Abstract
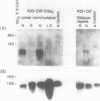
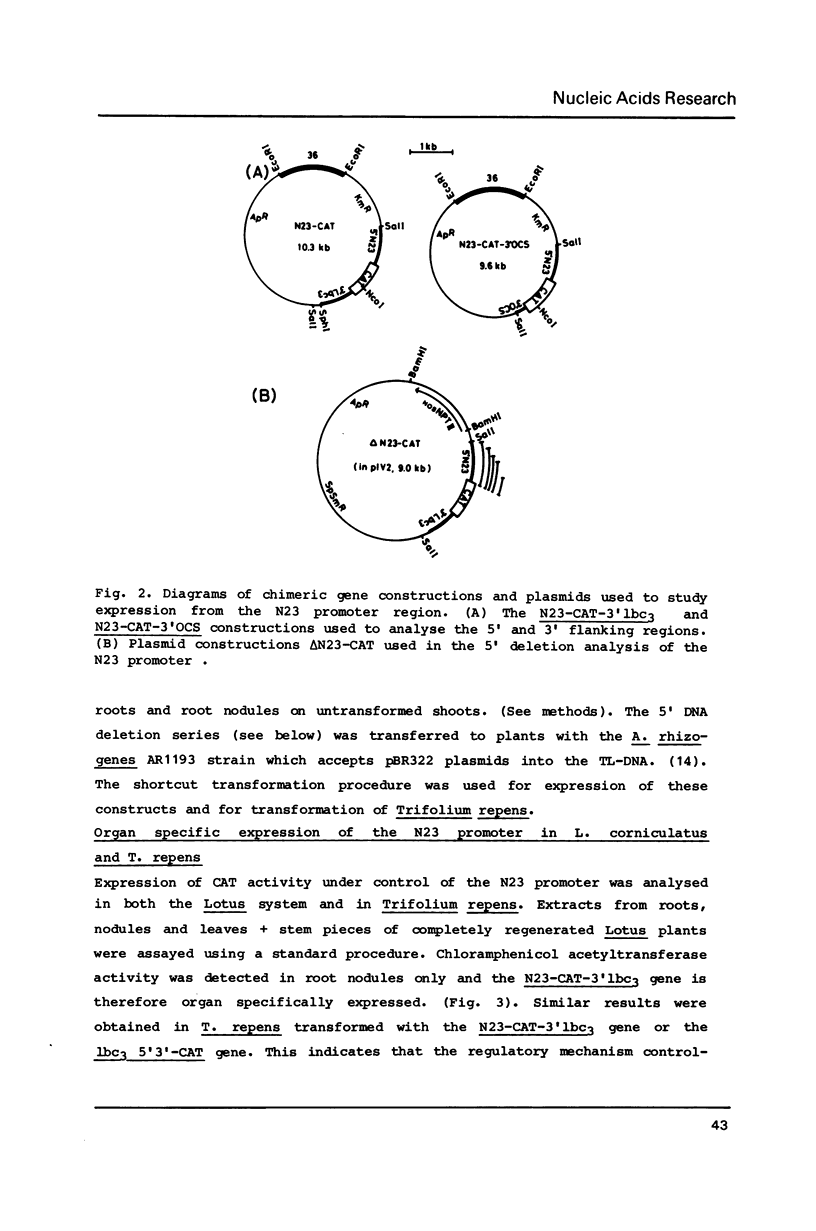
The nodulin N23 gene promoter was analysed in transgenic plants using the chloramphenicol acetyltransferase (CAT) coding sequence as a reporter. A 5' flanking region of less than 1 kb was sufficient for the organ-specific expression of a chimeric N23-CAT-3'lbc3 gene in root nodules formed on Lotus corniculatus and Trifolium repens after infection by their respective Rhizobium symbionts. Expression was regulated at the level of RNA in both species of transgenic plants. Promoter deletion analysis defined the 5' region required for high level expression and delimited two putative regulatory sequences involved in positive control of the N23 gene in L. corniculatus.
Full text
PDF





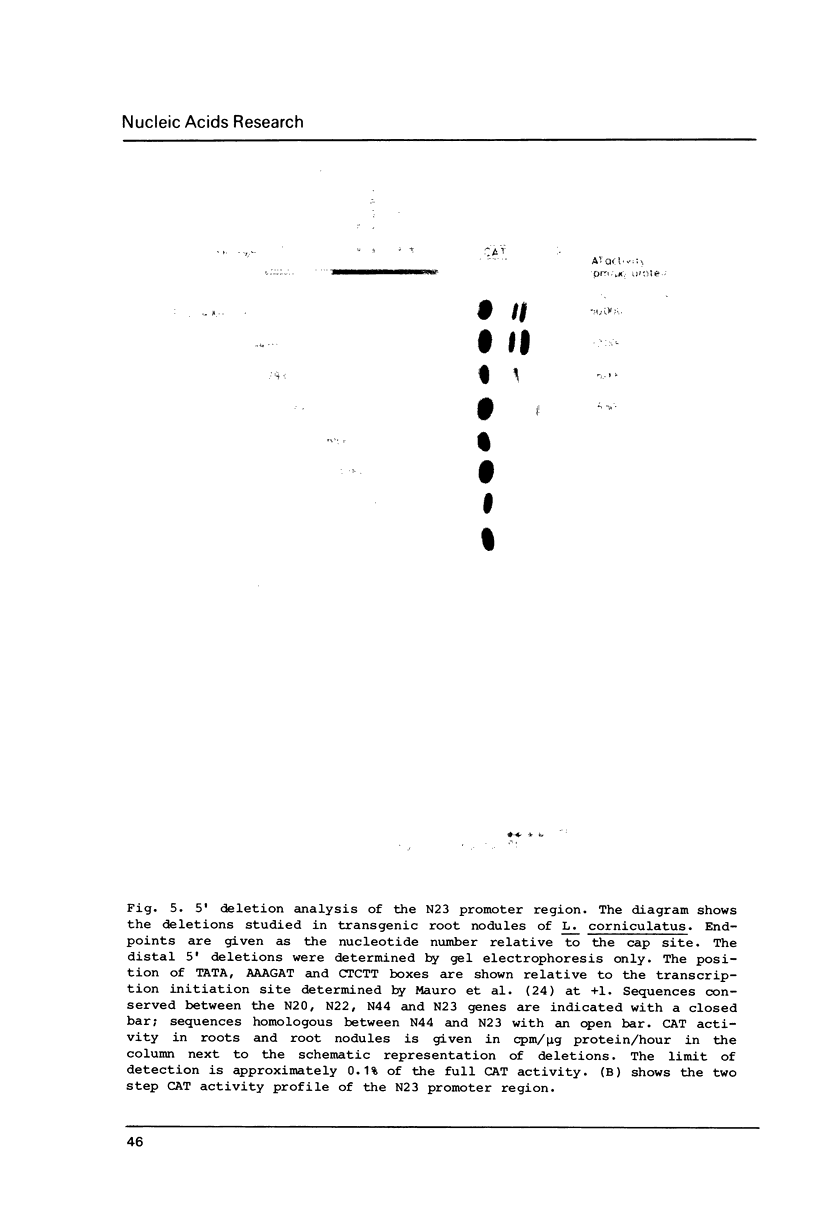




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann H., Preddie E., Verma D. P. Nodulin-35: a subunit of specific uricase (uricase II) induced and localized in the uninfected cells of soybean nodules. EMBO J. 1983;2(12):2333–2339. doi: 10.1002/j.1460-2075.1983.tb01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. L., Schuler M. A., Beachy R. N. Functional analysis of regulatory elements in a plant embryo-specific gene. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8560–8564. doi: 10.1073/pnas.83.22.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen T. M., Jochimsen B. U. Enzymes of ureide synthesis in pea and soybean. Plant Physiol. 1983 May;72(1):56–59. doi: 10.1104/pp.72.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R., Kuhlemeier C., Nagy F., Chua N. H. Organ-specific and light-induced expression of plant genes. Science. 1986 May 30;232(4754):1106–1112. doi: 10.1126/science.232.4754.1106. [DOI] [PubMed] [Google Scholar]
- Gausing K., Barkardottir R. Structure and expression of ubiquitin genes in higher plants. Eur J Biochem. 1986 Jul 1;158(1):57–62. doi: 10.1111/j.1432-1033.1986.tb09720.x. [DOI] [PubMed] [Google Scholar]
- Gielen J., De Beuckeleer M., Seurinck J., Deboeck F., De Greve H., Lemmers M., Van Montagu M., Schell J. The complete nucleotide sequence of the TL-DNA of the Agrobacterium tumefaciens plasmid pTiAch5. EMBO J. 1984 Apr;3(4):835–846. doi: 10.1002/j.1460-2075.1984.tb01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs F. A., Zhang M., Fortin M. G., Verma D. P. Several nodulins of soybean share structural domains but differ in their subcellular locations. Nucleic Acids Res. 1987 Feb 11;15(3):1271–1280. doi: 10.1093/nar/15.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen E. O., Marcker K. A., Villadsen I. S. Heme regulates the expression in Saccharomyces cerevisiae of chimaeric genes containing 5'-flanking soybean leghemoglobin sequences. EMBO J. 1986 May;5(5):843–847. doi: 10.1002/j.1460-2075.1986.tb04293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaulen Hildegard, Schell Jeff, Kreuzaler Fritz. Light-induced expression of the chimeric chalcone synthase-NPTII gene in tobacco cells. EMBO J. 1986 Jan;5(1):1–8. doi: 10.1002/j.1460-2075.1986.tb04169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. Identification of "nodule-specific" host proteins (nodoulins) involved in the development of rhizobium-legume symbiosis. Cell. 1980 May;20(1):153–163. doi: 10.1016/0092-8674(80)90243-3. [DOI] [PubMed] [Google Scholar]
- Marcker A., Lund M., Jensen E. Ø, Marcker K. A. Transcription of the soybean leghemoglobin genes during nodule development. EMBO J. 1984 Aug;3(8):1691–1695. doi: 10.1002/j.1460-2075.1984.tb02033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro V. P., Nguyen T., Katinakis P., Verma D. P. Primary structure of the soybean nodulin-23 gene and potential regulatory elements in the 5'-flanking regions of nodulin and leghemoglobin genes. Nucleic Acids Res. 1985 Jan 11;13(1):239–249. doi: 10.1093/nar/13.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell M., Copeland L. Sucrose synthase of soybean nodules. Plant Physiol. 1985 May;78(1):149–154. doi: 10.1104/pp.78.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell J. T., Nagy F., Chua N. H. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. 1985 Feb 28-Mar 6Nature. 313(6005):810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- Pankhurst C. E. Symbiotic effectiveness of antibiotic-resistant mutants of fast- and slow-growing strains of Rhizobium nodulating Lotus species. Can J Microbiol. 1977 Aug;23(8):1026–1033. doi: 10.1139/m77-152. [DOI] [PubMed] [Google Scholar]
- Sandal N. N., Bojsen K., Marcker K. A. A small family of nodule specific genes from soybean. Nucleic Acids Res. 1987 Feb 25;15(4):1507–1519. doi: 10.1093/nar/15.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J., Timko M. P., Cashmore A. R., Schell J., Montagu M. V., Herrera-Estrella L. Light-inducible and tissue-specific expression of a chimaeric gene under control of the 5'-flanking sequence of a pea chlorophyll a/b-binding protein gene. EMBO J. 1985 Nov;4(11):2723–2729. doi: 10.1002/j.1460-2075.1985.tb03995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stougaard J., Petersen T. E., Marcker K. A. Expression of a complete soybean leghemoglobin gene in root nodules of transgenic Lotus corniculatus. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5754–5757. doi: 10.1073/pnas.84.16.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiborg O., Pedersen M. S., Wind A., Berglund L. E., Marcker K. A., Vuust J. The human ubiquitin multigene family: some genes contain multiple directly repeated ubiquitin coding sequences. EMBO J. 1985 Mar;4(3):755–759. doi: 10.1002/j.1460-2075.1985.tb03693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. L., Verma D. P. Promoter analysis of a soybean nuclear gene coding for nodulin-23, a nodule-specific polypeptide involved in symbiosis with Rhizobium. EMBO J. 1985 Oct;4(10):2431–2438. doi: 10.1002/j.1460-2075.1985.tb03952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]