Abstract
Objective:
To assess the association of multiple sclerosis (MS) with concurrent restless legs syndrome (RLS) and daytime sleepiness. We also prospectively examined whether women with MS had an increased risk of developing RLS during 4 years of follow-up.
Methods:
The main analysis was based on a cross-sectional study of 65,544 women (aged 41−58 years) free of diabetes, arthritis, and pregnancy, who were participating in the Nurses' Health Study II cohort. Participants were considered to have RLS if they met 4 RLS diagnostic criteria recommended by the International Restless Leg Syndrome Study Group and had restless legs ≥5 times/month. MS was self-reported and confirmed by medical record review.
Results:
Among women with MS, the prevalence of RLS and severe RLS (15+ times/month) were 15.5% and 9.9% in 2005, respectively, relative to 6.4% and 2.6% among women without MS. After adjustment for potential confounders and the presence of other sleep disorders, women with MS had a higher likelihood of having RLS (odds ratio [OR] = 2.72, 95% confidence interval [CI] 1.89−3.93), severe RLS (OR = 4.12, 95% CI 2.65−6.42), and daily daytime sleepiness (OR = 2.11, 95% CI 1.31−3.42) compared with women without MS. Among the 172 women who had MS and were free of RLS in 2005, 9 developed RLS (5.2%) during a 4-year period and all had severe RLS. The adjusted relative risk of severe RLS was 3.58 (95% CI 1.53−8.35), comparing women with MS at baseline with those without MS.
Conclusion:
Women with MS had a significantly higher prevalence of RLS and daytime sleepiness and an increased risk of developing RLS in the future.
Multiple sclerosis (MS) is a T-cell−mediated demyelinating disease of the CNS. Restless legs syndrome (RLS) is a neurologic condition characterized by a distressing urge to move one's legs and is often accompanied by uncomfortable sensations.1 RLS and other sleep disorders have a negative impact on the overall health and quality of life of patients with MS.2,3 Among individuals with MS, RLS symptoms have been attributed to areas of demyelination.4 To date, a higher prevalence of RLS in patients with MS has been observed in some,5–9 but not all,10 clinic-based case-control studies. At present, no population-based studies on this topic have been conducted, and the association between MS and RLS has not been examined prospectively. In addition to RLS, other sleep disorders, including insomnia, nocturnal movement disorders, and sleep-disordered breathing, have also been observed in patients with MS.1,2 These chronic sleep disturbances result in daytime somnolence and fatigue, which may affect their daily activities.
The primary aim of this study was to assess the association between MS and RLS cross-sectionally as well as to prospectively examine whether individuals with MS had a higher risk of developing RLS. As a secondary aim, this study also explored the association between MS and daytime sleepiness.
METHODS
Study population.
The Nurses' Health Study (NHS) II is a large prospective cohort of 116,430 female registered nurses who were 25−42 years old at the start of the study in 1989. Follow-up questionnaires are mailed to the participants every 2 years (figure e-1 on the Neurology® Web site at www.neurology.org).
Assessment of RLS.
RLS was assessed based on the International Restless Leg Syndrome Study Group criteria11: “Do you have unpleasant leg sensations (like crawling, paresthesia, or pain) combined with motor restlessness and an urge to move?” (no, less than once/month, 2−4 times/month, 5−14 times/month, and 15+ times/month). Those who answered yes continued to the following questions: 1) “Do these symptoms occur only at rest and does moving improve them?”; and 2) “Are these symptoms worse in the evening/night compared with the morning?” A participant who had symptoms ≥5 times per month and answered yes to the subsequent questions was considered to have RLS; others were classified as not having RLS. Severe RLS was defined as having RLS more than 15 times per month. The questions on RLS were completed by 79,992 (82% of the 97,642 women who returned the 2005 questionnaire) women (figure e-1). Participants who did not complete the RLS questions had similar age (mean 50.4 vs 50.4 years) and BMI (27.1 vs 26.5 kg/m2) as those with RLS information.12
To reduce the misclassification of RLS, participants with diabetes and arthritis and those who were pregnant were excluded, leaving 65,554 women in the primary analysis. Similar RLS questions were administered in 2009 when the question “Do these symptoms occur only at rest and does moving improve them?” was split into the following 2 questions, “Do these symptoms occur only at rest?” and “Does moving improve them?”; 47,139 women free of RLS at baseline (2005) and free of diabetes or arthritis up to 2009 answered the questions. Among these, 172 women had MS at the baseline.
MS ascertainment.
Biennially since 1991 we asked NHS II participants whether they ever had physician-diagnosed MS (figure e-1). Women who self-reported the diagnosis of MS were followed up for confirmation. We asked their permission for study investigators to contact their neurologists and to obtain a copy of their medical records pertaining to the diagnosis. Women were considered as having definite or probable MS if so reported by their neurologist or, in the absence of the treating neurologist's diagnosis, if so determined after medical record review by our study neurologist.13 Among the 65,544 NHS II participants available for the primary analysis, we had 264 confirmed cases of MS (203 definite and 61 probable) between 1989 and June 2005.
Assessment of sleep complaints.
In 2001, we asked questions regarding sleep performance including sleep duration, snoring frequency, inadequate sleep (“Do you feel your sleep duration is adequate?”), and major reasons for inadequate sleep, including “Just cannot get to or stay asleep (worrying or insomnia).” We also asked about the frequency of “having difficulty falling asleep or staying asleep.”14 Regarding daytime sleepiness, participants were asked “How often are your daily activities affected because you are asleep during the day” (appendix e-1).
Assessment of other potential covariates.
Information on potential confounders, including age, body weight, smoking status, physical activity, use of antidepressants, use of tranquilizers, history of major chronic diseases, and use of nutritional supplements was collected via biennial questionnaires throughout the follow-up. We calculated body mass index (BMI) as weight (kg)/height (m2). We quantified physical activity as metabolic equivalents per week using reported time spent on various activities weighted by reported intensity level.15 The participants also recorded their body weight at age 18 in 1989. Ancestry was reported in 1989 as Scandinavian, Southern European, other Caucasian, or other.16 State of residence at age 15 was reported in 1993. Women were categorized into northern, middle, and southern tiers as described previously.13
Standard protocol approvals, registrations, and patient consents.
The institutional review board at Brigham and Women's Hospital and Harvard School of Public Health approved this study.
Statistical methods.
Statistical analyses were completed with SAS (version 9.1; SAS Institute, Inc., Cary, NC) for the cohort analyses and STATA (version 9.0; College Station, TX) for the meta-analysis. Continuous variables were compared between women with and without MS with the Student t test, and categorical variables were analyzed using the χ2 test.
Analysis 1.
We cross-sectionally examined the association of MS with RLS and other sleep disorders. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using multiple logistic regression, adjusting for age, body mass index (BMI2), BMI at age 18 years, ancestry, latitude of residence, smoking, physical activity, alcohol intake, menopause status, vitamin D supplement use, iron-specific supplement use, use of antidepressant medicine, use of tranquilizers, history of stroke, myocardial infarction, and hypertension, sleep duration, feeling sleep inadequate, difficulty falling or staying asleep, frequent snoring, and frequency of daytime sleepiness that affected their daily activities (appendix e-2).
We examined potential effect modification of the association between MS and RLS by age, BMI, menopause status, sleep duration, and use of antidepressant medicines. We tested for interaction between those risk factors and MS by creating categorical interaction terms and comparing the log likelihood of a model containing these interaction terms with a model that contained only the main effects (appendix e-2).
We did 2 sensitivity analyses: in the first analysis, we also included participants with arthritis. We then further included participants with diabetes, that is, including all participants with information on RLS and MS in the analysis.
We also examined the association between MS and other sleep disorders, including daytime sleepiness, short sleep time, feeling of inadequate sleep, difficulty falling or staying asleep, and frequent snoring. Because we collected the information on these sleep disorders in 2001, we conducted another sensitivity analysis by excluding the women with MS diagnosed at or after 2001 (appendix e-2).
Analysis 2.
We also conducted a prospective analysis among women free of RLS in 2005 to determine whether women with MS were at a higher risk of developing RLS using a multiple logistic regression model after adjustment for the same covariates as included in the cross-sectional analysis (figure e-1).
Analysis 3.
We conducted a meta-analysis of the current study together with published data on MS and RLS. Relevant studies were identified by searching the Medline, PubMed, EMBASE, and OMIM databases using the keywords “Restless legs syndrome OR RLS OR Ekbom syndrome” AND “Multiple sclerosis OR MS” for all published studies in English from 1966 through August 2011. In addition, the reference lists from the relevant publications were used to identify additional studies. Studies without control individuals assessed in the same study or patients with MS compared with historical or national databases were not considered in the analysis. Six studies were found eligible and were included in the meta-analysis (see Results). We used the Q statistic to examine heterogeneity among the studies, and the significance level was set at 0.1. We used a random-effects model to calculate the summary odds ratio (OR) as a significant heterogeneity was identified.
RESULTS
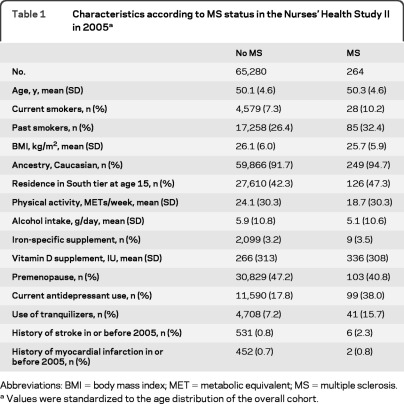
Compared with women without MS, those with MS were more likely to smoke, were less active, and were more likely to use antidepressant drugs (table 1).
Table 1.
Characteristics according to MS status in the Nurses' Health Study II in 2005a
Abbreviations: BMI = body mass index; MET = metabolic equivalent; MS = multiple sclerosis.
Values were standardized to the age distribution of the overall cohort.
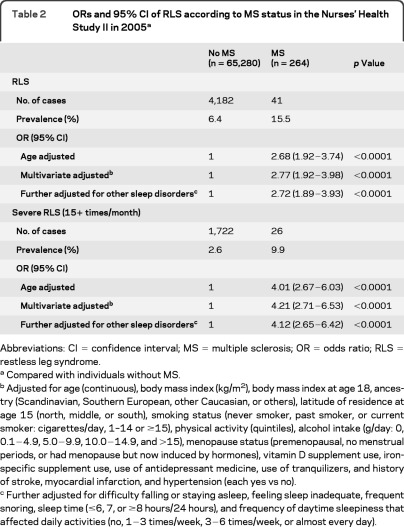
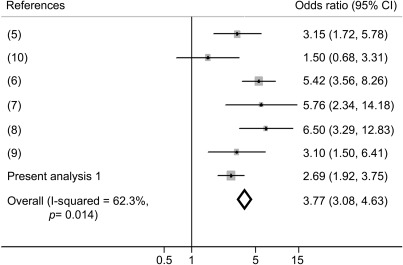
Among women with MS, the prevalence of RLS and severe RLS was15.5% and 9.9%, respectively, relative to 6.4% and 2.6% among individuals without MS (table 2). The crude OR of RLS was 2.69 (95% CI 1.92–3.75) comparing women with RLS and women without RLS. After adjustment for potential confounders, including age, BMI, lifestyle factors, and chronic conditions, women with MS had a higher odds of having RLS (OR = 2.77, 95% CI 1.92−3.98) and severe RLS (OR = 4.20, 95% CI 2.70−6.51) compared with those without MS. Further adjustment for the presence of other sleep disorders and daytime sleepiness slightly attenuated the OR of RLS (OR = 2.72. 95% CI 1.89−3.93) and severe RLS (OR = 4.12, 95% CI 2.65−6.42). We did a meta-analysis combining 5 previous cross-sectional studies7–11 with the present result, and the pooled OR of RLS was 3.77 (95% CI 3.08−4.63; p < 0.0001) comparing people with MS and individuals without MS (figure 1). We did not observe significant associations between MS duration and likelihood of having RLS or severe RLS. Among individuals with MS, the multiple adjusted OR was 1.05 (95% CI 0.94−1.17; p = 0.38) for RLS and 1.05 (95% CI 0.91−1.21; p = 0.53) for severe RLS with each 1-year increase of MS duration. Because in the primary analysis we excluded participants with arthritis or diabetes to minimize misclassifications of RLS assessment, we did 2 sensitivity analyses by further including these participants in the analyses. The full adjusted OR, comparing women with MS and women without MS, was 2.34 (95% CI 1.65−3.32) when we further included participants with arthritis in the analysis and was 2.27 (95% CI 1.62−3.19) when we included all participants with information on RLS and MS.
Table 2.
ORs and 95% CI of RLS according to MS status in the Nurses' Health Study II in 2005a
Abbreviations: CI = confidence interval; MS = multiple sclerosis; OR = odds ratio; RLS = restless leg syndrome.
Compared with individuals without MS.
Adjusted for age (continuous), body mass index (kg/m2), body mass index at age 18, ancestry (Scandinavian, Southern European, other Caucasian, or others), latitude of residence at age 15 (north, middle, or south), smoking status (never smoker, past smoker, or current smoker: cigarettes/day, 1–14 or ≥15), physical activity (quintiles), alcohol intake (g/day: 0, 0.1−4.9, 5.0−9.9, 10.0−14.9, and >15), menopause status (premenopausal, no menstrual periods, or had menopause but now induced by hormones), vitamin D supplement use, iron-specific supplement use, use of antidepressant medicine, use of tranquilizers, and history of stroke, myocardial infarction, and hypertension (each yes vs no).
Further adjusted for difficulty falling or staying asleep, feeling sleep inadequate, frequent snoring, sleep time (≤6, 7, or ≥8 hours/24 hours), and frequency of daytime sleepiness that affected daily activities (no, 1−3 times/week, 3−6 times/week, or almost every day).
Figure 1. Meta-analysis of the association between multiple sclerosis (MS) and restless leg syndrome (RLS).
The meta-analysis was based on the original numbers of cases of RLS and no RLS among people with and without MS because unadjusted/adjusted odds ratio were not reported among all publications included.
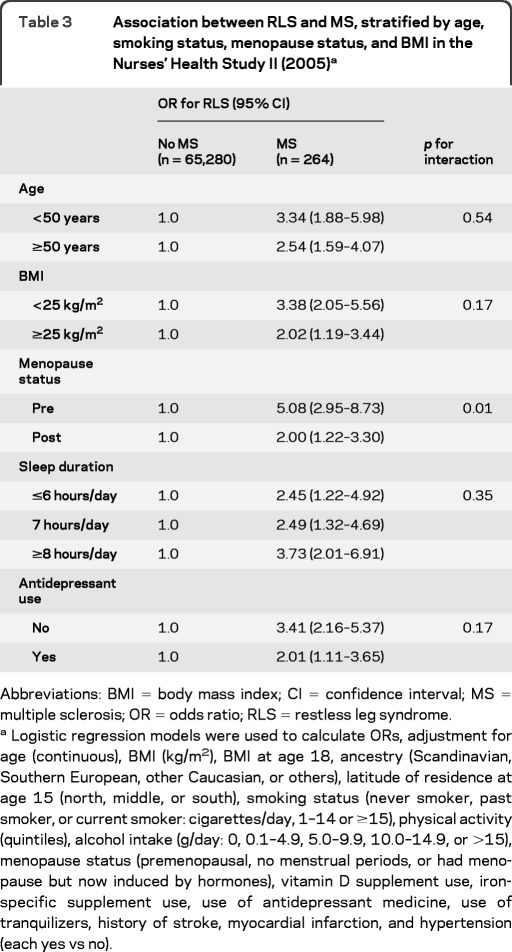
We observed a significant interaction between MS and menopause status in relation to the odds of having RLS (p for interaction = 0.01). The multiple adjusted OR of RLS was 5.08 (95% CI 2.95–8.73) among premenopausal women and 2.00 (95% CI 1.22–3.30) among women postmenopause (table 3). In contrast, the interactions between MS and other covariates (age, obesity, sleep duration, and use of antidepressant medicines) were not significant, and the association between MS and RLS persisted in subgroup analyses according to these variables (table 3).
Table 3.
Association between RLS and MS, stratified by age, smoking status, menopause status, and BMI in the Nurses' Health Study II (2005)a
Abbreviations: BMI = body mass index; CI = confidence interval; MS = multiple sclerosis; OR = odds ratio; RLS = restless leg syndrome.
Logistic regression models were used to calculate ORs, adjustment for age (continuous), BMI (kg/m2), BMI at age 18, ancestry (Scandinavian, Southern European, other Caucasian, or others), latitude of residence at age 15 (north, middle, or south), smoking status (never smoker, past smoker, or current smoker: cigarettes/day, 1–14 or ≥15), physical activity (quintiles), alcohol intake (g/day: 0, 0.1–4.9, 5.0–9.9, 10.0–14.9, or >15), menopause status (premenopausal, no menstrual periods, or had menopause but now induced by hormones), vitamin D supplement use, iron-specific supplement use, use of antidepressant medicine, use of tranquilizers, history of stroke, myocardial infarction, and hypertension (each yes vs no).
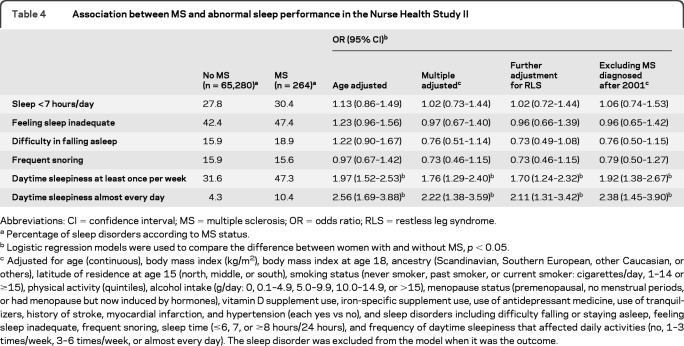
Among women with MS, 47.3% reported feeling sleepy during the daytime such that their daily activities were affected at least once per week and 10.4% almost every day, relative to 31.6% and 4.3% among women without MS (table 4). After adjustment for potential confounders, the OR was 2.22 (95% CI 1.38–3.59) for daily daytime sleepiness and 1.76 (95% CI 1.29–2.40) for daytime sleepiness weekly. The significant associations between MS and risk of having daytime sleepiness did not materially change when we further adjusted for the presence of RLS or excluded the individuals with MS diagnosed at or after 2001 (table 4). In contrast, although the frequencies of short sleep time, inadequate sleep, or difficulty falling or staying asleep were slightly higher among women with MS relative to those without MS, the differences were not significant. The difference in the total sleep time between women with and without MS was also not significant (7.5 vs 7.6 hours/day; p = 0.16).
Table 4.
Association between MS and abnormal sleep performance in the Nurse Health Study II
Abbreviations: CI = confidence interval; MS = multiple sclerosis; OR = odds ratio; RLS = restless leg syndrome.
Percentage of sleep disorders according to MS status.
Logistic regression models were used to compare the difference between women with and without MS, p < 0.05.
Adjusted for age (continuous), body mass index (kg/m2), body mass index at age 18, ancestry (Scandinavian, Southern European, other Caucasian, or others), latitude of residence at age 15 (north, middle, or south), smoking status (never smoker, past smoker, or current smoker: cigarettes/day, 1–14 or ≥15), physical activity (quintiles), alcohol intake (g/day: 0, 0.1–4.9, 5.0–9.9, 10.0–14.9, or >15), menopause status (premenopausal, no menstrual periods, or had menopause but now induced by hormones), vitamin D supplement use, iron-specific supplement use, use of antidepressant medicine, use of tranquilizers, history of stroke, myocardial infarction, and hypertension (each yes vs no), and sleep disorders including difficulty falling or staying asleep, feeling sleep inadequate, frequent snoring, sleep time (≤6, 7, or ≥8 hours/24 hours), and frequency of daytime sleepiness that affected daily activities (no, 1–3 times/week, 3–6 times/week, or almost every day). The sleep disorder was excluded from the model when it was the outcome.
We identified 1,291 incident cases of RLS during 4 years of follow-up (2005–2009). Among the 172 women with MS and free of RLS in 2005, 9 later developed RLS (5.2%), and all of those 9 women with MS reported having RLS symptoms ≥15 times/month (i.e., severe RLS), relative to 1.1% severe RLS incidence among women without MS at the baseline. After adjustment for potential confounders, women with MS had a higher risk of severe RLS (RR = 3.91, 95% CI 1.69–9.04; p = 0.001). Further adjustment for other sleep disorders and daytime sleepiness at baseline slightly attenuated the association (RR = 3.58; 95% CI 1.53–8.35; p = 0.003).
DISCUSSION
In this large cohort of women, we observed that women with MS had more than twice the prevalence of RLS, severe RLS, and daytime sleepiness that affected their daily activities relative to women without MS. Women with MS also had a higher risk of developing severe RLS than those without MS during 4 years of follow-up.
MS and RLS have only recently been recognized as comorbid conditions. Increased frequency of RLS among patients with MS was first reported in a French-Canadian population in 2005.5 A large multicenter case-control (861 subjects with MS) study was conducted in Italy and found a 5.4 times greater odds of RLS in patients with MS than that for control subjects. Similar significant results were also observed in 2 other studies.7–9 Although in one study in Spain the association between MS and RLS was not significant,10 a similar trend was seen. A meta-analysis of these studies and the current study supports the comorbidity of RLS and MS.
Although the biologic mechanism linking MS and RLS is unknown, one study comparing MRI parameters between patients with MS with and without RLS found no difference in brain MRI scans but did find more tissue damage in the cervical spinal cords of patients with MS with RLS compared with patients with MS without RLS.4 Low iron levels in the brain and iron deficiency have been associated with RLS.17–20 Iron may also play a role in the multifactorial initiation of myelination,21 and hypomyelination has been observed in both humans and animal models with chronic severe iron deficiency.22 Disturbance of myelination associated with progression of MS,23 as well as of many other neurologic diseases, may also induce RLS symptoms. In a recent clinical study including 23 subjects with RLS and 23 control subjects, a decrease in myelin was found in the brains of individuals with RLS.24 Iron deficiency anemia has been observed among patients with MS in different populations. In a population-based case-control study in Taiwan, patients with MS (n = 898) were almost 5 times more likely to have anemia compared with the randomly matched control subjects (n = 4,490).25 In another study in Spain including 72 patients with MS aged 24–61 years,26 the prevalence of iron deficiency was 39%, which is much higher than the prevalence in the general populations. Coincidence of MS and iron deficiency anemia was also observed in a family of Scottish descent.27 In the present study, we observed that the association between MS and RLS was more pronounced among premenopausal women than postmenopausal women, which may be due to the low iron levels that can occur with menstrual blood loss in premenopausal women. These observations imply that iron deficiency may have a role in the observed association between MS and RLS.
We also observed a significant association between MS and daytime sleepiness. Daily activities of almost half the women with MS were affected weekly by daytime sleepiness, which was twice that among individuals without MS. In a clinical study, severe daytime sleepiness was observed among 32% of the outpatients with MS,4 but the study had no comparable control data available. Furthermore, other studies report a correlation between excessive daytime sleepiness and fatigue.28–31 Daytime sleepiness in MS could be due to brainstem or diencephalic lesions affecting sleep-wake regulating structures.32 It may also be the result of sleep disturbance or a side effect of certain MS-related medications such as disease-modifying therapies and anticonvulsant and antispasticity agents.6 Collecting detailed information on medication among MS patients in future studies is warranted to further understand the underlying mechanisms of the association between MS and sleep disorders. In the present study, adjustment for RLS and other sleep disorders attenuated the association between MS and daytime sleepiness only slightly, implying that RLS and other sleep disorders could only partially explain the association between MS and daytime sleepiness.
Strengths of the current study include the facts that it is a population-based study with a large sample size and uses standardized questions to assess the presence of RLS. This prospective study provides evidence that MS could be a risk factor for RLS and suggests that demyelination in the CNS may have an important role in RLS etiology. Although we controlled for a number of potential confounders that are associated with both RLS and MS,33–36 residual confounding cannot be excluded because this is an observational study. Another limitation is the possibility that some RLS-like symptoms could be misclassified as an RLS diagnosis although we used the standard criteria of RLS diagnosis,37–39 and excluded women with diabetes, arthritis, and pregnancy from our primary analysis. Although we cannot exclude other common RLS mimics, such as positional discomfort and nocturnal cramps,37,38 these may not be an issue because there is no evidence that these 2 conditions are associated with MS. Thus, misclassification of RLS is likely to be random, which would probably attenuate the associations between MS and RLS. One other limitation is that our cohort represents a relatively healthy group of mostly Caucasian women, and the findings from this analysis might not be generalizable to other populations. However, the relative homogeneity of the study population in educational attainment and socioeconomic status enhances the internal validity of this study, which is the basis of generalizability. Although the significant association between MS and RLS is consistently observed in the primary analysis and sensitivity analyses, it must be emphasized that observational studies cannot prove causality, and residual confounding cannot be ruled out. Further, misclassification of exposures (i.e., RLS and common sleep disorders) is also inevitable. If the association between MS and RLS is confirmed in later studies based on objective measurements, also supported by future treatment studies, then we would suggest that clinicians should screen for RLS and other sleep disorders among patients with MS. Early RLS diagnosis in these patients may avoid ineffective chronic drug treatment in favor of more efficacious therapies.6
The results of this study indicate that women with MS had a significantly higher prevalence of RLS and daytime sleepiness. Further, women with MS were 4 times more likely to develop severe RLS during the 4-year follow-up. Further prospective studies using more precise diagnostic strategies for identifying RLS and other sleep disorders are warranted to clarify the association of MS with RLS and daytime sleepiness and to explore the relevant clinical implications.
Supplementary Material
GLOSSARY
- BMI
body mass index
- CI
confidence interval
- MS
multiple sclerosis
- NHS
Nurses' Health Study
- OR
odds ratio
- RLS
restless legs syndrome
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Conception and design: Dr. Li, Dr. Munger, Dr. Batool-Anwar, Dr. Ascherio, Dr. Gao. Analysis and interpretation of the data: Dr. Li, Dr. Munger, Dr. Batool-Anwar, K. De Vito, Dr. Gao. Drafting of the article: Dr. Li. Critical revision of the article for important intellectual content: Dr. Li, Dr. Munger, K. De Vito, Dr. Ascherio, Dr. Gao. Final approval of the article: Dr. Li, Dr. Munger, Dr. Batool-Anwar, K. De Vito, Dr. Ascherio, Dr. Gao. Provision of study materials or patients: Dr. Ascherio, Dr. Munger, Dr. Gao. Statistical expertise: Dr. Ascherio, Dr. Li, Dr. Munger, Dr. Gao. Collection and assembly of data: Dr. Ascherio, Dr. Munger, Dr. Gao. Obtaining of funding: Dr. Gao, Dr. Ascherio.
DISCLOSURE
Dr. Li, Dr. Munger, Dr. Batool-Anwar, and K. De Vito report no disclosures. Dr. Ascherio serves on a scientific advisory board for the Michael J. Fox Foundation; serves on the editorial boards of Neurology®, Annals of Neurology, and the American Journal of Epidemiology; has received speaker honoraria from Merck Serono; and receives research support from the NIH, the US Department of Defense, and the Michael J. Fox Foundation. Dr. Gao has received research support from the NIH/NINDS and reported consultancy relationship with Teva. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology: a report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health Sleep Med 2003;4:101–119 [DOI] [PubMed] [Google Scholar]
- 2. Fleming WE, Pollak CP. Sleep disorders in multiple sclerosis. Semin Neurol 2005;25:64–68 [DOI] [PubMed] [Google Scholar]
- 3. Ferini-Strambi L. Sleep disorders in multiple sclerosis. Handb Clin Neurol 2011;99:1139–1146 [DOI] [PubMed] [Google Scholar]
- 4. Manconi M, Rocca MA, Ferini-Strambi L, et al. Restless legs syndrome is a common finding in multiple sclerosis and correlates with cervical cord damage. Mult Scler 2008;14:86–93 [DOI] [PubMed] [Google Scholar]
- 5. Auger C, Montplaisir J, Duquette P. Increased frequency of restless legs syndrome in a French-Canadian population with multiple sclerosis. Neurology. 2005;65:1652–1653 [DOI] [PubMed] [Google Scholar]
- 6. Manconi M, Ferini-Strambi L, Filippi M, et al. Multicenter case-control study on restless legs syndrome in multiple sclerosis: the REMS study. Sleep 2008;31:944–952 [PMC free article] [PubMed] [Google Scholar]
- 7. Deriu M, Cossu G, Molari A, et al. Restless legs syndrome in multiple sclerosis: a case-control study. Mov Disord 2009;24:697–701 [DOI] [PubMed] [Google Scholar]
- 8. Fragoso YD, Finkelsztejn A, Gomes S, et al. Restless legs syndrome and multiple sclerosis: a Brazilian multicenter study and meta-analysis of the literature. Arq Neuropsiquiatr 2011;69:180–183 [DOI] [PubMed] [Google Scholar]
- 9. Aydar G, Kurt S, Karaer Unaldi H, Erkorkmaz U. Restless legs syndrome in multiple sclerosis. Eur Neurol 2011;65:302–306 [DOI] [PubMed] [Google Scholar]
- 10. Gómez-Choco MJ, Iranzo A, Blanco Y, Graus F, Santamaria J, Saiz A. Prevalence of restless legs syndrome and REM sleep behavior disorder in multiple sclerosis. Mult Scler 2007;13:805–808 [DOI] [PubMed] [Google Scholar]
- 11. National Institutes of Health Impotence. Natl Inst Health Consens Statement 1992;10:1–33 [PubMed] [Google Scholar]
- 12. Gao X, Schwarzschild MA, Wang H, Ascherio A. Obesity and restless legs syndrome in men and women. Neurology 2009;72:1255–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hernan MA, Olek MJ, Ascherio A. Geographic variation of MS incidence in two prospective studies of US women. Neurology 1999;53:1711–1718 [DOI] [PubMed] [Google Scholar]
- 14. Jenkins CD, Stanton B-A, Niemcryk SJ, Rose RM. A scale for the estimation of sleep problems in clinical research. J Clin Epidemiol 1988;41:313–321 [DOI] [PubMed] [Google Scholar]
- 15. Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–999 [DOI] [PubMed] [Google Scholar]
- 16. LeVine SM, Macklin WB. Iron-enriched oligodendrocytes: a reexamination of their spatial distribution. J Neurosci Res 1990;26:508–512 [DOI] [PubMed] [Google Scholar]
- 17. Salas RE, Gamaldo CE, Allen RP. Update in restless legs syndrome. Curr Opin Neurol 2010;23:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sikandar R, Khealani BA, Wasay M. Predictors of restless legs syndrome in pregnancy: a hospital based cross sectional survey from Pakistan. Sleep Med 2009;10:676–678 [DOI] [PubMed] [Google Scholar]
- 19. O'Keeffe ST, Gavin K, Lavan JN. Iron status and restless legs syndrome in the elderly. Age Ageing 1994;23:200–203 [DOI] [PubMed] [Google Scholar]
- 20. van Toorn R, Schoeman JF, Solomons R, Rensburg MA, van Rensburg SJ. Iron status in children with recurrent episodes of tumefactive cerebral demyelination. J Child Neurol 2010;25:1401–1407 [DOI] [PubMed] [Google Scholar]
- 21. Pinero D, Connor JR. Iron in the brain: an important contributor in normal and diseased states. Neuroscientist 2000;6:435–453 [Google Scholar]
- 22. Wang C, Wu C, Popescu DC, et al. Longitudinal near-infrared imaging of myelination. J Neurosci 2011;31:2382–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Connor JR, Ponnuru P, Lee BY, et al. Postmortem and imaging based analyses reveal CNS decreased myelination in restless legs syndrome. Sleep Med 2011;12:614–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ge Y, Jensen JH, Lu H, et al. Quantitative assessment of iron accumulation in the deep gray matter of multiple sclerosis by magnetic field correlation imaging. AJNR Am J Neuroradiol 2007;28:1639–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kang JH, Chen YH, Lin HC. Comorbidities amongst patients with multiple sclerosis: a population-based controlled study. Eur J Neurol 2010;17:1215–1219 [DOI] [PubMed] [Google Scholar]
- 26. Rodrigo L, Hernández-Lahoz C, Fuentes D, Alvarez N, López-Vázquez A, González S. Prevalence of celiac disease in multiple sclerosis. BMC Neurol 2011;11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rooney RN, Kotze MJ, de Villiers JN, Hillermann R, Cohen JA. Multiple sclerosis, porphyria-like symptoms, and a history of iron deficiency anemia in a family of Scottish descent. Am J Med Genet 1999;86:194–196 [DOI] [PubMed] [Google Scholar]
- 28. Taphoorn MJ, van Someren E, Snoek FJ, et al. Fatigue, sleep disturbances and circadian rhythm in multiple sclerosis. J Neurol 1993;240:446–448 [DOI] [PubMed] [Google Scholar]
- 29. Stanton BR, Barnes F, Silber E. Sleep and fatigue in multiple sclerosis. Mult Scler 2006;12:481–486 [DOI] [PubMed] [Google Scholar]
- 30. Stankoff B, Waubant E, Confavreux C, et al. Modafinil for fatigue in MS: a randomized placebo-controlled double-blind study. Neurology 2005;64:1139–1143 [DOI] [PubMed] [Google Scholar]
- 31. Culebras A. Neuroanatomic and neurologic correlates of sleep disturbances. Neurology 1992;42:19–27 [PubMed] [Google Scholar]
- 32. Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol 2010;9:599–612 [DOI] [PubMed] [Google Scholar]
- 33. Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann Neurol 2007;61:504–513 [DOI] [PubMed] [Google Scholar]
- 34. Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology 2008;70:35–42 [DOI] [PubMed] [Google Scholar]
- 35. Allen RP, Bharmal M, Calloway M. Prevalence and disease burden of primary restless legs syndrome: results of a general population survey in the United States. Mov Disord 2011;26:114–120 [DOI] [PubMed] [Google Scholar]
- 36. Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep 2009;32:589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Benes H, Walters AS, Allen RP, Hening WA, Kohnen R. Definition of restless legs syndrome, how to diagnose it, and how to differentiate it from RLS mimics. Mov Disord 2007;22(suppl 18):S401−S408 [DOI] [PubMed] [Google Scholar]
- 38. Hening WA, Allen RP, Washburn M, et al. The four diagnostic criteria for Restless Legs Syndrome are unable to exclude confounding conditions (“mimics”). Sleep Med 2009;10:976–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yeh P, Walters AS, Tsuang JW. Restless legs syndrome: a comprehensive overview on its epidemiology, risk factors, and treatment. Sleep Breath Epub 2011. October 26 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.