Abstract
Aims
Tenascin-C (TNC), a matricellular protein, is upregulated in atherosclerotic plaques. We investigated whether the deletion of TNC gene affects the development of atherosclerosis in a murine model.
Methods
TNC−/−/apo E−/− mice were generated and used for atherosclerosis studies. We compared these results to those observed in control groups of apo E−/− mice.
Results
The en face analysis of aortic area showed that the mean aortic lesion area of the double KO mice was significantly higher than control mice at different times after feeding of atherogenic diet; the accumulation of lesional macrophages and lipids were significantly higher, respectively. Analysis of cell adhesion molecules revealed that VCAM-1, but not ICAM-1, was upregulated 1 week after feeding of atherogenic diet in the double KO mouse as compared to apo E−/− mouse. Cell culture studies revealed that the expression of VCAM-1 in endothelial cells isolated from the double KO mouse is more sensitive to the TNFα stimulation than the cells isolated from apo E−/− mice. Cell adhesion studies showed that the adherence of RAW monocytic cells to the endothelial cells was significantly enhanced in the cultured endothelial cells from the TNC gene-deleted cells. Following the prolonged feeding of an atherogenic diet (28–30 weeks), the aortic and carotid atherosclerotic lesions frequently demonstrated large grossly visible areas of intraplaque hemorrhage in the double KO mice compared to control.
Conclusions
These data unveil a protective role for TNC in atherosclerosis and suggest that TNC signaling may have the potential to reduce atherosclerosis, in part by modulating VCAM-1 expression.
Keywords: tenascin, atherosclerosis, plaque hemorrhage, extracellular matrix, VCAM-1
1. Introduction
Atherosclerosis is a chronic inflammatory disease affecting the large and medium sized arteries. This disease process involves diverse cell types whose activities are regulated by soluble as well as insoluble molecules found in the plaque. One such class of insoluble molecules is matricellular proteins; these are extracellular matrix proteins that regulate cell–matrix interactions and cell functions, and they do not seem to have a direct structural role. Their expression is high during embryogenesis, but almost absent during normal postnatal life; however, they are re-expressed in response to injury in adult tissue.
Tenascin-C (TNC) is a member of the matricellular protein family. This protein is expressed in a rigidly controlled temporo-spatial pattern in the developing fetus, yet is undetectable or found only in low levels in the corresponding regions of the intact adult organs (for general discussion, see Midwood & Orend [1]). Multiple functions have been assigned to TNC based on in vitro studies, which may or may not truly reflect the activity of this protein in vivo.
We have previously shown that TNC is expressed in human vein grafts [2] and balloon catheterization induces arterial expression of new TNC isoforms [3, 4]. Further we showed that TNC is expressed in macrophage-rich regions of human atherosclerotic plaque [5]. Others have reported that TNC is an essential factor for neointimal hyperplasia after aortotomy [6]. Using cultured cells, we showed that factors which are important in neointima formation, such as angiotensin II [7] and PDGF-BB [8], regulate the expression of TNC in vascular smooth muscle cells (SMCs). To understand the mechanism of TNC activity in vitro, we cloned various domains of this molecule and showed that TNC interacts with SMCs through its fibrinogen-like domain and that this domain is important for the migration of cultured SMCs [9]. In addition, we reported that the epidermal growth factor domain of TNC induces apoptosis of SMCs [10]. Despite these findings the precise role of TNC in atherosclerosis in vivo remains unclear. Based on the expression pattern of TNC in vivo and in vitro, and activity of TNC in vitro we hypothesized that TNC expression may affect atherosclerosis. To explore this idea, we generated double knockout mice lacking TNC and apo E (TNC−/−/apo E−/−) and assessed the quantity and phenotype of atherosclerotic lesions in these mice, by comparing them to apo E−/− mice. We found that deletion of TNC gene accelerated lesion development in apo E−/− mice in the aortic sinuses, the brachiocephalic artery, and the whole aorta. Remarkably, we found frequent gross and large areas of intraplaque hemorrhage in TNC−/−/apo E−/− mice which were absent in apo E−/− mice. Furthermore, we noted that absence of TNC gene led to rapid upregulation of VCAM-1 in vivo which correlated with increased content of lesional macrophages, as compared to apo E−/− mice. In vitro studies supported a role for tenascin-C in regulating interaction of monocytes with endothelial cells. Therefore, our data suggest that TNC expression may have a protective role in atherosclerosis in part by controlling plaque inflammation.
Methods
2.1. Preparation of animals
Generation of double knockout mice— Apo E−/− mice were bred with TNC−/− mice (in C57BL/6 background) to generate double knockout mice and their littermate controls according to a protocol approved by the Cedars-Sinai Institutional Animal Care and Use Committee and this specific study was also approved by the Committee. TNC null mice were obtained from Dr. Kusakabi who generated the mice by TNC gene targeting in murine C57BL/6 ES cells [11]. Male apo E−/− mice (C57BL/6 background) were purchased from Jackson Labs and crossed with female TNC null mice. The apo E and TNC expression were identified by PCR using genomic DNA extracted from tail biopsies. The normal and mutant TNC alleles were identified by PCR using three primers in a single PCR reaction. The TNC upstream primer (TNUP; 5′-CTGCCAGGCATCTTTCTAGC-3′) and downstream primer (TNDN; 5′-TTCTGCAGGTTGGAGGCAAC-3′) bind to sequences in exon 2 of the mouse TNC gene and amplify a 420 bp long DNA fragment that is specific for the TNC wild type allele. A third primer binds to sequences in the neo gene (NEOPA; 5′-CTGCTCTTTACTGAAGGCTC-3′) and together with the TNDN primer amplifies a 340 bp fragment, which is specific for the mutant TNC allele. For the apo E gene, we used 3 primers: MR0180 (5′-GCC TAG CCG AGG GAG AGC CG-3′), MR0181 (5′-TGT GAC TTG GGA GCT CTG CAG C-3′), and MR0182(5′-GCC GCC CCG ACT GCA TCT-3′). The MR0180 and MR0181 primers amplify a 155 bp wild type band, and MR0180 and MR0182 primers amplify a 245 bp band from the targeted allele. The offspring were divided into subgroups based upon their sex (male/female) and their apo E and TNC genotypes. This initial genotyping was confirmed with Southern blotting. F1 mice were inter-bred and genotyped in order to obtain homozygous TNC−/−/apo E−/− double knockout mice.
2.2 Atherogenic diet feeding
Each mouse genotype was randomly divided into several groups of 10 mice and each group was fed a high fat diet at 6 weeks of age, with water taken ad libitum. Mean body weights of 6 weeks-old apo E −/− and TNC−/−/apo E−/− mice were similar (16±4 g for female and 19±5 g for male) and their weights did not significantly differ on the high fat diet for 18 weeks. For the high fat diet studies, the two mouse genotypes were placed on high fat diet (Western diet, Harlan), at 6 weeks of age for various times as indicated in the manuscript (10 mice/genotype/time point). After anesthetizing each group of mice with isoflurane, blood samples were collected by retro-orbital bleeding for the analysis of their plasma lipoprotein profiles. Terminal blood samples were collected by puncture of the right ventricle following euthanasia by an isoflurane overdose; heparinized tubes were used for blood collection. before centrifugation. Mice were perfused with PBS (20 ml.) via the left ventricle, while the perfusate drained from the punctured right atria. The heart and ascending aorta to the iliac bifurcation were removed. The heart was separated from the aorta at the base, embedded in OCT compound, and stored at −20°C. The aortic tissue was placed in freshly prepared paraformaldehyde (4% [wt/vol] in PBS) overnight at room temperature. After tissue fixation, the adventitial tissue was removed, and the luminal surface was exposed. The aortas were pinned to a dark surface, and images were captured by using a Spot digital camera (Diagnostic Instruments).
2.3. Preparation of tissues
The thoracic cavity was opened; the aortic arch as well as the aorta were exposed, and photographed under a dissecting microscope. After capturing the images, most of the vascular tree was dissected intact. The intact vascular tree of each mouse was quickly frozen in ethanol with dry ice, and mounted in OCT compound. Frozen sections were cut using a Leica cryostat and kept in a −70°C freezer for less than 1 month before use.
2.4. Atherosclerotic Lesion Analysis
The extent of the atherosclerotic lesions in the Oil red O-stained aorta were quantified, as described previously [12]. Regions of each aorta quantified were defined as follows: (1) thorax, from the arch to the intercostal artery branch; and (2) abdominal region, from the thorax to the branch of the iliac bifurcation. The lesion size for each aorta was measured by Image-Pro Plus (version 4, Media Cybernetics). Lesions were reported as percentage of the total aortic area consisting of thoracic aorta (ending at the final intercostal artery), and abdominal aorta (ending at the iliac bifurcation). The frozen tissues were serially sectioned into 10 μm sections from the aortic arch and aortic sinus. Each figure is representative of lesions in the two mouse genotypes.
2.5. Lipid and Lipoprotein Analysis
Serum cholesterol and triglyceride concentrations were measured by an enzymatic colorimetric assay (Thermo DMA, Sigma). Lipoprotein cholesterol distribution was determined in individual serum samples (50 μL) from 5 mice in each group after fractionation on a single Superose 6 column. Fractions were collected, and cholesterol concentrations were determined by an enzymatic colorimetric assay (Sigma), as described by the manufacturer.
2.6. Immunocytochemistry
Histochemical staining and immunostaining were performed essentially as described before [13]. Movat’s staining was used to analyze all connective tissue elements in a single section (SMCs=red, elastic lamina=dark blue-black, proteoglycan=blue-green). ICAM-1 and VCAM-1 staining were performed using 1:100 dilution of rat anti-ICAM-1 or rat anti-VCAM-1 (both from Bioscience) primary antibodies. Endothelial cells and macrophages were stained with anti-CD31antibodies and MOMA-2 antibodies, respectively. T cells and neutrophil staining were performed using In addition to macrophages, we determined the presence of T cells in the plaques by immunostaining using anti-CD4, anti-CD5, anti-CD8, and anti-CD90.2 antibodies. The presence of neutrophil in the plaques was assessed by anti-6G (LY6G) antibody.
2.7. Cell culture
The aortas were dissected from 4–6 weeks old mice and after removal of any residual fat, the vessels were cut into small segments and were placed on a tissue culture dish that had been covered with matrigel in a endothelial cell medium. The endothelial cells were passaged using collagen-coated culture dishes.
2.8. Monocyte adhesion assay
A monocyte adhesion assay was performed using a mouse monocytic cell line (RAW264.7). RAW cells were cultured in DMEM plus 10% heat-inactivated fetal bovine serum. Endothelial cells (passages 3–5) from TNC−/−/apoE−/− or apo E−/− mice were cultured to confluency in a 48-well plate. RAW cells were labeled with calcein-AM using standard methods described by the manufacturer (Molecular Probes). For the adhesion assay, endothelial cells were incubated with 35,000 calcein-labeled RAW cells/well for 30 mins. at 37°C. Nonadherent cells were rinsed, and adherent cells were fixed with 1% glutaraldehyde. The number of adherent monocytes within a 10 x 10 eyepiece grid at x40 magnification was counted using epifluorescence microscopy. The average of 4 fields was calculated. In some cases cells were treated with 2.5 units/ml recombinant murine TNFα (R&D Systems) for 4 hrs prior to the addition of monocytic cells.
2.9. Statistical Analysis
Statistical analysis was performed by Cedars-Sinai Statistical Analysis Group using SAS software version 9.3 (SAS Institute, Cary, North Carolina). The 2-way ANOVA Model P-value: p < 0.0001.
3. Results
3.1. TNC is expressed in atherosclerotic plaques of apo E−/− mice
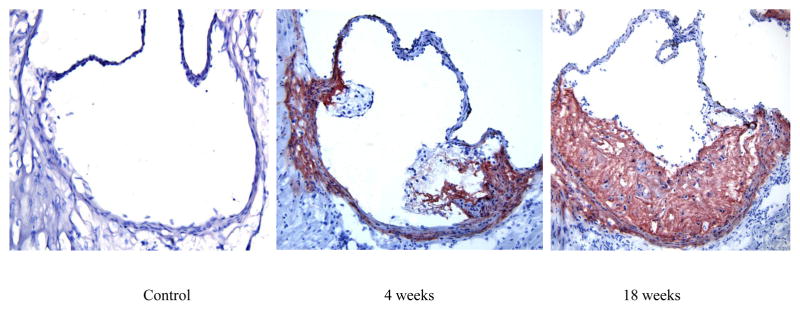
While we have previously shown that TNC is expressed in human plaque [5], nothing is known about the expression of this protein in mouse lesions. Therefore, as a first step to understand the role of TNC in atherosclerosis, we asked whether TNC is expressed in mouse plaque. Frozen sections from the aortic sinuses of apo E−/− mice on atherogenic diet for the indicated times were stained with an anti-TNC antibody (Fig. 1). While TNC expression was not detected in the aortic sinuses of mice fed a normal diet (Fig. 1A), a robust expression of TNC protein was detected in the mice fed atherogenic diet for 8 weeks (panel B) and the level of expression appeared to increase with the growth of the lesion (Panel C). The TNC expression was found throughout neointima and media, and some expression was also detected in the adventitia. These results combined with our human data suggest that TNC expression is a conserved feature of plaques implying that this protein may play a role in the pathogenesis of atherosclerosis.
Fig. 1. TNC expression in atherosclerotic plaques.
Apo E−/− mice were placed on a chow diet (panel A) or on a high fat diet for the indicated times. The mice were euthanized and their aortic sinuses were collected, snap-frozen, embedded in OCT. Frozen sections (6 μm) were used for immunostaining using anti-mouse TNC antibodies (1:200 dilution). Aortic sinus sections from TNC−/−/apo E−/− mice were used as a negative control (not shown). Original magnification 20x.
3.2. Deletion of TNC gene had no effect on lipoprotein profile in apo E−/− mice
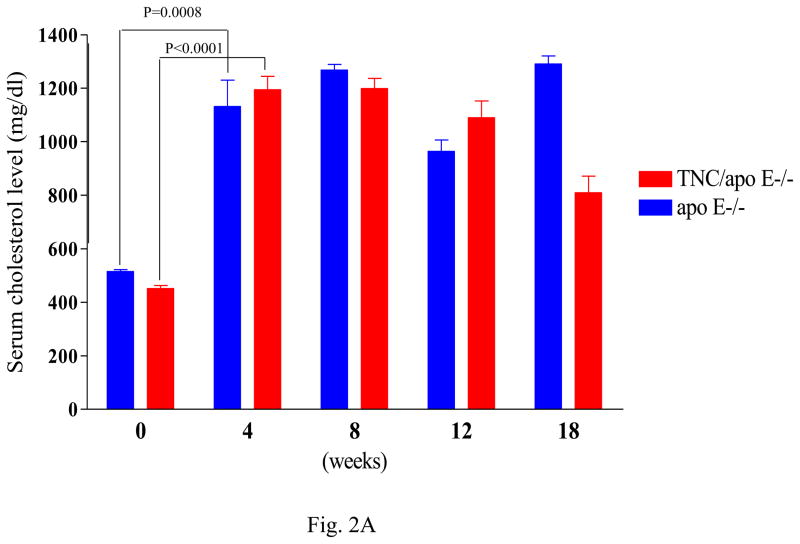
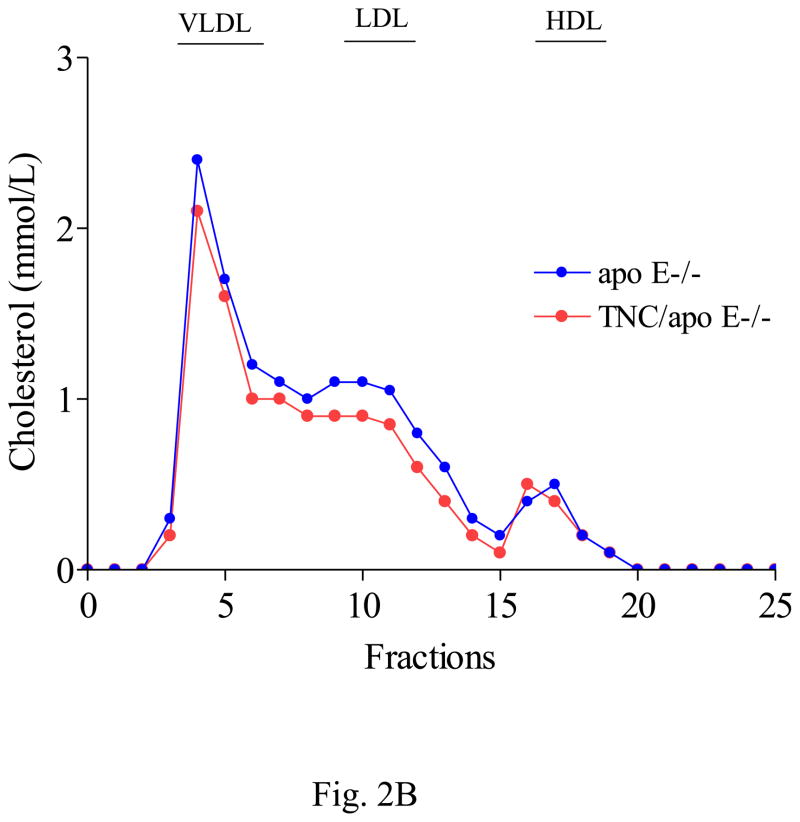
To gain insight into the role of TNC in atherosclerosis, we generated TNC−/−/apo E−/− mice and analyzed the extent and phenotype of atherosclerosis in these mice by comparing them to apo E−/− mice (control). Average body weight did not differ between the two mouse genotypes while on regular chow or an atherogenic diet. Different groups of mice were fed an atherogenic diet beginning at 6 weeks of age and were sacrificed at 4 weeks, 8 weeks, 12 weeks or 18 weeks later. To determine whether the absence of TNC gene affected the lipoprotein profile of apo E−/− mice, we compared the baseline plasma cholesterol levels between the two mouse genotypes; this showed there was no significant difference between them (Fig. 2A). There was no significant difference in the mean base-line plasma cholesterol levels between the two mouse geneotypes; however, after 4 weeks on atherogenic diet there was significant increase in the plasma cholesterol levels in the two mouse genotypes (Fig. 2A). These plasma cholesterol levels remained high and comparable when the mice were maintained on high fat diet for 18 weeks. Similarly, no significant difference in the lipoprotein profile was found between the two mouse genotypes (Fig 2B). The majority of plasma cholesterol was found in the VLDL fraction with no difference in the profile of LDL and HDL between the control and TNC−/−/apo E−/− mice (Fig. 2B). Likewise, the serum triglyceride levels remained comparable between the two mouse genotypes (Table 1). Likewise, glucose levels were similar (Table 1). These data show that deletion of TNC gene has no effect on the plasma cholesterol, triglyceride, and glucose levels in apo E −/− mice.
Fig. 2. Effect of TNC gene deletion on the lipoprotein profile of serum from apo E−/− mice.
Plasma were collected from the two mouse genotypes at the indicated times. Serum cholesterol (panel A) were measured by an enzymatic colorimetric assay (Thermo DMA, Sigma). Panel B shows FPLC analysis of lipoprotein profile in individual plasma samples (50 μL) from 5 mice/genotype in each group after fractionation on a single Superose 6 column. Most cholesterol was found in the VLDL fractions.
Table 1.
Plasma triglyceride
| Apo E | TNC/apo E |
|---|---|
| 175±150 mg/dl | 165±176 mg/dl |
| 4 weeks on HFD, 269 ± 154 mg/dl | 4 weeks on HFD, 265 ± 162 mg/dl |
| Plasma glucose | |
|---|---|
| 182±30 mg/dl, HFD 4 weeks | 145±45 mg/dl, HFD 4 weeks |
| 123±66 mg/dl, HFD 12 weeks | 120±30 mg/dl, HFD 12 weeks |
3.3. Effect of TNC gene deletion on atherosclerosis between the two mouse genotypes
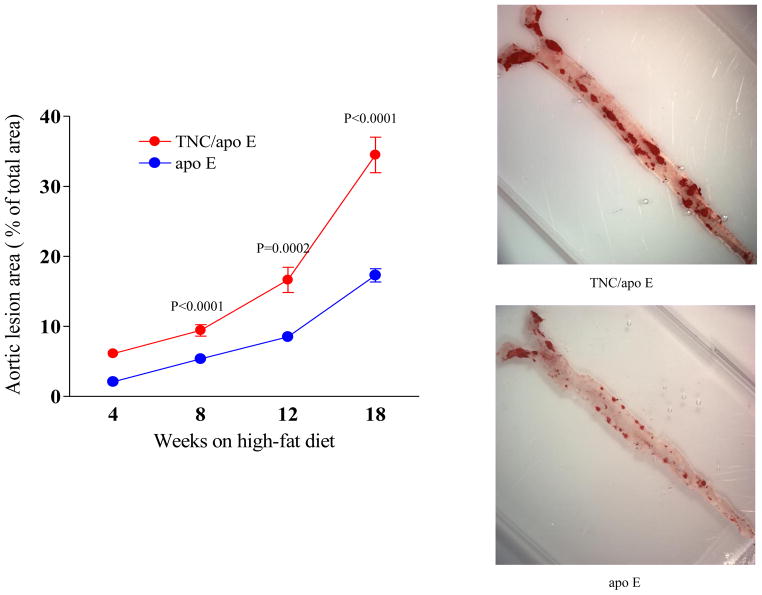
Since modulators of atherosclerosis and genetic backgrounds are known to differentially affect atherosclerosis in various vascular beds [14–16], we investigated the effect of TNC gene deletion on atherosclerosis in the mouse aorta, aortic sinuses, and brachiocephalic artery sites in the two mouse genotypes on an atherogenic diet for the indicated times. Compared to the control apo E mice group, the en face analysis of aortic area shown in Fig. 3 revealed that the deletion of TNC gene in apo E KO mice increased atherosclerosis at the four time points examined, suggesting that lesions develop earlier and their number is higher in the double KO mice.
Fig. 3. En face analysis of atherosclerosis between the two mouse genotypes.
Apo E−/− mice and TNC−/−/apo E−/− mice were fed atherogenic diet for the indicated times and their aortas were harvested and stained with Oil red O. Values are the mean ± SD of 10 mice /group/time point. Statistical significance is calculated at each time points between the two mouse genotypes. The representative staining from each mouse group is also shown.
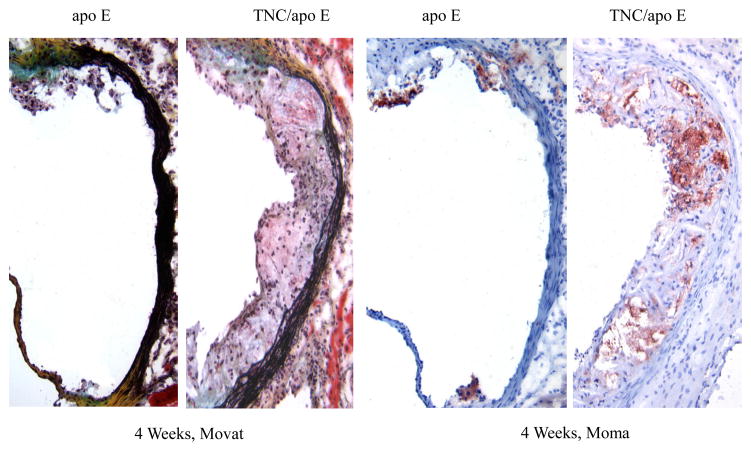
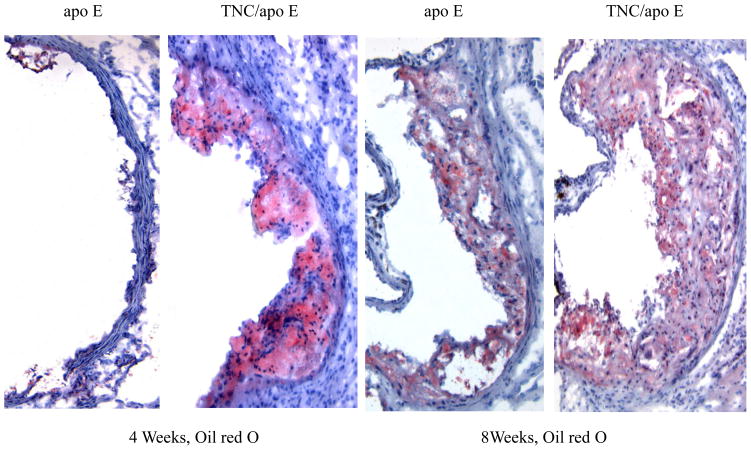
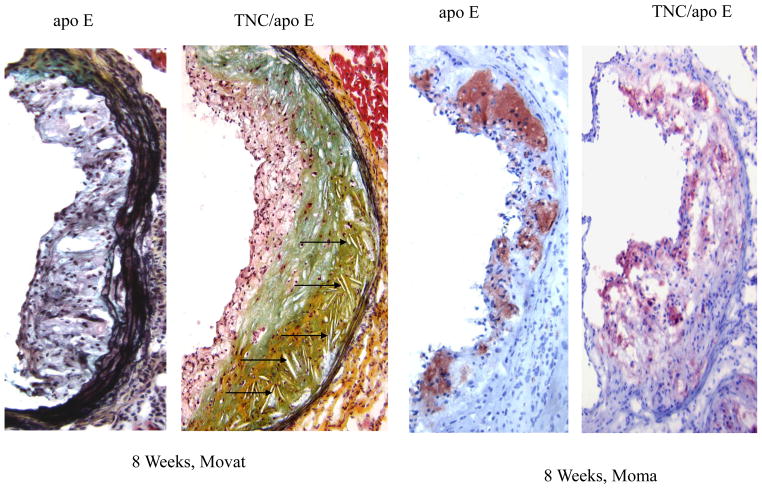
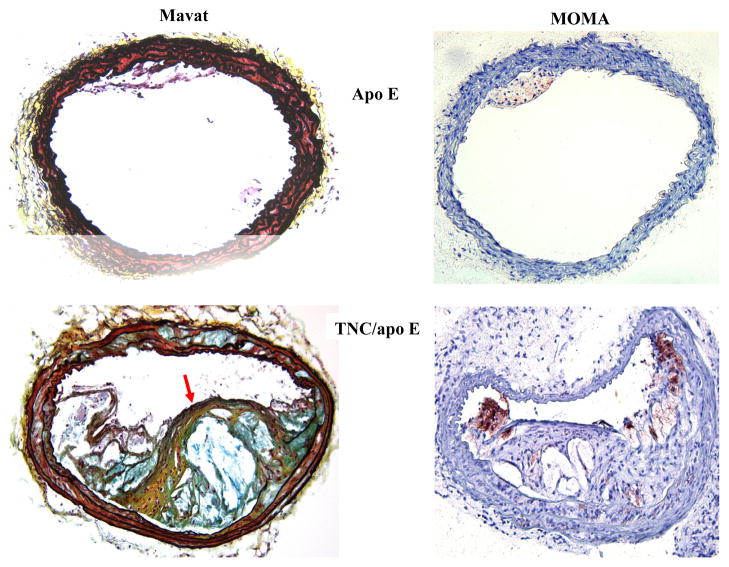
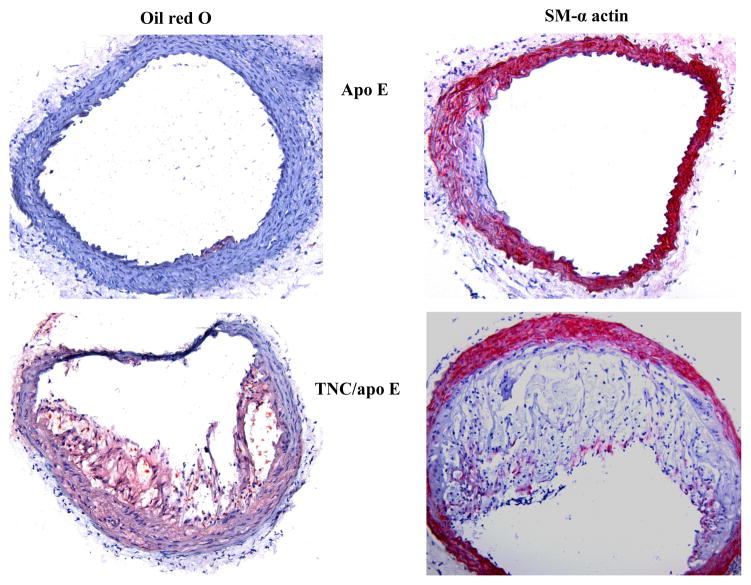
To determine the phenotypes of lesions, frozen sections from the aortic sinuses and brachiocephalic artery were analyzed. Representative images of the staining are shown in Figures 4 and 5. Movat staining of aortic sinuses showed that lesions from TNC−/−/apo E−/− mice on atherogenic diet for 4 weeks contained well defined plaques which were largely cellular with small amount of extracellular matrix (Fig. 4). In contrast, comparable lesions from apo E−/− mice had very small lesions. MOMA-2 staining of the lesions showed significant accumulation of macrophages in the double KO mice compared to the lesions from the control group. Simiraly, Oil red O staining showed that the lesions from the double KO mice contain extreculluar lipids. After 8 weeks on an atherogenic diet, the lesions found in the double KO mice were larger and they displayed the characteristics of an advanced plaque, i.e., the deep hypocellular regions containing largely proteoglycan (greenish color) and cholesterol clefts (arrows) with a hypercellular outer layer containing low levels of proteoglycans (Fig. 4). In contrast lesions from apo E−/− mice were largely hypercellular, and without a significant level of proteoglycans. MOMA-2 staining of sections showed a large number of macrophages were present in the lesions from the double KO mice compared to control group. Similarly, Oil red O staining showed accumulation of lipids in the lesions from the double KO mice compared to small lesions in the control group (Fig. 4). These results are consistent with those observations in aortic lesions (Fig. 3) suggesting that hypercholesterolemia in the absence of TNC gene leads to an early development of lesions in apo E −/− mice.
Fig. 4. Effect of TNC deletion on aortic sinus lesions.
The aortic sinuses frozen sections from the two mouse genotypes on atherogenic diet for 4 and 8 weeks were stained as indicated. Arrows indicate the cholesterol cleft.
Fig. 5. Effect of TNC deletion on brachiocephalic artery lesions.
Frozen sections from apo E−/− mice (upper panels) and TNC−/−/apo E−/− mice (lower panels) fed an atherogenic diet for 4 weeks were stained as indicated.
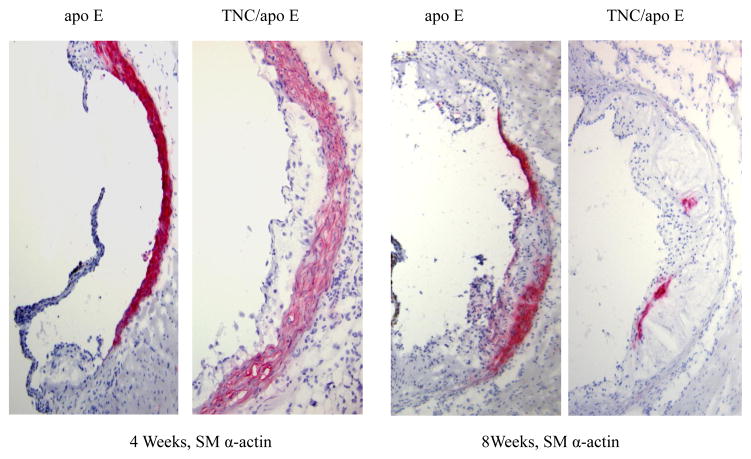
We also compared the expression pattern of SM α-actin staining in the aortic sinus lesions of the two mouse genotypes on atherogenic diet for 4 and 8 weeks (Fig. 4). We noted strong SM α-actin positivity in the tunica media of aortic sinuses in the two mouse genotypes. However, the SM α-actin positivity is significantly reduced in the lesional neointima of both groups. When the atherogenic diet were continued for 12 or 18 weeks, no SM α-actin positivity was detected in either tunica media or tunica intima of either mouse genotype groups (data not shown). These data suggest that TNC deficiency does not seem to affect activity of SMCs in vivo. To gather further evidence regarding the role of TNC in SMC activity, we isolated smooth muscle cells from the two mouse genotypes and their ability to migrate, to grow, and to adhere to extracellular matrix proteins were compared; these cell functions are important in the development of atherosclerotic plaques. We did not observe significant differences in the activities of SMCs isolated from the two mouse genotypes (see supplement).
We found similar results when the brachiocephalic artery lesions were examined. At 4 weeks on atherogenic diet, small lesions were detected in some of the control mice (Fig. 5), whereas significant lesions were detected in most of TNC−/−/apo E−/− mice group that contained well-formed lipid core and fibrous cap (red arrow). The MOMA-2 staining for macrophages showed that the lesions from the double KO mice are complex and they contain macrophages, especially in the shoulder regions. Oil red O staining showed significant accumulation of lipids in the lesions from the double KO. With respect to SM α-actin staining, we basically observed similar results that we described above for aortic sinus lesions (Fig. 4), i.e, the tunica media of brachiocephalic artery lesions were stained for the expression of SM α-actin while their tunica intima either did not stain or weakly/scarcely stained in the two mouse genotype groups on atherogenic diet for 4 and 8 weeks (Fig. 5). At later time points (12 or 18 weeks on atherogenic diet), SM α-actin staining was not detectable in tunica media of either mouse genotypes (data not shown). These results combine with data shown above indicated that deletion of TNC gene fosters a rapid development of lesions in all three anatomic sites in apo E −/− mice.
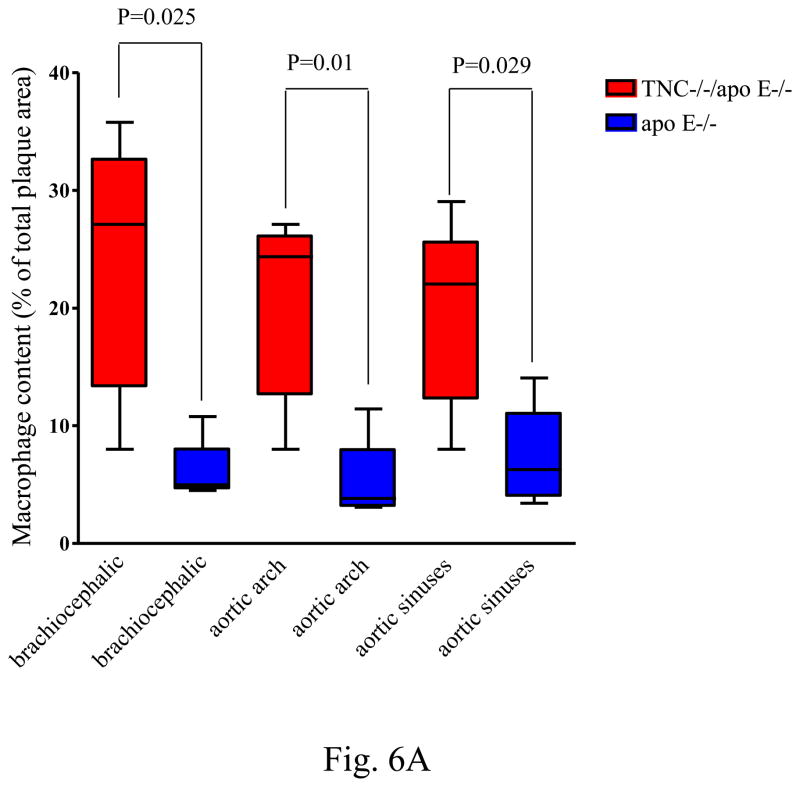
We next quantified the level of macrophages and lipid content of lesions in the aortic sinuses and brachiocephalic artery of the two mouse genotypes on atherogenic diet for 8 weeks. In addition, during the examination of lesions, we noted significant differences between the two genotype groups in the common carotid artery, and subclavian artery; therefore, to provide a comprehensive view of the difference in atherosclerosis between them, we combined the lesions from these two sites with those of brachiocephalic artery, and they were designated as aortic arch lesions. The average macrophage immunoreactive areas from the three anatomic sites in the double KO mice are significantly higher than those from corresponding sites in apo E mice (Fig. 6A).
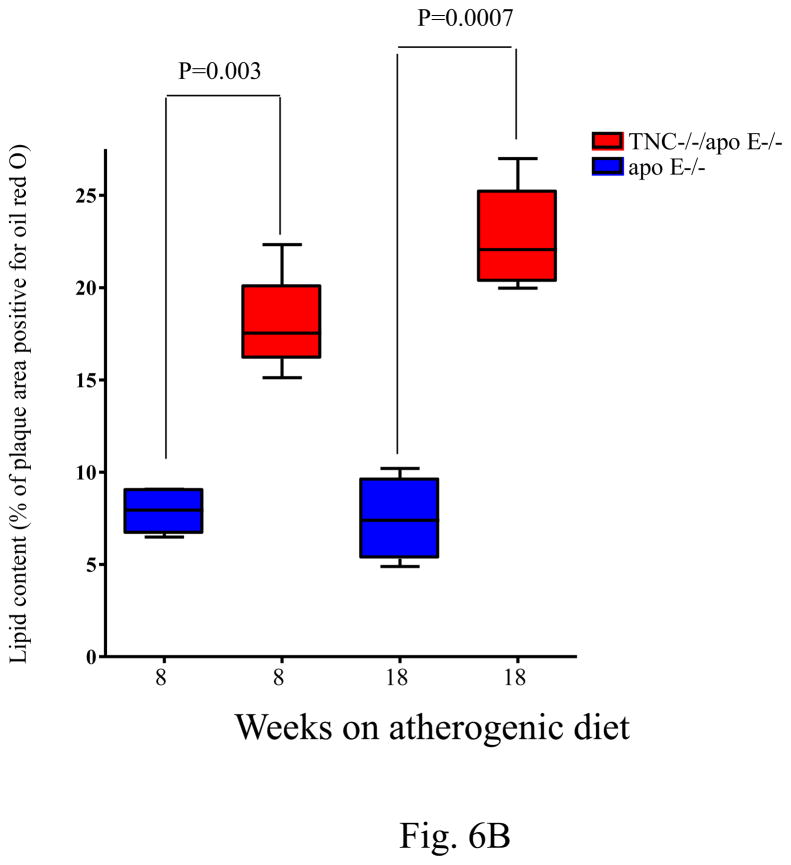
Fig. 6. Quantitative analysis of macrophage and lipid contents of the lesions.
Panel A shows the level of macrophage accumulation in different anatomic sites between the two mouse genotypes on atherogenic diet for 8 weeks. Panel B shows the extracellular accumulation of lipid as determined by Oil red O staining of aortic sinuses of TNC−/−/apo E−/− mice and apo E−/− mice on atherogenic diet for indicated times.
In addition to macrophages, we determined the presence of T cell subsets in these plaques by immunostaining using anti-CD4, anti-CD5, anti-CD8, and anti-CD90.2 antibodies. No significant differences in the lesional staining patterns were found between the two mouse genotypes (data not shown). Similarly, there was no significant difference in the neutrophil content of these lesions when comparing both genotype groups (data not shown). In addition, no differences between the animal groups were detected in the expression of TNFα, IL-1b, IL-6, IL-12, INFγ, MCP-1, CSF-M, and CD40 in the mouse plasma (not shown). We concluded that deletion of TNC gene seems to preferentially affect the accumulation of macrophages in the lesions in apo E−/− mice.
With respect to lipid accumulation, as with en face analysis shown in Fig. 3, we found a significant difference in the lipid content of aortic sinuses lesions between TNC−/−/apo E−/− mice and control groups on atherogenic diet for 8 weeks and 18 weeks (Fig. 6B). We could not accurately measure the lipid content of lesions in aortic arch sections because the lipid cores were dissolved out during the histological processing.
3.4. Expression of cell adhesion molecules
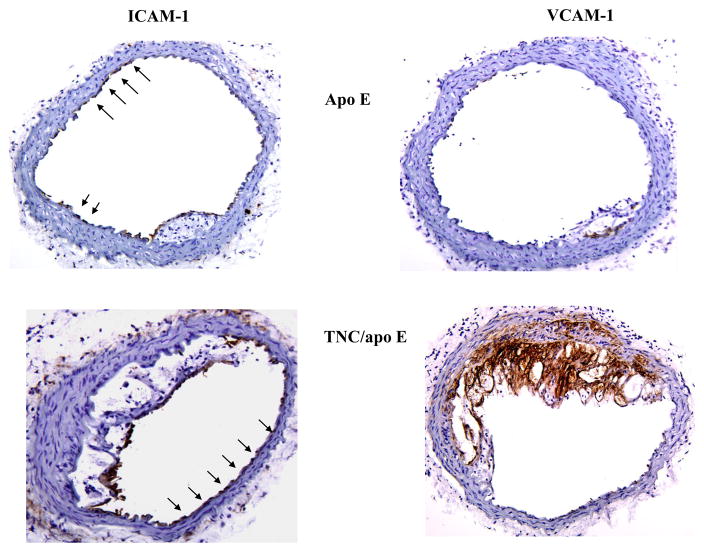
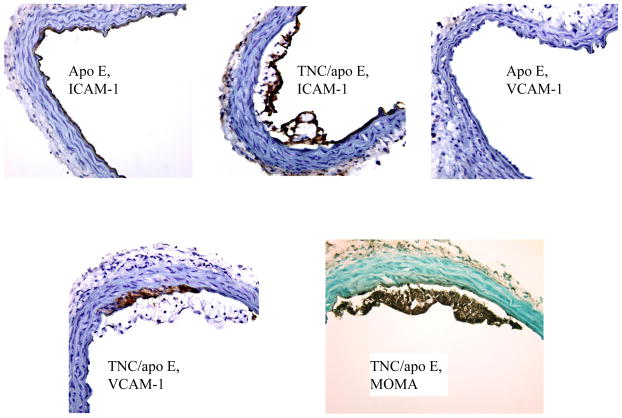
The recruitment of blood monocytes and lymphocytes to the arterial intima is one of the earliest events in the formation of an atherosclerotic lesion and persists even in advanced lesions [17]. We reasoned that accelerated lesion formation and accumulation of macrophages in the absence of TNC gene in apo E−/− mice is in part related to the rapid/enhanced interaction of monocytes with endothelial cells most likely through cell adhesion molecules. Since VCAM-1 is one of the best known adhesion molecules that is believed to be critical in lesion development[18], we determined its expression, along with ICAM-1, in the brachiocephalic artery frozen sections from the two mouse genotypes fed on atherogenic diet for 4 weeks. Immunostaining analysis showed that ICAM-1 is expressed in juxta-luminal cells in both plaque bearing (TNC and apo E double KO) as wells as plaque free (apo E single KO)) regions of arteries (Fig. 7, arrows). In contrast, VCAM-1 expression was found to be concentrated exclusively on the lesion bearing segment of arteries in both mouse groups (Fig. 7).
Fig. 7. Effect of TNC deletion on the expression of cell adhesion molecules in the brachiocephalic artery of two mouse genotype groups fed an atherogenic diet for 4 weeks.
The frozen sections from apo E KO mice (upper panels) and TNC/apo E KO mice (lower panels) were immunostained with antibodies specific to ICAM-I (right panels) or VCAM-1 (left panels). All antibodies were obtained from R&D Systems and diluted prior to use 1:200 (primary) and 1:1000 (secondary).
To further define the expression pattern of cell adhesion molecules in response to hypercholesterolemia in the double KO mice, the frozen sections from the two mouse genotypes on an atherogenic diet for one week were examined (Fig. 8). Again, ICAM-I was found to be uniformly expressed in both mouse genotypes with no obvious lesions (apo E−/− mice) or with lesions (TNC−/−/apo E −/−mice,), While VCAM-1 expression was not detected in sections from apo E−/− mice strong expression of VCAM-1 was detected in lesions from TNC and apo E double KO mice. In addition, we stained serial sections with an anti-MOMA-2 antibody to ask whether this rapid upregulation of VCAM-I contributes to the accumulation of macrophages. As shown in Fig. 8, macrophages accumulated in the VCAM-1-positive areas, suggesting that hypercholesterolemia in combination with TNC gene ablation enhances VCAM-1 expression and macrophage accumulation in apo E −/−mice.
Fig. 8. Expression of cell adhesion molecules in response to atherogenic diet feeding for one week.
Frozen sections from the two mouse genotype groups were stained with anti-ICAM-1 antibody or anti-VCAM-1 antibody. In the right lower panel, serial sections were stained with anti-MOMA-2 antibody to determine presence of macrophages.
3.5. Effect of deletion of TNC gene on the expression of VCAM-1 in cultured cells
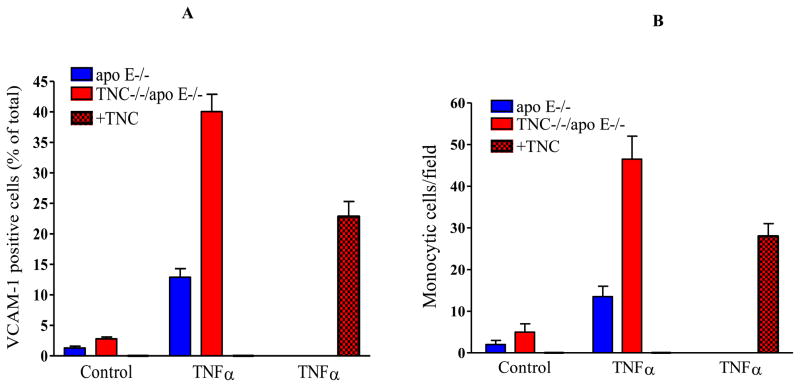
Next, we evaluated the expression of VCAM-1 in the cultured endothelial cells isolated from the two mouse genotypes by flow cytometry. Under basal conditions, VCAM-1 was expressed at a low level in the cells derived from either of the mouse genotypes (Fig. 9A). Following treatment with TNFα (2.5 units/ml), VCAM-1 expression was upregulated significantly in the cells from TNC−/−/apo E−/− group when compared to control group, 40±2.85% versus 12.9±1.4%. To confirm this effect was related to TNC, cells derived from the double KO mice were treated with TNC, 24 hrs. before treatment of cells with TNFα. This reduced VCAM-1 expression by 46%. We wanted to investigate whether this VCAM-1 induction led to an increased interaction of monocytes with endothelial cells. To explore this, RAW cells were labeled with a fluorescent dye and added to TNFα-stimulated cells; the number of adherent cells was determined. While the number of monoctytic cells that attached to the either control group or TNC−/−/apo E−/− group were small in the unstimulated cells, treatment of cells with TNFα increased the number of adherent monocytic cells from 13±3 monocytes/field for control group to 42±4 monocytes/field for cells derived from the double KO mice. The addition of monocytic cells to the endothelial cells that were cultured in the presence of TNC reduced adhesion of monocytic cells by 40% (Fig. 9B). These observations were consistent over three independent experiments. Collectively, these data suggest that while deletion of TNC gene does not seem to significantly affect expression of VCAM-1 and interaction of monocytes with endothelial cells, these events are significantly influenced by the absence and in some cases presence of TNC gene under inflammatory conditions.
Fig. 9. Deletion of TNC gene up-regulates VCAM-1 expression in endothelial cells.
(Panel A) Cultured endothelial cells isolated from the two mouse genotypes were analyzed by FACS using anti-VCAM-1 antibody. The cells were cultured on a collagen substrate and some cells were treated with 2.5 U/ml of TNFα for 4 hr. The cells were harvested and analyzed by FACA using anti-VCAM-1 antibody. (Panel B) The fluorescent-labeled RAW monocytic cells were added to unactivated (control) or TNFα-activated cells for 30 min. After several washes, the number of adherent cells was determined under the microscope. The data is the average of number of cells in 4 viewing fields.
3.6. Deletion of TNC gene did not affect adhesion, migration, and proliferation of SMCs
To gather further evidence for the role of TNC in regulating activity of SMCs, cells were cultured from the two mice genotypes and compared their ability to migrate, to proliferate, and to adhere to matrix proteins that are important in atherosclerosis. No significant effect was found in the activity of SMCs between the two mouse genotypes (see supplement).
3.7. Deficiency of TNC gene induced intraplaque hemorrhage
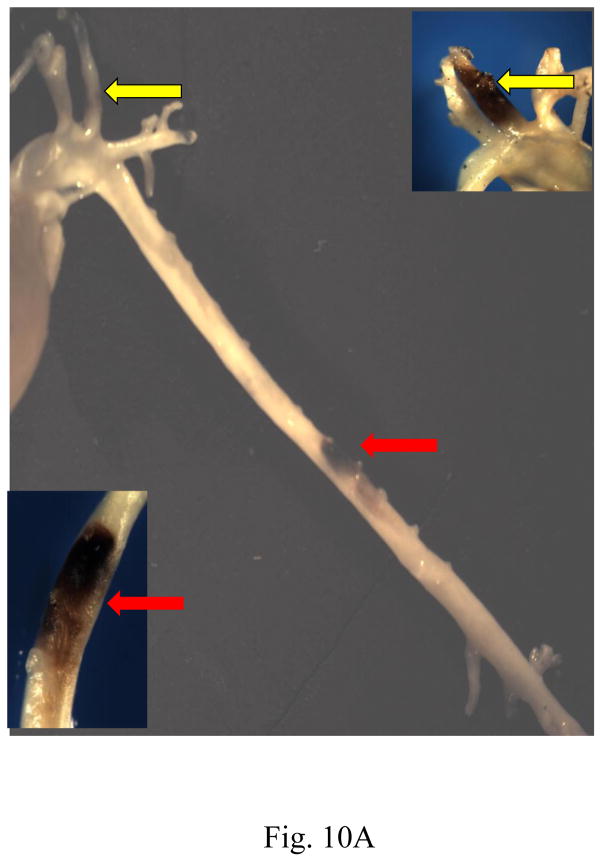
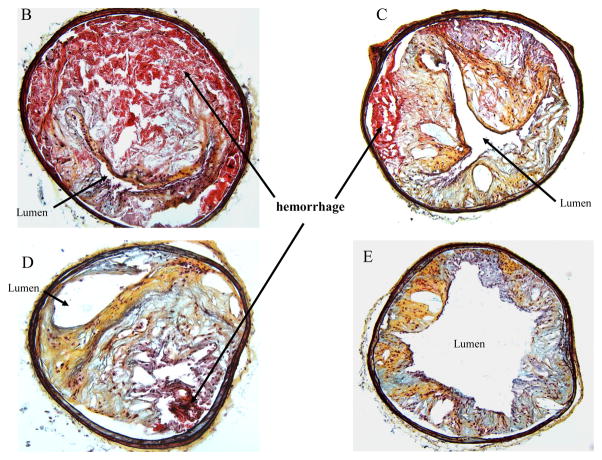
When the atherogenic diet was continued beyond 25 weeks (26–30 weeks), we noted a significant difference in the phenotype of lesions between the two mouse genotypes. In 6 out of 10 mice from the TNC−/−/apo E−/− group, gross analyses of the aortas and arch vessels revealed visibly large areas of hemorrhage in the aorta, carotid arteries, brachiocephalic arteries (Fig. 10A). No such hemorrhage was found in the aortas or arch vessels of apo E−/− mice. Movat staining of vascular segments proximal to large areas of hemorrhage showed that the lumen was almost completely occluded by the plaque and hemorrhage (panel B). As the sections from the areas distal to the hemorrhage site were examined, the plaques contained less intraplaque hemorrhage and the lumen was less occluded (panels C and D, respectively). No obvious rupture of fibrous caps was observed in these sections. In contrast, lesions from apo E−/− mice were relatively non-occlusive throughout aorta (both thoracic and abdominal) and none showed intraplaque hemorrhage under similar experimental conditions (panel E).
Fig. 10. Effect of TNC deletion on intraplaque hemorrhage.
(Panel A), the aorta of a TNC/apo E−/− mouse fed an atherogenic diet for 28 weeks showing visible plaque hemorrhage in carotid artery (yellow arrows) and abdominal aorta (red arrows). Insets shown at 10x magnification. The aortas were harvested, the fatty tissues and outer regions of adventitia around the aortas were removed, and the frozen sections were stained with Movat’s stain. Panels B–D shows sections from distal regions of the hemorrhagic area shown in panel A (red arrow). Panel E shows representative sections from similar regions of thoracic aorta of apo E−/− control group of mice. Hemorrhage areas (red color) are shown by arrows.
Discussion
We have previously reported that TNC, a matricellular protein, is expressed in human atheroma [2, 5], and in the current study we noted that TNC is also expressed in mouse atherosclerotic lesions, suggesting that the expression pattern of TNC is a conserved event in atherosclerosis. We noted TNC gene deletion significantly affected atherosclerosis in the three important anatomical sites (whole aorta, aortic sinuses and brachiocephalic arteries) of apo E−/− mice. The absence of TNC was associated with the rapid development of lesions in apo E−/− mice and the lesions exhibited a complex phenotype, after 4–8 weeks on atherogenic diet. This was demonstrated by the formation of cholesterol clefts and other features that are characteristic of advanced lesions.
We noted that VCAM-1 was rapidly upregulated in the lesions from the double KO mice compared to control groups and immunostaining showed that this expression is associated with the increased interaction of monocytes with endothelial cells, even when the mouse group was fed an atherogenic diet for as little as 1 week. This time course of VCAM-1 expression in mouse in reponse to atherogenic diet is similar to that observed in the rabbit [19]. When the atherogenic diet was continued for 4 weeks, we noted that the lesions in the double KO mice express VCAM- 1 throughout neointima and even in the media. In addition to endothelial cells, VCAM-1 is expressed by SMCs that may facilitate the accumulation of transmigrated leukocytes within the vascular wall [20]. Indeed, in advanced human plaques, VCAM-1 expression is infrequently found on the endothelial cells in the arterial lumen, but it is prevalent in the base of plaques [21, 22]. The VCAM-1 expression on SMCs in the intima and media is most prominent in fibrous plaques and advanced atherosclerotic lesions [23]. Macrophages are also known to express VCAM-1 in the lipid containing plaques [24]. Thus, the expression of VCAM-1 in the neointima of TNC and apo E double KO mice is an indicative of advanced nature of the lesion and most likely it is produced by a combination of cells. The effect of TNC on adhesion and accumulation of macrophages under atherosclerotic conditions seems to be specific to macrophages because we did not detect a significant difference in the accumulation of T cell subsets or neutrophils in the lesion between the two mouse genotypes.
The cell culture studies support our in vivo findings and suggest that the upregulation of VCAM-1 in the double KO mice requires the presence of an inflammatory condition, as cells derived from the two mouse genotypes showed low levels of VCAM-1 expression under a basal condition. The addition of a low dose of TNFα is required to enhance expression of VCAM-1 in cells derived from the two mouse genotypes, albeit, the level of VCAM-1 upregulation by TNFα was significantly higher in the TNC−/−/apo E−/− when compared to apo E null mice.
The underlying mechanism whereby TNC deficiency leads to an accumulation of macrophages in the lesions remains to be determined. The emigration of leukocytes in post-capillary venules and arteries is complex and involves several distinct interactions between leukocytes and endothelial cells [18, 25–28]. These include tethering and rolling, arrest, firm or stable adhesion and transendothelial migration (diapedesis). Each of these sequential steps is mediated by binding and detachment of adhesion molecules that are expressed on leukocytes and endothelium. Thus the enhanced interaction of monocytes with endothelial cells in the absence of TNC most likely is complex and mediated by number of factors affected by TNC gene deletion. This complexity of leukocyte emigration is further exacerbated by the complex nature of interaction of TNC with cells that involves both integrin as well as nonintergrin receptors [1].
We do not know the underlying mechanism by which deletion of TNC affects activity of SMCs in vivo and in vivo. Our data suggest that deletion of TNC has no obvious effect on activity of cells. These data seem to diverge from our own past reports as well as those from other investigators. There are, however, several major differences between our current studies and those past studies reported by us and other investigators. Chief among them is the use of cultured SMCs derived from TNC KO mice. In past studies by us and others, SMCs sources were either from human or wild type animal (porcine, rabbit, and rat). Since these isolated cells all express TNC, the extracellular matrix that is generated by these cells contains endogenous TNC. In such studies, exogenous TNC is generally added to wild type cultured cells and their response was compared to the control wild type cells without addition of exogenous TNC. Therefore, the difference between the two groups is not a complete absence of TNC gene; rather, presence (treated group) or absence (control group) of exogenous TNC. This cell culture system is fundamentally different from our system where SMCs are isolated from TNC KO mice. In our system, TNC expression is completely absent in the TNC KO cell culture system. This means that the interplay between the endogenous and exogenous proteins that are found in the wild type cell culture system is completely absent in the cells derived from TNC KO mice. In addition, unlike growth factors/cytokines that interact with cells as a soluble form, the extracellular matrix proteins such as TNC interact with cells as an insoluble substrate. Since information about the assembly of exogenously added TNC into insoluble extracellular matrix substrate is lacking, the significance of data obtained with the soluble TNC remains unclear.
Another difference between our system and those used in the past is the nature of exogenous TNC added to the cultured cells. The exogenous TNC that has been widely used is purified from human glioblastoma cell line that constitutively produces large quantity of mixture of different TNC isoforms. This source of TNC has several limitations. First, since expression of TNC isoforms is cell/tissue-specific, it follows that SMCs express their own version of TNC and most likely respond to those isoforms. Therefore, the relevance of activity of TNC isoform produced by glioblastoma cells to those of SMCs remains unclear. Second, alternatively spliced TNC mRNA is species-specific, i.e., human cells produce isoforms that are different from those of nonhuman origin. Indeed, murine TNC can use up to 6 alternatively spliced fibronectin type III domains which can independently exerted between the constitutively expressed fibronectin type III domains; theoretically, generating 64 different TNC isoforms [29]. Thus, TNC isoforms that is produced by human cells may exhibit different, possibly irrelevant activity, when used with nonhuman cells. Overall, our current studies suggest that while TNC is primarily expressed by mesenchymal cells in vivo, other cell type such as inflammatory cells may be the likely target. The role of TNC in modulating an inflammatory response seems to be complex, with both pro- and anti-inflammatory roles having been reported for this gene. With respect to the proinflammatory role, T cell activity seems to be stimulated by TNC gene [30] [31] and the expression of TNF-α, IL-6, and IL-8 seems to be upregulated by TNC gene through activation of Toll-like receptor 4 [32]. In addition to these pro-inflammatory activities, anti-inflammatory activities have also been reported for TNC, where this gene was found to inhibit T cell activation [33], to stimulate expression of anti-inflammatory cytokines such as IL-4 [34] and IL-13 [35], and to interfere with leukotriene B4-induced chemotaxis of PMNs [36]. With respect to the effect of TNC gene on activity of macrophages, an effect that is relevant to our observation, TNC is reported to inhibit monocyte chemotaxis [36] and the stromal compartment of TNC KO mice contains significantly more monocytes/macrophages than tumor stroma from wild-type mice [37]. These results are consistent with our findings as we have observed increased monocyte-endothelial interaction and trafficking in the absence of TNC gene. Our results also support a suggestion that the rapid production of TNC in inflamed tissues is part of a mechanism that controls the spread of inflammation [38].
One of the consequences of complex interaction of TNC with cells under an inflammatory condition seems to be the development of intraplaque hemorrhage. When the atherogenic diet was continued for 28–30 weeks, we noted that 60% of TNC−/−/apo E−/− mice demonstrated a complex phenotype including extensive intraplaque hemorrhage in any or all of the three key anatomical sites (the aorta, brachiocephalic artery or the carotid artery) whereas none of the apo E−/− mice showed intraplaque hemorrhage. The mechanism for development of intraplaque hemorrhage that we observed in the double KO mice remains to be determined. We were unable to verify presence of rupture of the fibrous cap in vascular segments proximal or distal to the areas of hemorrhage but we were also unable to examine sections within the central areas of hemorrhagic regions because of their fragile/friable nature. As seen in the proximal regions of hemorrhage areas, the lumen was nearly occluded and consequently these lesions could not be fixed through perfusion. Thus, we cannot completely exclude the possibility that the intraplaque blood may at least in part have originated from the lumen as a result of rupture/fissure of fibrous cap. Alternatively, the intraplaque hemorrhage may have originated from the plaque microvessels. This concept is based on the extensive data linking TNC and angiogenesis in ischemic tissues and in tumors [39–45]. However, like other investigators [46–52], we were unable to detect plaque vascularization. The reason for this is unclear but it may be related to the nature of mouse plaque or the small caliber of intraplaque capillaries that makes detection very difficult.
In summary, the deletion of the TNC gene in apo E−/− mice was found to accelerate lesion development; a unique feature being the frequent and extensive development of intraplaque hemorrhage. Mechanistic studies suggest that while SMCs may not be the target of TNC activity in vivo, activities of endothelial cells and inflammatory cells are influenced by this gene in vivo. These findings represent the first evidence that TNC may have an athero-protective effect and that the modulation of TNC expression may be an attractive target for the modulation of atherosclerosis.
Supplementary Material
Acknowledgments
This work was supported by grants from National Institute of Health ROI HL50566, The Eisner Foundation, The Heart Foundation, Spielberg Cardiovascular Research Fund, and the Corday Foundation, Sam Spaulding Fund and The California Agricultural Research Initiative grant # 03-4-089. BGS is established investigator of American Heart Association. We thank Ian Williamson for help in preparing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Midwood KS, Orend G. The role of tenascin-C in tissue injury and tumorigenesis. J Cell Commun Signal. 2009 doi: 10.1007/s12079-009-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallner K, Li C, Fishbein MC, Shah PK, Sharifi BG. Arterialization of human vein grafts is associated with tenascin-C expression. J Am Coll Cardiol. 1999;34:871–5. doi: 10.1016/s0735-1097(99)00272-7. [DOI] [PubMed] [Google Scholar]
- 3.Wallner K, Shah PK, Sharifi BG. Balloon catheterization induces arterial expression of new Tenascin-C isoform. Atherosclerosis. 2002;161:75–83. doi: 10.1016/s0021-9150(01)00627-x. [DOI] [PubMed] [Google Scholar]
- 4.Wallner K, Sharifi BG, Shah PK, Noguchi S, DeLeon H, Wilcox JN. Adventitial remodeling after angioplasty is associated with expression of tenascin mRNA by adventitial myofibroblasts. J Am Coll Cardiol. 2001;37:655–61. doi: 10.1016/s0735-1097(00)01117-7. [DOI] [PubMed] [Google Scholar]
- 5.Wallner K, Li C, Shah PK, Fishbein MC, Forrester JS, Kaul S, et al. Tenascin-C Is Expressed in Macrophage-Rich Human Coronary Atherosclerotic Plaque. Circulation. 1999;99:1284–9. doi: 10.1161/01.cir.99.10.1284. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto K, Onoda K, Sawada Y, Fujinaga K, Imanaka-Yoshida K, Shimpo H, et al. Tenascin-C is an essential factor for neointimal hyperplasia after aortotomy in mice. Cardiovasc Res. 2005;65:737–42. doi: 10.1016/j.cardiores.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Sharifi BG, LaFleur DW, Pirola CJ, Forrester JS, Fagin JA. Angiotensin II regulates tenascin gene expression in vascular smooth muscle cells. J Biol Chem. 1992;267:23910–5. [PubMed] [Google Scholar]
- 8.LaFleur DW, Fagin JA, Forrester JS, Rubin SA, Sharifi BG. Cloning and characterization of alternatively spliced isoforms of rat tenascin. Platelet-derived growth factor-BB markedly stimulates expression of spliced variants of tenascin mRNA in arterial smooth muscle cells. J Biol Chem. 1994;269:20757–63. [PubMed] [Google Scholar]
- 9.LaFleur DW, Chiang J, Fagin JA, Schwartz SM, Shah PK, Wallner K, et al. Aortic smooth muscle cells interact with tenascin-C through its fibrinogen-like domain. J Biol Chem. 1997;272:32798–803. doi: 10.1074/jbc.272.52.32798. [DOI] [PubMed] [Google Scholar]
- 10.Wallner K, Li C, Shah PK, Wu K-J, Schwartz SM, Sharifi BG. EGF-Like Domain of Tenascin-C Is Proapoptotic for Cultured Smooth Muscle Cells. Arterioscler Thromb Vasc Biol. 2004;24:1416–21. doi: 10.1161/01.ATV.0000134299.89599.53. [DOI] [PubMed] [Google Scholar]
- 11.Steindler DA, Settles D, Erickson HP, Laywell ED, Yoshiki A, Faissner A, et al. Tenascin knockout mice: barrels, boundary molecules, and glial scars. J Neurosci. 1995;15:1971–83. doi: 10.1523/JNEUROSCI.15-03-01971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–8. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 13.Song L, Wang L, Shah PK, Chaux A, Sharifi BG. Bioengineered vascular graft grown in the mouse peritoneal cavity. J Vasc Surg. 2010 doi: 10.1016/j.jvs.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.VanderLaan PA, Reardon CA, Getz GS. Site Specificity of Atherosclerosis: Site-Selective Responses to Atherosclerotic Modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 15.Reardon CA, Blachowicz L, White T, Cabana V, Wang Y, Lukens J, et al. Effect of immune deficiency on lipoproteins and atherosclerosis in male apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1011–6. doi: 10.1161/01.atv.21.6.1011. [DOI] [PubMed] [Google Scholar]
- 16.Reardon CA, Blachowicz L, Lukens J, Nissenbaum M, Getz GS. Genetic Background Selectively Influences Innominate Artery Atherosclerosis: Immune System Deficiency as a Probe. Arterioscler Thromb Vasc Biol. 2003;23:1449–54. doi: 10.1161/01.ATV.0000079793.58054.2E. [DOI] [PubMed] [Google Scholar]
- 17.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 18.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842–51. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Cybulsky MI, Gimbrone MA, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arteriosclerosis, Thrombosis, and Vascular Biology. 1993;13:197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- 20.Libby P, Li H. Vascular cell adhesion molecule-1 and smooth muscle cell activation during atherogenesis. The Journal of Clinical Investigation. 1993;92:538–9. doi: 10.1172/JCI116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien KD, McDonald TO, Chait A, Allen MD, Alpers CE. Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation. 1996;93:672–82. doi: 10.1161/01.cir.93.4.672. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien KD, Allen MD, McDonald TO, Chait A, Harlan JM, Fishbein D, et al. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993;92:945–51. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasper HU, Schmidt A, Roessner A. Expression of the adhesion molecules ICAM, VCAM, and ELAM in the arteriosclerotic plaque. Gen Diagn Pathol. 1996;141:289–94. [PubMed] [Google Scholar]
- 24.Davies MJ, Gordon JL, Gearing AJH, Pigott R, Woolf N, Katz D, et al. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. The Journal of Pathology. 1993;171:223–9. doi: 10.1002/path.1711710311. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Cybulsky MI, Gimbrone MA, Jr, Libby P. Inducible expression of vascular cell adhesion molecule-1 by vascular smooth muscle cells in vitro and within rabbit atheroma. Am J Pathol. 1993;143:1551–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Collins RG, Velji R, Guevara NV, Hicks MJ, Chan L, Beaudet AL. P-Selectin or Intercellular Adhesion Molecule (Icam)-1 Deficiency Substantially Protects against Atherosclerosis in Apolipoprotein E†“Deficient Mice. The Journal of Experimental Medicine. 2000;191:189–94. doi: 10.1084/jem.191.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aiello RJ, Bourassa P-AK, Lindsey S, Weng W, Natoli E, Rollins BJ, et al. Monocyte Chemoattractant Protein-1 Accelerates Atherosclerosis in Apolipoprotein E-Deficient Mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19:1518–25. doi: 10.1161/01.atv.19.6.1518. [DOI] [PubMed] [Google Scholar]
- 28.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joester A, Faissner A. Evidence for Combinatorial Variability of Tenascin-C Isoforms and Developmental Regulation in the Mouse Central Nervous System. Journal of Biological Chemistry. 1999;274:17144–51. doi: 10.1074/jbc.274.24.17144. [DOI] [PubMed] [Google Scholar]
- 30.Nakahara H, Gabazza EC, Fujimoto H, Nishii Y, D’Alessandro-Gabazza CN, Bruno NE, et al. Deficiency of tenascin C attenuates allergen-induced bronchial asthma in the mouse. Eur J Immunol. 2006;36:3334–45. doi: 10.1002/eji.200636271. [DOI] [PubMed] [Google Scholar]
- 31.El-Karef A, Yoshida T, Gabazza EC, Nishioka T, Inada H, Sakakura T, et al. Deficiency of tenascin-C attenuates liver fibrosis in immune-mediated chronic hepatitis in mice. J Pathol. 2007;211:86–94. doi: 10.1002/path.2099. [DOI] [PubMed] [Google Scholar]
- 32.Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15:774–80. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 33.Ruegg CR, Chiquet-Ehrismann R, Alkan SS. Tenascin, an extracellular matrix protein, exerts immunomodulatory activities. Proc Natl Acad Sci U S A. 1989;86:7437–41. doi: 10.1073/pnas.86.19.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makhluf HA, Stepniakowska J, Hoffman S, Smith E, LeRoy EC, Trojanowska M. IL-4 upregulates tenascin synthesis in scleroderma and healthy skin fibroblasts. J Invest Dermatol. 1996;107:856–9. doi: 10.1111/1523-1747.ep12331160. [DOI] [PubMed] [Google Scholar]
- 35.Phipps S, Ying S, Wangoo A, Ong YE, Levi-Schaffer F, Kay AB. The relationship between allergen-induced tissue eosinophilia and markers of repair and remodeling in human atopic skin. J Immunol. 2002;169:4604–12. doi: 10.4049/jimmunol.169.8.4604. [DOI] [PubMed] [Google Scholar]
- 36.Loike JD, Cao L, Budhu S, Hoffman S, Silverstein SC. Blockade of alpha 5 beta 1 integrins reverses the inhibitory effect of tenascin on chemotaxis of human monocytes and polymorphonuclear leukocytes through three-dimensional gels of extracellular matrix proteins. J Immunol. 2001;166:7534–42. doi: 10.4049/jimmunol.166.12.7534. [DOI] [PubMed] [Google Scholar]
- 37.Talts JF, Wirl G, Dictor M, Muller WJ, Fassler R. Tenascin-C modulates tumor stroma and monocyte/macrophage recruitment but not tumor growth or metastasis in a mouse strain with spontaneous mammary cancer. J Cell Sci. 1999;112 ( Pt 12):1855–64. doi: 10.1242/jcs.112.12.1855. [DOI] [PubMed] [Google Scholar]
- 38.Chiquet-Ehrismann R, Chiquet M. Tenascins: regulation and putative functions during pathological stress. J Pathol. 2003;200:488–99. doi: 10.1002/path.1415. [DOI] [PubMed] [Google Scholar]
- 39.Ballard VL, Sharma A, Duignan I, Holm JM, Chin A, Choi R, et al. Vascular tenascin-C regulates cardiac endothelial phenotype and neovascularization. Faseb J. 2006;20:717–9. doi: 10.1096/fj.05-5131fje. [DOI] [PubMed] [Google Scholar]
- 40.Jallo GI, Friedlander DR, Kelly PJ, Wisoff JH, Grumet M, Zagzag D. Tenascin-C expression in the cyst wall and fluid of human brain tumors correlates with angiogenesis. Neurosurgery. 1997;41:1052–9. doi: 10.1097/00006123-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Castellon R, Caballero S, Hamdi HK, Atilano SR, Aoki AM, Tarnuzzer RW, et al. Effects of tenascin-C on normal and diabetic retinal endothelial cells in culture. Invest Ophthalmol Vis Sci. 2002;43:2758–66. [PubMed] [Google Scholar]
- 42.Paik DC, Fu C, Bhattacharya J, Tilson MD. Ongoing angiogenesis in blood vessels of the abdominal aortic aneurysm. Exp Mol Med. 2004;36:524–33. doi: 10.1038/emm.2004.67. [DOI] [PubMed] [Google Scholar]
- 43.Zagzag D, Friedlander DR, Dosik J, Chikramane S, Chan W, Greco MA, et al. Tenascin-C expression by angiogenic vessels in human astrocytomas and by human brain endothelial cells in vitro. Cancer Res. 1996;56:182–9. [PubMed] [Google Scholar]
- 44.Zagzag D, Friedlander DR, Miller DC, Dosik J, Cangiarella J, Kostianovsky M, et al. Tenascin expression in astrocytomas correlates with angiogenesis. Cancer Res. 1995;55:907–14. [PubMed] [Google Scholar]
- 45.Zagzag D, Shiff B, Jallo GI, Greco MA, Blanco C, Cohen H, et al. Tenascin-C Promotes Microvascular Cell Migration and Phosphorylation of Focal Adhesion Kinase. Cancer Res. 2002;62:2660–8. [PubMed] [Google Scholar]
- 46.Eitzman DT, Westrick RJ, Shen Y, Bodary PF, Gu S, Manning SL, et al. Homozygosity for factor V Leiden leads to enhanced thrombosis and atherosclerosis in mice. Circulation. 2005;111:1822–5. doi: 10.1161/01.CIR.0000160854.75779.E8. [DOI] [PubMed] [Google Scholar]
- 47.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson J, Carson K, Williams H, Karanam S, Newby A, Angelini G, et al. Plaque rupture after short periods of fat feeding in the apolipoprotein E-knockout mouse: model characterization and effects of pravastatin treatment. Circulation. 2005;111:1422–30. doi: 10.1161/01.CIR.0000158435.98035.8D. [DOI] [PubMed] [Google Scholar]
- 49.Johnson JL, Baker AH, Oka K, Chan L, Newby AC, Jackson CL, et al. Suppression of atherosclerotic plaque progression and instability by tissue inhibitor of metalloproteinase-2: involvement of macrophage migration and apoptosis. Circulation. 2006;113:2435–44. doi: 10.1161/CIRCULATIONAHA.106.613281. [DOI] [PubMed] [Google Scholar]
- 50.Johnson JL, George SJ, Newby AC, Jackson CL. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc Natl Acad Sci U S A. 2005;102:15575–80. doi: 10.1073/pnas.0506201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khallou-Laschet J, Caligiuri G, Tupin E, Gaston AT, Poirier B, Groyer E, et al. Role of the intrinsic coagulation pathway in atherogenesis assessed in hemophilic apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2005;25:e123–6. doi: 10.1161/01.ATV.0000171995.22284.9a. [DOI] [PubMed] [Google Scholar]
- 52.Rodgers KJ, Watkins DJ, Miller AL, Chan PY, Karanam S, Brissette WH, et al. Destabilizing role of cathepsin S in murine atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2006;26:851–6. doi: 10.1161/01.ATV.0000203526.75772.4b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.