Abstract
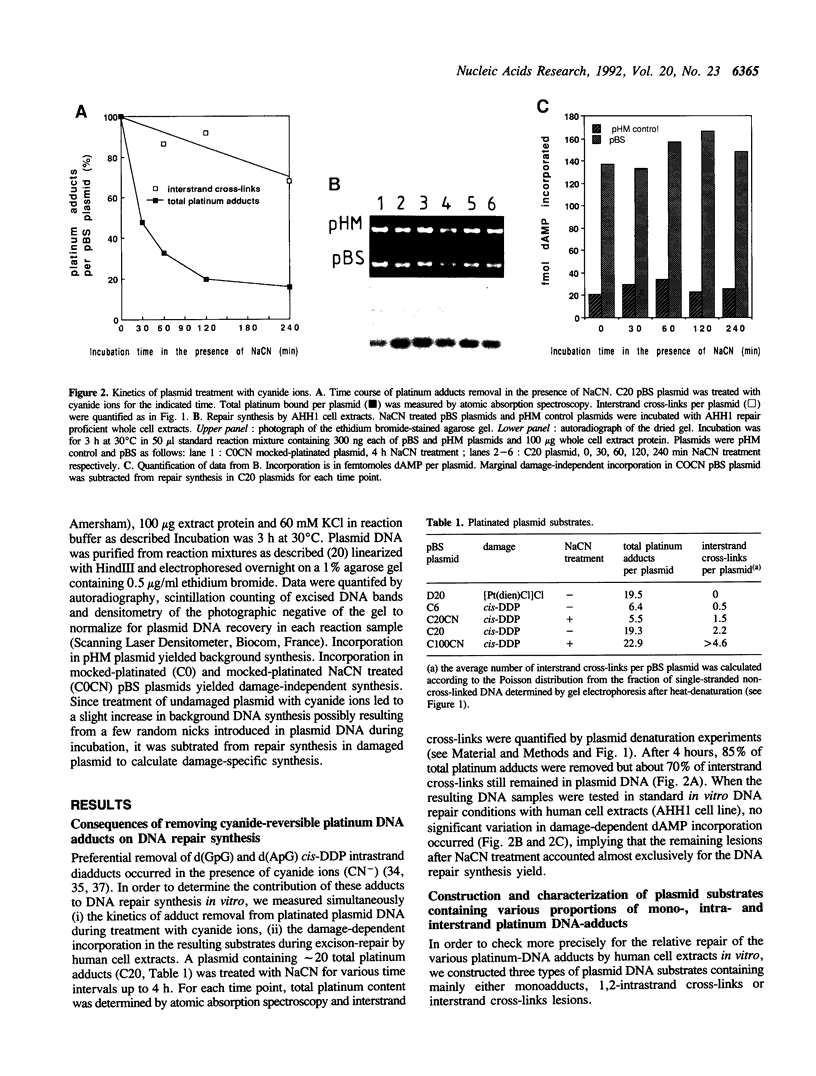
During reaction of cis-diamminedichloroplatinum(II) (cis-DDP) with DNA, a number of adducts are formed which may be discriminated by the excision-repair system. An in vitro excision-repair assay with human cell-free extracts has been used to assess the relative repair extent of monofunctional adducts, intrastrand and interstrand cross-links of cis-DDP on plasmid DNA. Preferential removal of cis-DDP 1,2-intrastrand diadducts occurred in the presence of cyanide ions. In conditions where cyanide treatment removed 85% of total platinum adducts while approximately 70% of interstrand cross-links remained in plasmid DNA, no significant variation in repair synthesis by human cell extracts was observed. Then, we constructed three types of plasmid DNA substrates containing mainly either monoadducts, 1,2-intrastrand cross-links or interstrand cross-links lesions. The three plasmid species were modified in order to obtain the same extent of total platinum DNA adducts per plasmid. No DNA repair synthesis was detected with monofunctional adducts during incubation with human whole cell extracts. However, a two-fold increase in repair synthesis was found when the proportion of interstrand cross-links in plasmid DNA was increased by 2-3 fold. These findings suggest that (i) cis-DDP 1,2-intrastrand diadducts are poorly repaired by human cell extracts in vitro, (ii) among other minor lesions potentially cyanide-resistant, cis-DDP interstrand cross-links represent a major lesion contributing to the repair synthesis signal in the in vitro assay. These results could account for the drug efficiency in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedford P., Fichtinger-Schepman A. M., Shellard S. A., Walker M. C., Masters J. R., Hill B. T. Differential repair of platinum-DNA adducts in human bladder and testicular tumor continuous cell lines. Cancer Res. 1988 Jun 1;48(11):3019–3024. [PubMed] [Google Scholar]
- Biggerstaff M., Robins P., Coverley D., Wood R. D. Effect of exogenous DNA fragments on human cell extract-mediated DNA repair synthesis. Mutat Res. 1991 May;254(3):217–224. doi: 10.1016/0921-8777(91)90059-x. [DOI] [PubMed] [Google Scholar]
- Cheng S., Sancar A., Hearst J. E. RecA-dependent incision of psoralen-crosslinked DNA by (A)BC excinuclease. Nucleic Acids Res. 1991 Feb 11;19(3):657–663. doi: 10.1093/nar/19.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli R. B., Solomon M. J., Varshavsky A., Lippard S. J. In vivo effects of cis- and trans-diamminedichloroplatinum(II) on SV40 chromosomes: differential repair, DNA-protein cross-linking, and inhibition of replication. Biochemistry. 1985 Dec 17;24(26):7533–7540. doi: 10.1021/bi00347a005. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Jen J., Charles W. C., Mitchell D. L. Cyclobutane dimers and (6-4) photoproducts in human cells are mended with the same patch sizes. Photochem Photobiol. 1991 Sep;54(3):393–402. doi: 10.1111/j.1751-1097.1991.tb02033.x. [DOI] [PubMed] [Google Scholar]
- Dijt F. J., Fichtinger-Schepman A. M., Berends F., Reedijk J. Formation and repair of cisplatin-induced adducts to DNA in cultured normal and repair-deficient human fibroblasts. Cancer Res. 1988 Nov 1;48(21):6058–6062. [PubMed] [Google Scholar]
- Donahue B. A., Augot M., Bellon S. F., Treiber D. K., Toney J. H., Lippard S. J., Essigmann J. M. Characterization of a DNA damage-recognition protein from mammalian cells that binds specifically to intrastrand d(GpG) and d(ApG) DNA adducts of the anticancer drug cisplatin. Biochemistry. 1990 Jun 19;29(24):5872–5880. doi: 10.1021/bi00476a032. [DOI] [PubMed] [Google Scholar]
- Eastman A., Jennerwein M. M., Nagel D. L. Characterization of bifunctional adducts produced in DNA by trans-diamminedichloroplatinum(II). Chem Biol Interact. 1988;67(1-2):71–80. doi: 10.1016/0009-2797(88)90087-7. [DOI] [PubMed] [Google Scholar]
- Eastman A. Reevaluation of interaction of cis-dichloro(ethylenediamine)platinum(II) with DNA. Biochemistry. 1986 Jul 1;25(13):3912–3915. doi: 10.1021/bi00361a026. [DOI] [PubMed] [Google Scholar]
- Eastman A., Schulte N. Enhanced DNA repair as a mechanism of resistance to cis-diamminedichloroplatinum(II). Biochemistry. 1988 Jun 28;27(13):4730–4734. doi: 10.1021/bi00413a022. [DOI] [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., van der Veer J. L., den Hartog J. H., Lohman P. H., Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry. 1985 Jan 29;24(3):707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., van der Veer J. L., den Hartog J. H., Lohman P. H., Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry. 1985 Jan 29;24(3):707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- Gibbons G. R., Page J. D., Mauldin S. K., Husain I., Chaney S. G. Role of carrier ligand in platinum resistance in L1210 cells. Cancer Res. 1990 Oct 15;50(20):6497–6501. [PubMed] [Google Scholar]
- Hansson J., Grossman L., Lindahl T., Wood R. D. Complementation of the xeroderma pigmentosum DNA repair synthesis defect with Escherichia coli UvrABC proteins in a cell-free system. Nucleic Acids Res. 1990 Jan 11;18(1):35–40. doi: 10.1093/nar/18.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson J., Keyse S. M., Lindahl T., Wood R. D. DNA excision repair in cell extracts from human cell lines exhibiting hypersensitivity to DNA-damaging agents. Cancer Res. 1991 Jul 1;51(13):3384–3390. [PubMed] [Google Scholar]
- Hansson J., Munn M., Rupp W. D., Kahn R., Wood R. D. Localization of DNA repair synthesis by human cell extracts to a short region at the site of a lesion. J Biol Chem. 1989 Dec 25;264(36):21788–21792. [PubMed] [Google Scholar]
- Hansson J., Wood R. D. Repair synthesis by human cell extracts in DNA damaged by cis- and trans-diamminedichloroplatinum(II). Nucleic Acids Res. 1989 Oct 25;17(20):8073–8091. doi: 10.1093/nar/17.20.8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. A., Berardini M. D., Souhami R. L. An agarose gel method for the determination of DNA interstrand crosslinking applicable to the measurement of the rate of total and "second-arm" crosslink reactions. Anal Biochem. 1991 Feb 15;193(1):131–134. doi: 10.1016/0003-2697(91)90052-u. [DOI] [PubMed] [Google Scholar]
- Heiger-Bernays W. J., Essigmann J. M., Lippard S. J. Effect of the antitumor drug cis-diamminedichloroplatinum(II) and related platinum complexes on eukaryotic DNA replication. Biochemistry. 1990 Sep 11;29(36):8461–8466. doi: 10.1021/bi00488a037. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Bootsma D. Molecular genetics of eukaryotic DNA excision repair. Cancer Cells. 1990 Oct;2(10):311–320. [PubMed] [Google Scholar]
- Hoeijmakers J. H. How relevant is the Escherichia coli UvrABC model for excision repair in eukaryotes? J Cell Sci. 1991 Dec;100(Pt 4):687–691. doi: 10.1242/jcs.100.4.687. [DOI] [PubMed] [Google Scholar]
- Hoffmann J. S., Johnson N. P., Villani G. Conversion of monofunctional DNA adducts of cis-diamminedichloroplatinum (II) to bifunctional lesions. Effect on the in vitro replication of single-stranded DNA by Escherichia coli DNA polymerase I and eukaryotic DNA polymerases alpha. J Biol Chem. 1989 Sep 5;264(25):15130–15135. [PubMed] [Google Scholar]
- Hospers G. A., Mulder N. H., De Vries E. G. Mechanisms of cellular resistance to cisplatin. Med Oncol Tumor Pharmacother. 1988;5(3):145–151. doi: 10.1007/BF02986437. [DOI] [PubMed] [Google Scholar]
- Huang J. C., Svoboda D. L., Reardon J. T., Sancar A. Human nucleotide excision nuclease removes thymine dimers from DNA by incising the 22nd phosphodiester bond 5' and the 6th phosphodiester bond 3' to the photodimer. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3664–3668. doi: 10.1073/pnas.89.8.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. C., Zhen W. P., Reed E., Parker R. J., Sancar A., Bohr V. A. Gene-specific formation and repair of cisplatin intrastrand adducts and interstrand cross-links in Chinese hamster ovary cells. J Biol Chem. 1991 Apr 15;266(11):7101–7107. [PubMed] [Google Scholar]
- Lemaire M. A., Schwartz A., Rahmouni A. R., Leng M. Interstrand cross-links are preferentially formed at the d(GC) sites in the reaction between cis-diamminedichloroplatinum (II) and DNA. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1982–1985. doi: 10.1073/pnas.88.5.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn M. M., Rupp W. D. Interaction of the UvrABC endonuclease with DNA containing a psoralen monoadduct or cross-link. Differential effects of superhelical density and comparison of preincision complexes. J Biol Chem. 1991 Dec 25;266(36):24748–24756. [PubMed] [Google Scholar]
- Page J. D., Husain I., Sancar A., Chaney S. G. Effect of the diaminocyclohexane carrier ligand on platinum adduct formation, repair, and lethality. Biochemistry. 1990 Jan 30;29(4):1016–1024. doi: 10.1021/bi00456a026. [DOI] [PubMed] [Google Scholar]
- Pil P. M., Lippard S. J. Specific binding of chromosomal protein HMG1 to DNA damaged by the anticancer drug cisplatin. Science. 1992 Apr 10;256(5054):234–237. doi: 10.1126/science.1566071. [DOI] [PubMed] [Google Scholar]
- Pinto A. L., Lippard S. J. Sequence-dependent termination of in vitro DNA synthesis by cis- and trans-diamminedichloroplatinum (II). Proc Natl Acad Sci U S A. 1985 Jul;82(14):4616–4619. doi: 10.1073/pnas.82.14.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plooy A. C., van Dijk M., Berends F., Lohman P. H. Formation and repair of DNA interstrand cross-links in relation to cytotoxicity and unscheduled DNA synthesis induced in control and mutant human cells treated with cis-diamminedichloroplatinum(II). Cancer Res. 1985 Sep;45(9):4178–4184. [PubMed] [Google Scholar]
- Plooy A. C., van Dijk M., Lohman P. H. Induction and repair of DNA cross-links in chinese hamster ovary cells treated with various platinum coordination compounds in relation to platinum binding to DNA, cytotoxicity, mutagenicity, and antitumor activity. Cancer Res. 1984 May;44(5):2043–2051. [PubMed] [Google Scholar]
- Reardon J. T., Spielmann P., Huang J. C., Sastry S., Sancar A., Hearst J. E. Removal of psoralen monoadducts and crosslinks by human cell free extracts. Nucleic Acids Res. 1991 Sep 11;19(17):4623–4629. doi: 10.1093/nar/19.17.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles B., Butour J. L., Lesca C., Macquet J. P. cis-Pt(NH3)2Cl2 and trans-Pt(NH3)2Cl2 inhibit DNA synthesis in cultured L1210 leukemia cells. Biochem Biophys Res Commun. 1983 Apr 29;112(2):555–563. doi: 10.1016/0006-291x(83)91500-0. [DOI] [PubMed] [Google Scholar]
- Shivji K. K., Kenny M. K., Wood R. D. Proliferating cell nuclear antigen is required for DNA excision repair. Cell. 1992 Apr 17;69(2):367–374. doi: 10.1016/0092-8674(92)90416-a. [DOI] [PubMed] [Google Scholar]
- Sibghat-Ullah, Sancar A. Substrate overlap and functional competition between human nucleotide excision repair and Escherichia coli photolyase and (a)BC excision nuclease. Biochemistry. 1990 Jun 19;29(24):5711–5718. doi: 10.1021/bi00476a011. [DOI] [PubMed] [Google Scholar]
- Sibghatullah, Husain I., Carlton W., Sancar A. Human nucleotide excision repair in vitro: repair of pyrimidine dimers, psoralen and cisplatin adducts by HeLa cell-free extract. Nucleic Acids Res. 1989 Jun 26;17(12):4471–4484. doi: 10.1093/nar/17.12.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek F. M., Munn M. M., Rupp W. D., Howard-Flanders P. In vitro repair of psoralen-DNA cross-links by RecA, UvrABC, and the 5'-exonuclease of DNA polymerase I. J Biol Chem. 1989 Apr 25;264(12):6755–6765. [PubMed] [Google Scholar]
- Sorenson C. M., Barry M. A., Eastman A. Analysis of events associated with cell cycle arrest at G2 phase and cell death induced by cisplatin. J Natl Cancer Inst. 1990 May 2;82(9):749–755. doi: 10.1093/jnci/82.9.749. [DOI] [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Holbrook S. R., Hearst J. E., Sancar A. Action mechanism of ABC excision nuclease on a DNA substrate containing a psoralen crosslink at a defined position. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8077–8081. doi: 10.1073/pnas.83.21.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houten B. Nucleotide excision repair in Escherichia coli. Microbiol Rev. 1990 Mar;54(1):18–51. doi: 10.1128/mr.54.1.18-51.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. D. Repair of pyrimidine dimer ultraviolet light photoproducts by human cell extracts. Biochemistry. 1989 Oct 17;28(21):8287–8292. doi: 10.1021/bi00447a005. [DOI] [PubMed] [Google Scholar]
- Wood R. D., Robins P., Lindahl T. Complementation of the xeroderma pigmentosum DNA repair defect in cell-free extracts. Cell. 1988 Apr 8;53(1):97–106. doi: 10.1016/0092-8674(88)90491-6. [DOI] [PubMed] [Google Scholar]
- Zhen W., Link C. J., Jr, O'Connor P. M., Reed E., Parker R., Howell S. B., Bohr V. A. Increased gene-specific repair of cisplatin interstrand cross-links in cisplatin-resistant human ovarian cancer cell lines. Mol Cell Biol. 1992 Sep;12(9):3689–3698. doi: 10.1128/mcb.12.9.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graeff A., Slebos R. J., Rodenhuis S. Resistance to cisplatin and analogues: mechanisms and potential clinical implications. Cancer Chemother Pharmacol. 1988;22(4):325–332. doi: 10.1007/BF00254240. [DOI] [PubMed] [Google Scholar]