Abstract
Objective:
The objective of this work was to determine the impact of therapeutic hypothermia (TH) on the magnitude and time course of mean diffusivity (MD) changes following hypoxic-ischemic encephalopathy (HIE) in newborns.
Methods:
Cerebral MRI scans of infants undergoing whole body TH for HIE from 2007 to 2010 were retrospectively reviewed. The data were analyzed identically to a control group of newborns with HIE previously published, prior to the development of TH. Anatomic injury was defined on T1- and T2-weighted (“late”) MRI obtained after the fifth day of life. Since MD values vary regionally, the ratios of MD values for injured and normal tissue were calculated for areas of injury. Normal values were obtained from corresponding brain regions of 12 infants undergoing TH who had no injury on MRI studies.
Results:
Twenty-three of 59 infants who underwent TH and MRI displayed cerebral injury on late MRI and were included in the study. MD ratios were decreased in all injured infants within the first 7 days of life. The return of MD to normal (pseudonormalization) occurred after the tenth day as compared to 6–8 days in the control group. Infants with severest injury demonstrated greater reduction in MD, but no difference in time to pseudonormalization.
Conclusion:
TH slows the evolution of diffusion abnormalities on MRI following HIE in term infants.
Hypoxic-ischemic encephalopathy (HIE) remains a major cause of adverse outcome in the term born infant,1–3 and therapeutic hypothermia (TH) has been shown to safely improve neurodevelopmental outcome.4 Infants with HIE typically undergo MRI studies to assess prognosis, as the anatomic pattern of lesions is a powerful predictor of neurodevelopmental outcome,5,6 including those treated with TH.7 Previous MRI studies of infants treated with TH have demonstrated a reduction in markers of neuronal8,9 and subcortical white matter injury.10 TH does not affect either the predictive value of late MRI7 or the recommendations for the timing for acquiring an MRI.11 While conventional MRI are useful for the evaluation of brain injury in term HIE12,13 they often only show subtle changes in response to injury during the first days following injury.14 Findings on diffusion-weighted imaging (DWI) are more conspicuous and seen earlier—during the first hours to days—after injury in normothermic patients.15,16 Thereafter, the initially low mean diffusivity (MD) values increase and reach normal values (“pseudonormalize”) by the end of the first week following injury.17–21 We aimed to evaluate the time course of diffusion imaging values in infants with HIE who underwent TH. This time course was compared with data from a previous study of injured infants who were not treated with TH.17 We hypothesized that TH slows the evolution of MD changes, delaying pseudonormalization.
METHODS
Subjects.
Cerebral MRI scans of infants undergoing whole body TH for HIE from April 2007 to October 2010 at St. Louis Children's Hospital, St. Louis, MO, were retrospectively reviewed. The selection criteria for infants to receive TH were as follows:
≥35 weeks' gestation
≤6 hours of age
Clinical encephalopathy (abnormal tone, poor responsiveness, or excessive irritability)
Known or suspected perinatal ischemic events evidenced by either catastrophic sentinel event or 2 criteria from the following: fetal or neonatal acidemia (pH <7.0 or based deficit >12 mEq/L), Apgar score of <5 at 5 minutes, or ventilatory support for ≥5 minutes.
Exclusion criteria included age over 6 hours, weight under 2 kg, presence of major congenital abnormalities, and imminent death. Infants were cooled for 72 hours with a target core temperature of 33.5°C.
All infants with HIE who underwent TH and had an MRI within the 2 first weeks of life were eligible for enrollment. The clinical team caring for the infant determined the timing of the MRI study. The infants were imaged without sedation.22
Standard protocol approvals, registrations, and patient consents.
The Human Research Protection Office (Washington University School of Medicine) approved the study.
MRI.
Clinical MRI scans were performed on 3T Trio Siemens scanners (Erlangen, Germany). The imaging sessions included axial, magnetization-prepared rapid gradient echo T1-weighted (T1W) images (repetition time [TR]/echo time [TE] 1,500/3 msec, voxel size 1 × 0.7 × 1 mm3) and turbo spin echo T2-weighted (T2W) images (TR/TE 8,600/160 msec, voxel size 1 × 1 × 1 mm3, echo train length 17). Due to the retrospective nature of the study, there was some variability in the DWI sequences. DWI was obtained using a single-shot, echoplanar imaging sequence with diffusion sensitization obtained in 3–12 different directions and b values of 0 and 1,000 s/mm2. Spatial resolution was 1.5 × 1.5 × 3 mm3.
MD maps were computed and diffusion values were measured in regions of anatomic injury. The brain injury regions of interest (ROI) were defined by evaluating “late” MRI (T1W, T2W, DWI) obtained after day of life (DOL) 5 except for 3 symptomatic infants who had global injury (GI) with diffuse abnormality and did not survive to DOL 5. These ROIs were identified and analyzed by a board-certified neuroradiologist (J.S.) and pediatric neurologists (N.B., A.M.) who were blinded to the patient's history. With use of the anatomic information from these conventional images, the corresponding brain area on the diffusion map was chosen and the injured region identified. ROIs were drawn within the injured lesions and diffusion values sampled using Citrix (Fort Lauderdale, FL). For patients with GI, ROIs were placed within every injured region, and the lowest value was selected for the study. Since MD values vary regionally in normal brain, the ratios of MD values for injured and normal tissue were calculated for areas of injury. Normal values were obtained from corresponding brain regions of 12 infants scanned twice undergoing hypothermia who had no injury on MRI studies with diffusion coefficients value within the normative ranges described previously.23 Differences were resolved by consensus among the 3 readers. These values were compared to those obtained in a previous study17 in 10 newborns who were not treated by TH.
MRI scoring system.
The patterns of injury on the DWI, T1W, and T2W studies were categorized as white matter (WM), focal cortical (Cx), deep nuclear gray matter (DNGM), and GI. GI was defined by the presence of 3 or more patterns of injury and involvement of both hemispheres. If the patient had more than 1 lesion the lowest value of MD was used. WM lesions were defined by loss of cerebral cortical gray–white differentiation on T1W or T2W images and a decreased MD in a border-zone region; Cx injury was defined by high cortical signal on T1W imaging and a decreased MD in the cortical ribbon; DNGM lesions were identified by high signal on T1W imaging or a decreased signal on T2W imaging in basal ganglia or a loss of the normal low T2W signal in the posterior limb internal capsule (PLIC) or ventral nuclei of the thalami (figure 1).
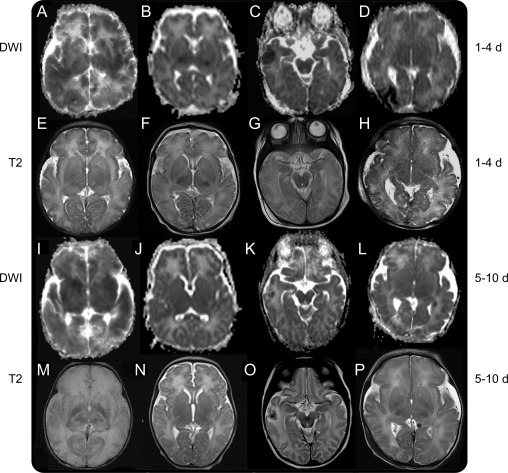
Figure 1. Images from 4 patients scanned at 2 timepoints.
The first presented with global injury (A, E, I, and M); the second presented with basal ganglia and posterior limb internal capsule (PLIC) injuries (B, F, J, and N); the third presented with cortical injury (C, G, K, and O); the fourth presented with border zone injury (D, H, L, and P). Diffusion imaging during the first 4 days after birth (A–D) shows respectively global (cortex and deep nuclear gray matter), bilateral PLIC, right temporal cortex, and right occipital border zone reductions in mean diffusivity (MD) with resolving reductions in MD seen in the delayed images obtained at 5–10 days after birth (I–L). The most persistent reduction in MD is seen in the global injury case (I). Corresponding T2-weighted images during the first 4 days after birth (E–H) showed mild lesions, more conspicuous in T2 images obtained after day 4 (M–P), demonstrating respectively a complete loss of cortical ribbon and an absence of differentiation between white and gray matter, absence of normal ventral thalami nuclei and PLIC signal (M), an absence of normal signal in the PLIC (N), a hypointensity surrounded by hyperintensity in right temporal lobe suggestive of hemorrhagic infarct (O), and subtle signal abnormality in the right occipital region (P). DWI = diffusion-weighted imaging.
Anatomic and diffusion scans were graded using a qualitative injury scale, a modification of a previously published method.24 Regions were scored independently for signal abnormality in each hemisphere on T1W, T2W, and DWI sequences, thus creating a 6-part score (left and right T1W, T2W, and DWI). Most regions were scored on a scale of 1 to 4 (except the brainstem [BS], scaled 1 to 3 due to its small size), with higher scores reflecting greater severity of injury (1 = normal; 2 = mild focal abnormality affecting <25% of the region; 3 = moderate multifocal abnormality affecting 25%–50% of the region; 4 = severe widespread abnormality affecting >50% of the region). The regions graded on this scale (range 6–24) were used to create component scores, including a WM score (WMS), Cx score (CxS), and cerebellum score (CbS). The BS score (BSS) ranged from 6 to 18. The scores from the caudate, putamen/globus pallidus, thalamus, and PLIC were added into 1 basal ganglia score (BGS, range 24–96). The global score (GS) was calculated as the sum of the 5 subscores (range 48–186):
Note that the BGS is intentionally more heavily weighted in the GS due to the severity of neurodevelopmental deficits associated with injury to this region.
Overall, injury was considered mild if the GS was between 49 and 59, moderate if between 60 and 80, and severe if ≥81.
Diffusion timeline estimation.
In order to obtain quantitative estimates of the diffusion as a function of time, the diffusion changes were modeled as a γ-variate function of the form:
with t being the time in days. The constants N, α, and β were estimated using a least squares approach. The γ-variate function was selected since it represents the diffusion changes through most of the time course, although it cannot model the pseudonormalization time point accurately because it does not cross zero. The time at which pseudonormalization took place was approximated as the point at which the MD ratio reached 0.9.
Statistical analysis.
Statistical analysis was performed using the Statistical Package for the Social Sciences version 18 (SPSS, Chicago, IL). Student t test and analysis of variance were used to compare the differences between outcomes among groups. Two-sided p values of <0.05 were used to define statistical significance for all comparisons. Data are expressed as mean ± SD.
RESULTS
Population.
Sixty-six term babies underwent TH. Ten patients died and 7 did not undergo an MRI scan due to severity of illness. Three infants, included in the study, who died, had an early MRI (1–3 days, 4 scans) showing GI with extensive cortical, DNGM, and BS injuries.
Of the 59 infants who underwent MRI, 20 had brain injury detected on MRI obtained after DOL 5. These 20 patients, plus the 3 who had GI, underwent 37 MRI studies, and 12 had serial studies (10 and 2 patients had respectively 2 and 3 studies). The age at MRI scan was 6.2 ± 4.1 days (range 0–17 days). The demographic information for these 23 patients is summarized in table 1. None of these patients had proven sepsis, viral infection, or hypoglycemia during their hospitalization.
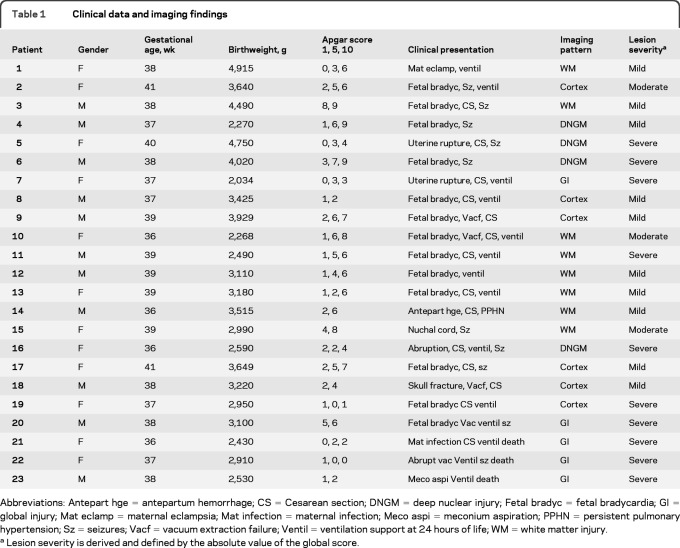
Table 1.
Clinical data and imaging findings
Abbreviations: Antepart hge = antepartum hemorrhage; CS = Cesarean section; DNGM = deep nuclear injury; Fetal bradyc = fetal bradycardia; GI = global injury; Mat eclamp = maternal eclampsia; Mat infection = maternal infection; Meco aspi = meconium aspiration; PPHN = persistent pulmonary hypertension; Sz = seizures; Vacf = vacuum extraction failure; Ventil = ventilation support at 24 hours of life; WM = white matter injury.
Lesion severity is derived and defined by the absolute value of the global score.
MRI description.
The pattern and the severity of the lesions for each patient are detailed in table 1. WM (35%) and DNGM (19%) lesions were the most common. The mean qualitative injury scores are as follows: BGS = 39.9 ± 24.8 (range 24–96); WMS = 12.4 ± 5.8 (6–24); CxS = 12.8 ± 6.9 (6–24); BSS = 9 ± 5.2 (6–18); CbS = 8.1 ± 2.5 (6–20); GS = 81.5 ± 41.6 (49–178).
The MRI characteristics of patients scanned more than once are summarized in table e-1 on the Neurology® Web site at www.neurology.org. Early DWI failed to show injury identified on conventional images at DOL 10 in 1 moderately injured patient (patient h, table e-1). In 2 patients (patients d [severe injury] and f [mild injury], table e-1), early DWI (DOL 1 and 5) demonstrated injury that was not detected by conventional imaging.
MD, magnitude, and time course.
Timeline of alteration in diffusivity.
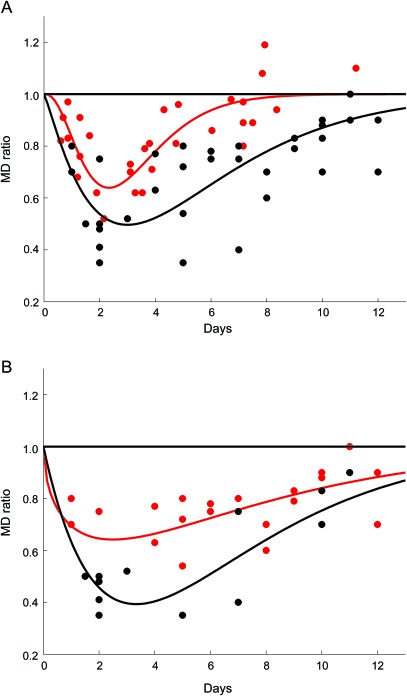
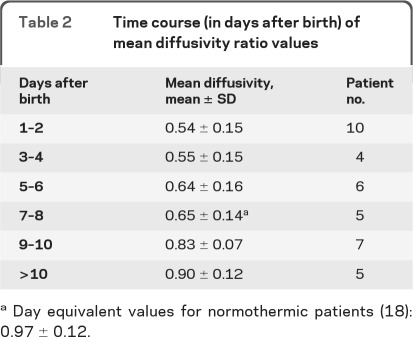
As shown in figure 2, A and B, and table 2, the values were lowest between DOL 2 and 6 (0.58 ± 0.16). Fitting the data to a γ-variate function (figure 2, A and B) predicts that pseudonormalization takes place at approximately DOL 11 and 12, and does not depend on the severity of injury (figure 2B). Pseudonormalization did not depend on the pattern of injury. MD ratios after DOL 6 were respectively 0.83 ± 0.13, 0.79 ± 0.08, 0.83 ± 0.11 for WM, Cx, and DNGM lesions. Six infants exhibited increased MD ratio (days 10 to 12), not reflected in figure 2, due to our choice of using the lowest MD when more than 1 lesion is present (see MRI scoring system).
Figure 2. Time course of the changes in the MD ratio over the 2 weeks following injury.
In (A) the red circles are reproduced from figure 1 in reference 17 and represent the mean diffusivity (MD) ratio in normothermic patients. The black circles represent the MD ratio in patients treated by hypothermia. Superposed on the figure are γ-variate functions that approximate the time course of the data. The red line represents the time course for the normothermic cohort, and the black line approximates the time course for the hypothermic group. Notice the delay in time to pseudonormalization in the hypothermia cohort as compared to the normothermia group. In (B) only the hypothermic cohort is presented and is divided into 2 groups. The red circles represent the mild/moderate injured group, and the black circles represent the severely injured group. The red line is the γ-variate approximation for the mild/moderate injured group, and the black line represents the time course for the severely injured cohort. Notice that the MD ratio is lower on average in the severely injured group but there is no difference in the time of pseudonormalization in the 2 groups.
Table 2.
Time course (in days after birth) of mean diffusivity ratio values
Day equivalent values for normothermic patients (18): 0.97 ± 0.12.
Relationship to pattern of injury.
The size of the lesion was incorporated through the scoring system with a larger score given to larger lesions. The MD ratio did not differ in relation to the regional pattern of the lesion in WM: 0.63 ± 0.15, DNGM: 0.63 ± 0.17, Cx: 0.64 ± 0.23, but differed in the presence of a GI: 0.45 ± 0.10 (p = 0.049) (these data are from the first 7 days, date of the last scanned global injured patient). The pattern of injury did not affect the initial drop of the MD in WM: 0.63 ± 0.14, DNGM: 0.64 ± 0.22, Cx: 0.54 ± 0.16 (these data are from the first 5 days after birth).
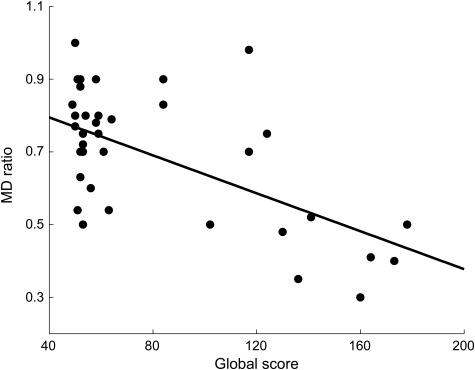
The MD ratio was strongly related to the GS (Pearson r = −0.59, p < 0.001, figure 3). The major determinant of the MD ratio was severity of injury. At its nadir, the MD ratio was lower for the severely injured group (0.59 ± 0.06, p = 0.02) than for the mild/moderately injured group (0.74 ± 0.13, figure 2B).
Figure 3. Correlation between global score (horizontal axis) and MD ratio (vertical axis).
A highly significant negative linear correlation is seen between the global score and the mean diffusivity (MD) ratio (Pearson correlation coefficient, r = −0.59).
DISCUSSION
This study provides the time course for the changes in MD following injury in a cohort of term infants receiving TH, showing that the time course of the MD reduction is delayed, with a slower recovery to normal compared to normothermic infants. Pseudonormalization takes place at approximately 6–8 days17,19,25 for normothermic infants, vs a prediction of 11–12 days for TH-treated infants. It should be noted that other authors report longer pseudonormalization times (8–10 days) in normothermic patients.20
Our data confirm that the severity of brain injury is strongly related to the magnitude of reduction in MD. The severest forms of brain injury demonstrated the largest reduction in MD, though the time to pseudonormalization was equivalent for the severely and mild/moderately affected groups. The evidence that MD is associated with severity of injury and neurodevelopmental outcome has been mixed. Only 1 published study in newborn infants treated with whole-body hypothermia compared MD values in the thalami and lentiform nuclei and found no difference between favorable and unfavorable outcome groups.26 However, the analysis was performed during the second week after the injury, possibly after the pseudonormalization. Several studies, performed in normothermic patients with HIE, have evaluated the association between MD reduction and outcome.12,15,26,27 Studies which have reported an association between a MD reduction and disability have usually been performed during the first week of life.15,27,28 Recent publications argue that measurement 6 days after the injury is necessary to demonstrate a correlation between MD reduction and outcome.25,29 Our data also demonstrate the importance of the timing of the MRI in relation to injury, with its greatest reduction apparent between days 2 and 6.
In this study, the early fall in MD after injury was not altered by TH, as the pattern was similar to that previously documented in the normothermic infants.17 This suggests that the early phase of the HI process is not affected by TH.4 However, the subsequent time course of MD changes is delayed. The mechanisms by which TH slows the time course of MD changes following injury are unknown. It is difficult to speculate on this mechanism because the means by which hypothermia is neuroprotective are unknown. Further, the neuroprotective mechanisms of hypothermia may be of limited relevance to the time course of MD ratio changes in injured tissue because the areas in which the prolonged reduction in MD is present are not protected by definition—they go on to show injury on late MRI. Nevertheless, there may be some value in examining mechanisms of injury and the effects of hypothermia.
Hypoxic ischemia induces a biphasic injury with an immediate followed by a delayed phase of energy failure.30 It has been suggested that hypothermia acts by a variety of mechanisms: reducing cerebral metabolism,31 stabilizing the blood–brain barrier,31 decreasing the inflammatory and excitatory amino acids response,32 or suppressing apoptosis.33 Of note, these are all processes potentially involved in the delayed phase of cerebral injury.34 It is conceivable that hypothermia converts areas destined for necrosis into areas undergoing prolonged apoptosis or that it prolongs the duration of the secondary energy failure in areas destined to show injury on late MRI. MD reduction is correlated with increased caspase 3 activity, a quantitative marker of apoptotic injury, 24 hours after HI in newborn rodents.35 Thus, the prolonged reduction in MD ratio may reflect prolonged apoptotic-like change with hypothermia. Brain immunohistologic studies of the postnatal day 9 HI mouse, mimicking lesions observed in term neonates,36 would be helpful to corroborate this hypothesis.
There are some limitations to our study, though they do not negate its principal findings. The first is that the interpretation of the time course of the MD is complicated by a lack of precise knowledge of the timing of the injury. The data are reported in relation to the time of birth, although only a small fraction (<10%) of HIE is associated with a clear sentinel event. The majority of cases involve a combination of prenatal and perinatal compromise that may have occurred for days prior to delivery.37–39 In addition, our retrospective design has unavoidable variability in the timing of the MRI scans and in the acuity of the injury (acute, repetitive, or protracted). The nature of the injury in our study is also variable and includes mild/moderate hypoperfusion with secondary energy failure, severe global hypoperfusion with acute necrosis, and focal/embolic injury. Further, the MRI reviews were done by 3 people, with differences resolved by consensus, and therefore do not provide an opportunity for assessing intraobserver and interobserver variability.
MD has a weak dependence on temperature (2.4% per 1°C change) and thus diffusion measurements made during the cooling period are expected to be lower than those performed at normal body temperature.40 The additional decrease in the MD in the first 3 days is calculated to be 8.5%, which would change figure 2 and table 2 slightly, but would not alter the conclusions.
The distribution of injuries to our population is consistent with that in some other studies. Nine out of 23 patients (39%) had DNGM injury (4 as the primary injury and 5 as part of GI), as compared with 75% and 50% reported in a population that underwent, respectively, whole body and selective head cooling.9 Due to different practices, our population is likely to have different patterns and severity of injury than those seen in other hospitals. Our population of infants appeared less severely injured than another reported population.9 An additional limitation of our study is its retrospective design, with the control group performed years before the study group. In comparison to another study,17 our cohort had several patients with GI, an injury pattern not included in the prior study. However, a prospective study in which subjects are randomized to normothermia and TH is not ethically feasible.
Finally, our assessment of injury severity was based on late MRI results. In the future, it will be important to determine severity of injury through neurodevelopmental follow-up as well. TH in term infants may not alter the initial decline of MD ratio after brain injury, but does prolong the time to pseudonormalization. This altered time course extends the window during which lesions are visible on DWI and has implications for the interpretation of these studies. In addition, quantitative measurement of MD during the first week of life has the potential to help quantify the severity of brain injury. Importantly, these findings raise the possibility of a prolonged therapeutic window following TH, although this would require further study for confirmation.
Supplementary Material
GLOSSARY
- BGS
basal ganglia score
- BS
brainstem
- BSS
BS score
- CbS
cerebellum score
- Cx
cortical
- CxS
cortical score
- DNGM
deep nuclear gray matter
- DOL
day of life
- DWI
diffusion-weighted imaging
- GI
global injury
- GS
global score
- HIE
hypoxic-ischemic encephalopathy
- MD
mean diffusivity
- PLIC
posterior limb internal capsule
- ROI
region of interest
- T1W
T1-weighted
- T2W
T2-weighted
- TE
echo time
- TH
therapeutic hypothermia
- TR
repetition time
- WM
white matter
- WMS
white matter score
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Bednarek contributed to the design of the study, to analysis and interpretation of the data, and to drafting and revising of the manuscript. Dr. Mathur contributed to the analysis of the data and to the revising of the manuscript. Dr. Inder contributed to the design and conceptualization of the study, to the interpretation of the data, and to the drafting and revising of the manuscript. J. Wilkinson contributed to the analysis and interpretation of the data. Dr. Neil contributed to the design and conceptualization of the study, to the interpretation of the data, and to the drafting and revising of the manuscript. Dr. Shimony contributed to the design and conceptualization of the study, to the analysis and interpretation of the data, and to the drafting and revising of the manuscript.
DISCLOSURE
Dr. Bednarek is receiving research support from American Memorial Hospital Inc (American Memorial Hospital, Reims, France) and reports no financial relationships for herself or her immediate family members for the past 2 years. Dr. Mathur is receiving support from the Thrasher Foundation and reports no financial relationships for himself or his immediate family members for the past 2 years. Dr. Inder is receiving support from National Institute of Health and reports no financial relationships for herself or her immediate family members for the past 2 years. J. Wilkinson reports no disclosures. Dr Neil is receiving support from National Institute of Health and the Green fund for Pediatric Neurology. He reports no financial relationships for himself or his immediate family members for the past 2 years. Dr Shimony is receiving support from the National Institute of Health and reports no financial relationships for himself or his immediate family members for the past 2 years. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Volpe J. Neurology of the Newborn. Philadelphia: Saunders; 2008 [Google Scholar]
- 2. Badawi N, Felix JF, Kurinczuck JJ, et al. Cerebral palsy following newborn encephalopathy: a population-based study. Dev Med Child Neurol 2005; 47: 293– 298 [DOI] [PubMed] [Google Scholar]
- 3. Nelson KB. Neonatal encephalopathy: etiology and outcome. Dev Med Child Neurol 2005; 47: 293– 298 [DOI] [PubMed] [Google Scholar]
- 4. Jacobs SE, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for Newborns with Hypoxic Ischaemic Encephalopathy. Hoboken, NJ: John Wiley & Sons; 2009 [Google Scholar]
- 5. Rutherford M, Pennock JM, Counsell SJ. Abnormal resonance magnetic signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics 1998; 102: 323– 328 [DOI] [PubMed] [Google Scholar]
- 6. Miller SP, Ramaswamy V, Michelson D, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr 2005; 146: 453– 460 [DOI] [PubMed] [Google Scholar]
- 7. Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol 2010; 9: 39– 45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inder TE, Hunt RW, Morley CJ, et al. Randomized trial of systemic hypothermia selectively protects the cortex on MRI in term hypoxic-ischemic encephalopathy. J Pediatr 2004; 145: 835– 837 [DOI] [PubMed] [Google Scholar]
- 9. Rutherford M, Azzopardi D, Whitelaw A, et al. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics 2005; 116: 1001– 1006 [DOI] [PubMed] [Google Scholar]
- 10. Parikh NA, Lasky RE, Garza CN, Bonfante-Mejia E, Shankaran S, Tyson JE. Volumetric and anatomical MRI for hypoxic-ischemic encephalopathy: relationship to hypothermia therapy and neurosensory impairments. J Perinatol 2009; 29: 143– 149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wintermark P, Hansen A, Soul J, Labrecque M, Robertson RL, Warfield SK. Early versus late MRI in asphyxiated newborns treated with hypothermia. Arch Dis Child Fetal Neonatal Ed 2010; 96: F36– F44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuenzle C, Baenziger O, Martin E, et al. Prognostic value of early MR imaging in term infants with severe perinatal asphyxia. Neuropediatrics 1994; 25: 191– 200 [DOI] [PubMed] [Google Scholar]
- 13. Barkovich AJ, Westmark K, Partridge C, Sola A, Ferriero DM. Perinatal asphyxia: MR findings in the first 10 days. AJNR Am J Neuroradiol 1995; 16: 427– 438 [PMC free article] [PubMed] [Google Scholar]
- 14. Barkovich AJ, Miller SP, Bartha A, et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol 2006; 27: 533– 547 [PMC free article] [PubMed] [Google Scholar]
- 15. Wolf RL, Zimmerman RA, Clancy R, Haselgrove JH. Quantitative apparent diffusion coefficient measurements in term neonates for early detection of hypoxic-ischemic brain injury: initial experience. Radiology 2001; 218: 825– 833 [DOI] [PubMed] [Google Scholar]
- 16. Vermeulen RJ, Fetter WP, Hendrikx L, Van Schie PE, van der Knaap MS, Barkhof F. Diffusion-Weighted MRI in severe neonatal hypoxic-ischaemia: the white cerebrum. Neuropediatrics 2003; 34: 72– 76 [DOI] [PubMed] [Google Scholar]
- 17. McKinstry RC, Miller JH, Snyder AZ, et al. A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology 2002; 59: 824– 833 [DOI] [PubMed] [Google Scholar]
- 18. Mader I, Schöning M, Klose U, Küker W. Neonatal cerebral infarction diagnosed by diffusion-weighted MRI: pseudonormalization occurs early. Stroke 2002; 33: 1142– 1145 [DOI] [PubMed] [Google Scholar]
- 19. Rutherford M, Counsell S, Allsop J, et al. Diffusion-weighted magnetic resonance imaging in term perinatal brain injury: a comparison with site of lesion and time from birth. Pediatrics 2004; 114: 1004– 1014 [DOI] [PubMed] [Google Scholar]
- 20. Winter JD, Lee DS, Hung RM, et al. Apparent diffusion coefficient pseudonormalization time in neonatal hypoxic-ischemic encephalopathy. Pediatr Neurol 2007; 37: 255– 262 [DOI] [PubMed] [Google Scholar]
- 21. Van Pul C, Buijs J, Janssen A, Roos GF, Vlaardingerbroek M, Wijn PF. Selecting the best index for following the temporal evolution of apparent diffusion coefficient and diffusion anisotropy after hypoxic-ischemic white matter in neonates. AJNR Am J Neuroradiol 2005; 26: 469– 481 [PMC free article] [PubMed] [Google Scholar]
- 22. Mathur AM, Neil JJ, McKinstry RC, Inder TE. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol 2008; 38: 260– 264 [DOI] [PubMed] [Google Scholar]
- 23. Neil JJ, Shiran SI, McKinstry RC, et al. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology 1998; 209: 57– 66 [DOI] [PubMed] [Google Scholar]
- 24. Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 2006; 355: 685– 694 [DOI] [PubMed] [Google Scholar]
- 25. Boichot C, Walker PM, Durand C, et al. Term neonate prognoses after perinatal asphyxia: contributions of MR imaging, MR spectroscopy, relaxation times, and apparent diffusion coefficients. 2006; 239: 839– 848 [DOI] [PubMed] [Google Scholar]
- 26. Massaro AN, Kadom N, Chang T, Glass P, Nelson K, Baumgart S. Quantitative analysis of magnetic resonance images and neurological outcome in encephalopathic neonates treated with whole-body hypothermia. J Perinatal 2010; 30: 596– 603 [DOI] [PubMed] [Google Scholar]
- 27. Hunt RW, Neil JJ, Coleman LT, Kean MJ, Inder TE. Apparent diffusion coefficient in the posterior limb of the internal capsule predicts outcome after perinatal asphyxia. Pediatrics 2004; 114: 999– 1003 [DOI] [PubMed] [Google Scholar]
- 28. Twomey E, Twomey A, Ryan S, Murphy J, Donoghue VB. MR imaging of term infants with hypoxic-ischaemic encephalopathy as a predictor of neurodevelopmental outcome and late MRI appearances. Pediatr Radiol 2010; 40: 1526– 1535 [DOI] [PubMed] [Google Scholar]
- 29. Brissaud O, Amirault M, Villega F, Periot O, Chateil JF, Allard M. Efficiency of fractional anisotropy and apparent diffusion coefficient on diffusion tensor imaging in prognosis of neonates with hypoxic-ischemic encephalopathy: a methodologic prospective pilot study. AJNR Am J Neuroradiol 2010; 31: 282– 287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lorek A, Takey Y, Cady EB, et al. Delayed (“secondary”) cerebral energy failure after acute hypoxia-ischemia in the new born piglet: continuous 48 hours studies by phosphorus magnetic resonance spectroscopy. Pediatr Res 1994; 36: 699– 706 [DOI] [PubMed] [Google Scholar]
- 31. Thoresen M, Penrice J, Lorek A, et al. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res 1995; 37: 667– 670 [DOI] [PubMed] [Google Scholar]
- 32. Thorensen M, Satas S, Puka-Sundvall M, et al. Post-hypoxic hypothermia reduces cerebrocortical release of NO and excitotoxins. Neuroreport 1997; 8: 3359– 3362 [DOI] [PubMed] [Google Scholar]
- 33. Edwards AD, Yue X, Squier MV, et al. Specific inhibition of apoptosis after cerebral hypoxic-ischemia by moderate post-insult hypothermia. Biochem Biophys Res Commun 1995; 217: 1193– 1199 [DOI] [PubMed] [Google Scholar]
- 34. Blomgren K, Hagberg H. Free Radicals, mitochondria, and hypoxia-ischemia in the developing brain. Free Radic Biol Med 2006; 40: 388– 397 [DOI] [PubMed] [Google Scholar]
- 35. Wendland MF, Faustino J, West T, Manabat C, Holtzman DM, Vexler ZS. Early diffusion-weighted MRI as a predictor of Caspase-3 activation after hypoxic-ischemic insult in neonatal rodents. Stroke 2008; 39: 1862– 1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic ischemic brain damage in the rat. Ann Neurol 1981; 9: 131– 141 [DOI] [PubMed] [Google Scholar]
- 37. Badawi N, Kurinczuk JJ, Keogh JM, et al. Antepartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ 1998; 317: 1549– 1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cowan F, Rutherford M, Groenendaal F, et al. Origin and timing of brain lesions in term infants with neonatal encephalopathy 2003; 361: 736– 742 [DOI] [PubMed] [Google Scholar]
- 39. Himmelmann K, Hagberg G, Beckung E, Hagberg B, Uvebrant P. The changing panorama of cerebral palsy in Sweden: IX: prevalence and origin in the birth-year period 1995–1998. Acta Pediatr 2005; 94: 287– 294 [DOI] [PubMed] [Google Scholar]
- 40. Le Bihan D, Turner D, MacFall JR. Temperature mapping with MR imaging of molecular diffusion: application to hyperthermia. Radiology 1989; 171: 853– 857 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.