear gene regulates epidermal and dermal melanocyte development. J. Invest. Dermatol. 2007;127:421–428. doi: 10.1038/sj.jid.5700566. [DOI] [PubMed] [Google Scholar]
- 134.Nguyen T, Novak EK, Kermani M, Fluhr J, Peters LL, Swank RT, Wei ML. Melanosome morphologies in murine models of Hermansky-Pudlak syndrome reflect blocks in organelle development. J. Invest. Dermatol. 2002;119:1156–1164. doi: 10.1046/j.1523-1747.2002.19535.x. [DOI] [PubMed] [Google Scholar]
- 135.Noebels JL, Sidman RL. Persistent hypersynchronization of neocortical neurons in the mocha mutant of mouse. J. Neurogenet. 1989;6:53–56. doi: 10.3109/01677068909107100. [DOI] [PubMed] [Google Scholar]
- 136.Novikoff AB, Albala A, Biempica L. Ultrastructural and cytochemical observations on B-16 and Harding-Passey mouse melanomas. The origin of premelanosomes and compound melanosomes. J. Histochem. Cytochem. 1968;16:299–319. doi: 10.1177/16.5.299. [DOI] [PubMed] [Google Scholar]
- 137.Oiso N, Riddle SR, Serikawa T, Kuramoto T, Spritz RA. The rat Ruby (R) locus is Rab38: identical mutations in Fawn-hooded and Tester-Moriyama rats derived from an ancestral Long Evans rat sub-strain. Mamm. Genome. 2004;15:307–314. doi: 10.1007/s00335-004-2337-9. [DOI] [PubMed] [Google Scholar]
- 138.Ooi CE, Moreira JE, Dell'Angelica EC, Poy G, Wassarman DA, Bonifacino JS. Altered expression of a novel adaptin leads to defective pigment granule biogenesis in the Drosophila eye color mutant garnet. EMBO J. 1997;16:4508–4518. doi: 10.1093/emboj/16.15.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Orlow SJ. Melanosomes are specialized members of the lysosomal lineage of organelles. J. Invest. Dermatol. 1995;105:3–7. doi: 10.1111/1523-1747.ep12312291. [DOI] [PubMed] [Google Scholar]
- 140.Pastural E, Barrat FJ, Dufourcq-Lagelouse R, Certain S, Sanal O, Jabado N, Seger R, Griscelli C, Fischer A, de Saint Basile G. Griscelli disease maps to chromosome 15q21 and is associated with mutations in the myosin-Va gene. Nat Genet. 1997;16:289–92. doi: 10.1038/ng0797-289. [DOI] [PubMed] [Google Scholar]
- 141.Peden AA, Oorschot V, Hesser BA, Austin CD, Scheller RH, Klumperman J. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J. Cell Biol. 2004;164:1065–1076. doi: 10.1083/jcb.200311064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Peplowska K, Markgraf DF, Ostrowicz CW, Bange G, Ungermann C. The CORVET tethering complex interacts with the yeast Rab5 homolog Vps21 and is involved in endolysosomal biogenesis. Dev. Cell. 2007;12:739–750. doi: 10.1016/j.devcel.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 143.Piccirillo R, Palmisano I, Innamorati G, Bagnato P, Altimare D, Schiaffino MV. An unconventional dileucine-based motif and a novel cytosolic motif are required for the lysosomal and melanosomal targeting of OA1. J. Cell Sci. 2006;119:2003–2014. doi: 10.1242/jcs.02930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Poupon V, Stewart A, Gray SR, Piper RC, Luzio JP. The role of mVps18p in clustering, fusion, and intracellular localization of late endocytic organelles. Mol. Biol. Cell. 2003;14:4015–4027. doi: 10.1091/mbc.E03-01-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pulipparacharuvil S, Akbar MA, Ray S, Sevrioukov EA, Haberman AS, Rohrer J, Kramer H. Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. J. Cell Sci. 2005;118:3663–3673. doi: 10.1242/jcs.02502. [DOI] [PubMed] [Google Scholar]
- 146.Ramalho JS, Tolmachova T, Hume AN, McGuigan A, Gregory-Evans CY, Huxley C, Seabra MC. Chromosomal mapping, gene structure and characterization of the human and murine RAB27B gene. BMC Genet. 2001;2:2. doi: 10.1186/1471-2156-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Raposo G, Marks MS. Melanosomes - dark organelles enlighten endosomal membrane transport. Nat. Rev. Mol. Cell Biol. 2007;8:786–798. doi: 10.1038/nrm2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Raposo G, Marks MS, Cutler DF. Lysosome-related organelles: driving post-golgi compartments into specialisation. Curr. Opin. Cell Biol. 2007;19:394–401. doi: 10.1016/j.ceb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Raposo G, Tenza D, Murphy DM, Berson JF, Marks MS. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J. Cell Biol. 2001;152:809–823. doi: 10.1083/jcb.152.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Richardson SC, Winistorfer SC, Poupon V, Luzio JP, Piper RC. Mammalian late vacuole protein sorting orthologues participate in early endosomal fusion and interact with the cytoskeleton. Mol. Biol. Cell. 2004;15:1197–1210. doi: 10.1091/mbc.E03-06-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Richmond B, Huizing M, Knapp J, Koshoffer A, Zhao Y, Gahl WA, Boissy RE. Melanocytes derived from patients with HermanskyPudlak Syndrome types 1, 2, and 3 have distinct defects in cargo trafficking. J. Invest. Dermatol. 2005;124:420–427. doi: 10.1111/j.0022-202X.2004.23585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Robila V, Ostankovitch M. Altrich-Vanlith ML, Theos AC, Drover S, Marks MS, Restifo N, Engelhard VH. MHC class II presentation of gp100 epitopes in melanoma cells requires the function of conventional endosomes and is influenced by melanosomes. J. Immunol. 2008;181:7843–7852. doi: 10.4049/jimmunol.181.11.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Robinson MS. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 154.Rodriguez-Fernandez IA, Dell'angelica EC. A data-mining approach to rank candidate protein-binding partners-The case of biogenesis of lysosome-related organelles complex-1 (BLOC-1) J. Inherit. Metab. Dis. 2009;32:190–203. doi: 10.1007/s10545-008-1014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rosengren Pielberg G, Golovko A, Sundström E, Curik I, Lennartsson J, Seltenhammer MH, Druml T, Binns M, Fitzsimmons C, Lindgren G, Sandberg K, Baumung R, Vetterlein M, Strömberg S, Grabherr M, Wade C, Lindblad-Toh K, Pontén F, Heldin CH, Sölkner J, Andersson L. A cis-acting regulatory mutation causes premature hair graying and susceptibility to melanoma in the horse. Nature Genet. 2008;40:1004–1009. doi: 10.1038/ng.185. [DOI] [PubMed] [Google Scholar]
- 156.Salazar G, Craige B, Styers ML, Newell-Litwa KA, Doucette MM, Wainer BH, Falcon-Perez JM, Dell'Angelica EC, Peden AA, E. Werner E, Faundez V. BLOC-1 complex deficiency alters the targeting of Adaptor Protein complex-3 cargoes. Mol. Biol. Cell. 2006;17:4014–4026. doi: 10.1091/mbc.E06-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-Like Receptor 9 signaling by Adaptor Protein 3. Science. 2010;329:1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schiaffino MV, Baschirotto C, Pellegrini G, Montalti S, Tacchetti C, De Luca M, Ballabio A. The ocular albinism type 1 gene product is a membrane glycoprotein localized to melanosomes. Proc. Natl. Acad. Sci. USA. 1996;93:9055–9060. doi: 10.1073/pnas.93.17.9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Seabra MC, Mules EH, Hume AN. Rab GTPases, intracellular traffic and disease. Trends Mol. Med. 2002;8:23–30. doi: 10.1016/s1471-4914(01)02227-4. [DOI] [PubMed] [Google Scholar]
- 160.Seals DF, Eitzen G, Margolis N, Wickner WT, Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9402–9407. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Seiji M, Fitzpatrick TM, Simpson RT, Birbeck MSC. Chemical composition and terminology of specialized organelles (melanosomes and melanin granules) in mammalian melanocytes. Nature. 1963;197:1082–1084. doi: 10.1038/1971082a0. [DOI] [PubMed] [Google Scholar]
- 162.Setty SRG, Tenza D, Sviderskaya EV, Bennett DC, Raposo G, Marks MS. Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes. Nature. 2008;454:1142–1146. doi: 10.1038/nature07163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Setty SRG, Tenza D, Truschel ST, Chou E, Sviderskaya EV, Theos AC, Lamoreux ML, Di Pietro SM, Starcevic M, Bennett DC, Dell'Angelica EC, Raposo G, Marks MS. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol. Biol. Cell. 2007;18:768–780. doi: 10.1091/mbc.E06-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sevrioukov EA, He JP, Moghrabi N, Sunio A, Kramer H. A role for the deep orange and carnation eye color genes in lysosomal delivery in Drosophila. Mol. Cell. 1999;4:479–486. doi: 10.1016/s1097-2765(00)80199-9. [DOI] [PubMed] [Google Scholar]
- 165.Simmen T, Schmidt A, Hunziker W, Beermann F. The tyrosinase tail mediates sorting to the lysosomal compartment in MDCK cells via a di-leucine and a tyrosine-based signal. J. Cell Sci. 1999;112:45–53. doi: 10.1242/jcs.112.1.45. [DOI] [PubMed] [Google Scholar]
- 166.Simon JD, Peles D, Wakamatsu K, Ito S. Current challenges in understanding melanogenesis: bridging chemistry, biological control, morphology and function. Pigment Cell Melanoma Res. 2009;22:563–579. doi: 10.1111/j.1755-148X.2009.00610.x. [DOI] [PubMed] [Google Scholar]
- 167.Simpson F, Peden AA, Christopoulou L, Robinson MS. Characterization of the adaptor-related protein complex, AP-3. J. Cell Biol. 1997;137:835–845. doi: 10.1083/jcb.137.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sitaram A, Piccirillo R, Palmisano I, Harper DC, Dell'Angelica EC, Schiaffino MV, Marks MS. Localization to mature melanosomes by virtue of cytoplasmic dileucine motifs is required for human OCA2 function. Mol. Biol. Cell. 2009;20:1464–1477. doi: 10.1091/mbc.E08-07-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sriram V, Krishnan KS, Mayor S. deep-orange and carnation define distinct stages in late endosomal biogenesis in Drosophila melanogaster. J. Cell Biol. 2003;161:593–607. doi: 10.1083/jcb.200210166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 171.Sugita M, Cao X, Watts GFM, Rogers RA, Bonifacino JS, Brenner MB. Failure of trafficking and antigen presentation by CD1 in AP-3-deficient cells. Immunity. 2002;16:697–706. doi: 10.1016/s1074-7613(02)00311-4. [DOI] [PubMed] [Google Scholar]
- 172.Sundström E, Komisarczuk AZ, Jiang L, Golovko A, Navratilova P, Rinkwitz S, Becker TS, Andersson L. Identification of a melanocyte-specific, microphthalmia-associated transcription factor-dependent regulatory element in the intronic duplication causing hair greying and melanoma in horses. Pigment Cell Melanoma Res. 2012;25:28–36. doi: 10.1111/j.1755-148X.2011.00902.x. [DOI] [PubMed] [Google Scholar]
- 173.Suzuki T, Oiso N, Gautam R, Novak EK, Panthier JJ, Suprabha PG, Vida T, Swank RT, Spritz RA. The mouse organellar biogenesis mutant buff results from a mutation in Vps33a, a homologue of yeast vps33 and Drosophila carnation. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1146–1150. doi: 10.1073/pnas.0237292100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Suzuki T, Li W, Zhang Q, Karim A, Novak EK, Sviderskaya EV, Hill SP, Bennett DC, Levin AV, Nieuwenhuis H, K., Fong C-T, Castellan C, Miterski B, Swank RT, Spritz RA. Hermansky-Pudlak syndrome is caused by mutations in HPS4, the human homolog of the mouse light-ear gene. Nature Genet. 2002;30:321–324. doi: 10.1038/ng835. [DOI] [PubMed] [Google Scholar]
- 175.Swank RT, Reddington M, Howlett O, Novak EK. Platelet storage pool deficiency associated with inherited abnormalities of the inner ear in the mouse pigment mutants muted and mocha. Blood. 1991;78:2036–2044. [PubMed] [Google Scholar]
- 176.Tamura K, Ohbayashi N, Ishibashi K, Fukuda M. Structure-function analysis of VPS9-ankyrin-repeat protein (Varp) in the trafficking of tyrosinase-related protein 1 in melanocytes. J. Biol. Chem. 2011;286:7507–7521. doi: 10.1074/jbc.M110.191205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tamura K, Ohbayashi N, Maruta Y, Kanno E, Itoh T, Fukuda M. Varp is a novel Rab32/38-binding protein that regulates Tyrp1 trafficking in melanocytes. Mol. Biol. Cell. 2009;20:2900–2908. doi: 10.1091/mbc.E08-12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Theos AC, Truschel ST, Raposo G, Marks MS. The Silver locus product Pmel17/ gp100/ Silv/ ME20: Controversial in name and in function. Pigment Cell Res. 2005;18:322–336. doi: 10.1111/j.1600-0749.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Theos AC, Truschel ST, Tenza D, Hurbain I, Harper DC, Berson JF, Thomas PC, Raposo G, Marks MS. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev. Cell. 2006;10:343–354. doi: 10.1016/j.devcel.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Theos AC, Berson JF, Theos SC, Herman KE, Harper DC, Tenza D, Sviderskaya EV, Lamoreux ML, Bennett DC, Raposo G, Marks MS. Dual loss of ER export and endocytic signals with altered melanosome morphology in the silver mutation of Pmel17. Mol. Biol. Cell. 2006;17:3598–3612. doi: 10.1091/mbc.E06-01-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Theos AC, Tenza D, Martina JA, Hurbain I, Peden AA, Sviderskaya EV, Stewart A, Robinson MS, Bennett DC, Cutler DF, Bonifacino JS, Marks MS, Raposo G. Functions of AP-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol. Biol. Cell. 2005;16:5356–5372. doi: 10.1091/mbc.E05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Toyofuku K, Wada I, Spritz RA, Hearing VJ. The molecular basis of oculocutaneous albinism type 1 (OCA1): sorting failure and degradation of mutant tyrosinases results in a lack of pigmentation. Biochem. J. 2001;355:259–269. doi: 10.1042/0264-6021:3550259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Toyofuku K, Wada I, Valencia JC, Kushimoto T, Ferrans VJ, Hearing VJ. Oculocutaneous albinism types 1 and 3 are ER retention diseases: mutation of tyrosinase or Tyrp1 can affect the processing of both mutant and wild-type proteins. FASEB J. 2001;15:2149–2161. doi: 10.1096/fj.01-0216com. [DOI] [PubMed] [Google Scholar]
- 184.Toyofuku K, Valencia JC, Kushimoto T, Costin G-E, Virador VM, Vieira WD, Ferrans VJ, Hearing VJ. The etiology of oculocutaneous albinism (OCA) type II: the Pink protein controls the transport and processing of tyrosinase. Pigment Cell Res. 2002;15:218–227. doi: 10.1034/j.1600-0749.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- 185.Truschel ST, SImoes S, Setty SRG, Harper DC, Tenza D, Thomas PC, Herman KE, Sackett SD, Cowan DC, Theos AC, Raposo G, Marks MS. ESCRT-1 function is required for Tyrp1 transport from early endosomes to the melanosome limiting membrane. Traffic. 2009;10:1318–1336. doi: 10.1111/j.1600-0854.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Valencia JC, Watabe H, Chi A, Rouzaud F, Chen KG, Vieira WD, Takahashi K, Yamaguchi Y, Berens W, Nagashima K, Shabanowitz J, Hunt DF, Appella E, Hearing VJ. Sorting of Pmel17 to melanosomes through the plasma membrane by AP1 and AP2: evidence for the polarized nature of melanocytes. J. Cell Sci. 2006;119:1080–1091. doi: 10.1242/jcs.02804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Valencia JC, Rouzaud F, Julien S, Chen KG, Passeron T, Yamaguchi Y, Abu-Asab M, Tsokos M, Costin GE, Yamaguchi H, Miller Jenkins LM, Nagashima K, Appella E, Hearing VJ. Sialylated core 1 O-glycans influence the sorting of Pmel17/gp100 and determine its capacity to form fibrils. J. Biol. Chem. 2007;282:11266–11280. doi: 10.1074/jbc.M608449200. [DOI] [PubMed] [Google Scholar]
- 188.van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G. The Tetraspanin CD63 integrates ESCRT-independent and dependent sorting at a common endosome. Dev. Cell. 2011 doi: 10.1016/j.devcel.2011.08.019. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vijayasaradhi S, Xu YQ, Bouchard B, Houghton AN. Intracellular sorting and targeting of melanosomal membrane proteins: identification of signals for sorting of the human brown locus protein, gp75. J. Cell Biol. 1995;130:807–820. doi: 10.1083/jcb.130.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vites O, Rhee JS, Schwarz M, Rosenmund C, Jahn R. Reinvestigation of the role of snapin in neurotransmitter release. J. Biol. Chem. 2004;279:26251–26256. doi: 10.1074/jbc.M404079200. [DOI] [PubMed] [Google Scholar]
- 191.Wang N, Hebert DN. Tyrosinase maturation through the mammalian secretory pathway: bringing color to life. Pigment Cell Res. 2006;19:3–18. doi: 10.1111/j.1600-0749.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 192.Wasmeier C, Romao M, Plowright L, Bennett DC, Raposo G, Seabra MC. Rab38 an Rab32 control early post-Golgi trafficking of melanogenic enzymes. J. Cell Biol. 2006;175:271–281. doi: 10.1083/jcb.200606050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Watt B, Raposo G, Marks MS. Pmel17: An amyloid determinant of organelle structure. In: Bucciantini M, editor. Functional Amyloid Aggregation. Research Signpost; Trivandrum, Kerala, India: 2010. [Google Scholar]
- 194.Watt B, van Niel G, Fowler DM, Hurbain I, Luk KC, Stayrook SE, Lemmon MA, Raposo G, Shorter J, Kelly JW, Marks MS. N-terminal domains elicit formation of functional Pmel17 amyloid fibrils. J. Biol. Chem. 2009;284:35543–35555. doi: 10.1074/jbc.M109.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wei ML. Hermansky–Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19:19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 196.Wickner W. Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles. Annu. Rev. Cell Dev. Biol. 2010;26:115–136. doi: 10.1146/annurev-cellbio-100109-104131. [DOI] [PubMed] [Google Scholar]
- 197.Wilson SM, Yip R, Swing DA, O'Sullivan TN, Zhang Y, Novak EK, Swank RT, Russell LB, Copeland NG, Jenkins NA. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc Natl Acad Sci U S A. 2000;97:7933–8. doi: 10.1073/pnas.140212797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wu X, Rao K, Bowers MB, Copeland NG, Jenkins NA, Hammer JA. Rab27a enables myosin Va-dependent melanosome capture by recruiting the myosin to the organelle. J. Cell Sci. 2001;114:1091–1100. doi: 10.1242/jcs.114.6.1091. [DOI] [PubMed] [Google Scholar]
- 199.Wu XS, Rao K, Zhang H, Wang F, Sellers JR, Matesic LE, Copeland NG, Jenkins NA, Hammer JAr. Identification of an organelle receptor for myosin-Va. Nature Cell Biol. 2002;4:271–278. doi: 10.1038/ncb760. [DOI] [PubMed] [Google Scholar]
- 200.Yamasaki A, Menon S, Yu S, Barrowman J, Meerloo T, Oorschot V, Klumperman J, Satoh A, Ferro-Novick S. mTrs130 is a component of a mammalian TRAPPII complex, a Rab1 GEF that binds to COPI-coated vesicles. Mol. Biol. Cell. 2009;20:4205–4215. doi: 10.1091/mbc.E09-05-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yang W, Li C, Ward DM, Kaplan J, Mansour SL. Defective organellar membrane protein trafficking in Ap3b1-deficient cells. J. Cell Sci. 2000;113:4077–4086. doi: 10.1242/jcs.113.22.4077. [DOI] [PubMed] [Google Scholar]
- 202.Zhang Q, Zhen L, Li W, Novak EK, Collinson LM, Jang EK, Haslam RJ, Elliott RW, Swank RT. Cell-specific abnormal prenylation of Rab proteins in platelets and melanocytes of the gunmetal mouse. Br J Haematol. 2002;117:414–23. doi: 10.1046/j.1365-2141.2002.03444.x. [DOI] [PubMed] [Google Scholar]
- 203.Zhang Q, Zhao B, Li W, Oiso N, Novak EK, Rusiniak ME, Gautam R, Chintala S, O'Brien EP, Zhang Y, Roe BA, Elliott RW, Eicher EM, Liang P, Kratz C, Legius E, Spritz RA, O'Sullivan TN, Copeland NG, Jenkins NA, Swank RT. Ru2 and Ru encode mouse orthologs of the genes mutated in human Hermansky-Pudlak syndrome types 5 and 6. Nature Genet. 2003;33:145–153. doi: 10.1038/ng1087. [DOI] [PubMed] [Google Scholar]
- 204.Zhen L, Jiang S, Feng L, Bright NA, Peden AA, Seymour AB, Novak EK, Elliott R, Gorin MB, Robinson MS, Swank RT. Abnormal expression and subcellular distribution of subunit proteins of the AP-3 adaptor complex lead to platelet storage pool deficiency in the pearl mouse. Blood. 1999;94:146–155. [PubMed] [Google Scholar]
- 205.Zhu Y, Traub LM, Kornfeld S. High-affinity binding of the AP-1 adaptor complex to trans-golgi network membranes devoid of mannose 6-phosphate receptors. Mol. Biol. Cell. 1999;10:537–549. doi: 10.1091/mbc.10.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zong M, Wu XG, Chan CW, Choi MY, Chan HC, Tanner JA, Yu S. The adaptor function of TRAPPC2 in mammalian TRAPPs explains TRAPPC2-associated SEDT and TRAPPC9-associated congenital intellectual disability. PLoS One 6. 2011:e23350. doi: 10.1371/journal.pone.0023350. [DOI] [PMC free article] [PubMed] [Google Scholar]