Abstract
Water stress affects many agronomic traits that may be regulated by the phytohormone abscisic acid (ABA). Within these traits, loss of fruit quality becomes important in many citrus cultivars that develop peel damage in response to dehydration. To study peel dehydration transcriptional responsiveness in harvested citrus fruit and the putative role of ABA in this process, this study performed a comparative large-scale transcriptional analysis of water-stressed fruits of the wild-type Navelate orange (Citrus sinesis L. Osbeck) and its spontaneous ABA-deficient mutant Pinalate, which is more prone to dehydration and to developing peel damage. Major changes in gene expression occurring in the wild-type line were impaired in the mutant fruit. Gene ontology analysis revealed the ability of Navelate fruits to induce the response to water deprivation and di-, tri-valent inorganic cation transport biological processes, as well as repression of the carbohydrate biosynthesis process in the mutant. Exogenous ABA triggered relevant transcriptional changes and repressed the protein ubiquitination process, although it could not fully rescue the physiological behaviour of the mutant. Overall, the results indicated that dehydration responsiveness requires ABA-dependent and -independent signals, and highlight that the ability of citrus fruits to trigger molecular responses against dehydration is an important factor in reducing their susceptibility to developing peel damage.
Keywords: ABA-deficient mutant fruit, abiotic stress, abscisic acid (ABA), citrus, gene expression, microarray, peel damage, water stress
Introduction
Plant growth, crop agricultural productivity, and quality are adversely affected by both biotic and abiotic stress factors. The effect of water stress on physiological and molecular responses of model plants has been widely described (Bray et al., 2000; Bartels and Sunkar, 2005; Seki et al., 2007). However, in spite of the relevance of this environmental factor on fruit quality, knowledge of these mechanisms in fruits is limited. Transcriptomic studies conducted in grapes indicate that genes, gene categories, and regulatory elements are affected differently by dehydration occurring before or after harvesting the fruit and also by the stress severity (Grimplet et al., 2007; Deluc et al., 2009; Rizzini et al., 2009; Zamboni et al., 2010).
Studies conducted in plants show that water stress causes removal of water from the cytoplasm to the extracellular space, causing a reduction in the cytosolic and vacuolar volumes and an alteration of reactive oxygen species homeostasis, which causes accumulation of toxic substances and also the production of signal transduction molecules (Miller et al., 2010). Accumulation of sugars, poly-alcohols, amino acids, amines, and abscisic acid (ABA) in response to water stress has been demonstrated in the model plant Arabidopsis thaliana and in a number of important horticultural crops (Bartels and Sunkar, 2005; Seki et al., 2007). As these metabolites function as osmolytes, antioxidants, scavengers, and/or signalling molecules that can help plants to tolerate abiotic stresses, changes in their homeostasis are thought to be associated with maintenance of the structure and function of cellular component networks. Therefore, the metabolic pathways of these compounds have been widely investigated (Seki et al., 2007), although regulatory networks and cross-talk between their components need further investigation (Yamaguchi-Shinozaki and Shinozaki, 2006; Shinozaki and Yamaguchi-Shinozaki, 2007). Deregulation of these water-stress metabolites and/or responsive genes can be manifested finally as damaged cellular tissues (Alférez et al., 2008). Moreover, mechanisms occurring in grape berries dehydrated after harvest (Grimplet et al., 2007; Zamboni et al., 2010) or in berries from water-stressed vines (Deluc et al., 2009) have indicated that dehydration may have a profound effect on the expression of genes associated with the biosynthesis of relevant compounds that ultimately impact on fruit quality. Functional characterization of the stress-induced genes also highlights the relevance of secondary metabolism, which may be affected by the rate and intensity of dehydration (Rizzini et al., 2009). Furthermore, the relevance of fruit surface properties in the dehydration of detached fruits should also be considered.
The tight relationship between ABA and dehydration is well known (Bartels and Sunkar, 2005; Shinozaki and Yamaguchi-Shinozaki, 2007), although ABA-independent pathways may also operate in response to dehydration (Riera et al., 2005). Plant hormone mutants have been used extensively to elucidate signal transduction pathways and to define the involvement of hormones in physiological processes. Focusing on ABA, natural and induced knockout and overexpressing mutants of biosynthetic and signalling transduction genes in Arabidopsis (Armstrong et al., 1995; Koornneef et al., 2004) and other plant species (Pena-Cortes et al., 1989; Groot and Karssen, 1992; Schwartz et al., 1997; Burbidge et al., 1999) have been characterized. However, the availability of artificially generated mutants is uncommon in woody plants. Therefore, access to spontaneous fruit hormone mutants is of particular scientific interest. A spontaneous fruit-specific ABA-deficient mutant from the wild-type Navelate orange (Citrus sinensis L. Osbeck), named Pinalate, has been described (Rodrigo et al., 2003). Pinalate orange presents distinctive yellow-coloured fruits because of a partial blockage of the carotenoid biosynthetic pathway causing a fruit-specific ABA deficiency. Moreover, harvested Pinalate fruits show higher dehydration and much higher susceptibility than its parental fruits to developing peel depressions, which in advanced stages become bronze and necrotic (Alférez et al., 2005; Sala et al., 2005). This physiological disorder, known as non-chilling peel pitting (NCPP), rind breakdown, or rind staining (Agustí et al., 2001; Lafuente and Sala, 2002), occurs in many citrus cultivars at temperatures above 11 °C, with water stress being an important causal factor in both attached and detached fruits (Alférez et al., 2003; Lafuente and Zacarías, 2006). Therefore, because of its higher susceptibility to developing NCPP and to dehydration, and its fruit-specific ABA deficiency, Pinalate fruit is a valuable experimental system to understand the involvement of ABA in the molecular mechanisms underlying the response of citrus fruits to water stress, eventually causing peel damage.
In the last decade, ‘omics’ tools have been widely used to characterize regulatory networks involved in plant abiotic stress responses (Urano et al., 2010). Numerous transcriptomic studies have been conducted to analyse model and crop plant transcriptomes under various stress conditions and have identified thousands of stress-responsive genes (Vij and Tyagi, 2007). Genome-wide studies have been also carried out in fruits with the aim of characterizing ripening or their responses to several stresses or hormone treatments (Maul et al., 2008; Ziliotto et al., 2008; Liu et al., 2009), but information on changes occurring in the transcriptome of water-stressed fruits to date is limited to grapes (Grimplet et al., 2007; Deluc et al., 2009; Rizzini et al., 2009). In recent years, the Spanish Citrus Functional Genomic Project (CFGP) has generated useful tools for citrus transcriptomic research. Citrus cDNA microarrays have been developed in this consortium (Forment et al., 2005; Martínez-Godoy et al., 2008), and the latest generation contains 21 081 (20K) putative citrus unigenes, which offers a good representation of the citrus genome. In the framework of the CFGP, important insights into citrus biology have been already achieved (Cercós et al., 2006; Gandía et al., 2007; Agustí et al., 2008; Alós et al., 2008; Huerta et al., 2008; Brumós et al., 2009; Ballester et al., 2011). Global changes in gene expression in response to drought have been characterized in citrus seedlings (Gimeno et al., 2009). However, in spite of the relevance of dehydration in fruit quality, a large-scale transcriptomic profile of citrus fruit in response to this stress has not been conducted so far.
With the aim of characterizing the molecular mechanisms involved in the response of harvested citrus fruits to dehydration and the potential role of ABA in this process, as well as elucidating the possible relationship existing between these two components and the occurrence of NCPP, a large-scale transcriptional analysis of the flavedo of Navelate and its mutant Pinalate oranges was conducted using the CFGP 20K microarray. To this end, fruits from both cultivars were stored at a temperature and relative humidity causing moderate water stress and the appearance of peel damage, and transcriptomic changes occurring in Pinalate fruit treated with ABA were examined.
Materials and methods
Plant material and ABA treatment
Full mature fruits of Navelate (Citrus sinensis L. Osbeck) orange and its spontaneous ABA-deficient mutant Pinalate were harvested randomly from adult trees grown in experimental orchards under normal cultural practices at The Spanish Citrus Germoplasm Bank at Instituto Valenciano de Investigaciones Agrarias (Moncada, Valencia, Spain). After harvest, fruits without any damage or visual defects were delivered immediately to the laboratory. To test whether application of ABA modified the post-harvest response of Pinalate fruit to dehydration, fruits from both cultivars were divided into two groups. The first group was treated with ABA (Sigma-Aldrich, St Louis, MO, USA) by dipping the fruits for 1 min in an aqueous solution of 1 mM ABA containing 0.7% ethanol to dissolve the hormone, while fruits of the second group were treated with water containing 0.7% ethanol following the same procedure. Fruits were dried at room temperature and then stored in the dark at 12 °C and 70–75% relative humidity for up to 6 weeks. The ABA treatment was repeated every 2 weeks to ensure high ABA levels during fruit storage. Likewise, Pinalate and Navelate control fruits were dipped into 0.7% ethanol at these times. Periodically, flavedo (outer coloured part of the peel) samples were collected from the total surface of fruits, frozen and homogenized in liquid nitrogen, and kept at –80 °C for later analysis. Three biological replicates, each consisting of five fruits, were collected during each sampling period.
Peel damage incidence and water loss measurement
A visual rating scale from 0 (no peel damage) to 4 (severe damage), based on surface necrosis and intensity of peel browning, was used to evaluate the incidence of NCPP in fruits stored at 12 °C and 70–75% relative humidity. The mean NCPP index was calculated by summing the products of the number of fruits in each category by the value assigned to each category in the rating scale and then dividing the resulting sum by the total number of fruits evaluated. In citrus fruit, water is lost mainly through the peel surface. Therefore, the cumulative percentage of fruit weight loss occurring during storage was expressed per cm2 of fruit surface area. Fruit surface was estimated using Turrell’s tables after measuring the diameter and height of the fruits (Turrell, 1946). The results are the means of three replicates of ten fruits each ±SE.
RNA isolation, cDNA labelling, and microarray hybridization
Total RNA was extracted from frozen flavedo samples by a modified method of that previously described by Rodrigo et al. (2004), as reported by Ballester et al. (2006). Total RNA was treated with ribonuclease-free DNase (Ambion/Applied Biosystems, Austin, TX, USA) following the manufacturer’s instructions for removing possible genomic DNA contamination. Thereafter, the amount of RNA was measured by spectrophotometric analysis (Nanodrop; Thermo Fisher Scientific, Madrid, Spain) and its quality was verified by agarose gel electrophoresis and ethidium bromide staining. cDNA synthesis and purification, dye coupling, and labelled cDNA purification were accomplished using the method described by Forment et al. (2005). cDNA samples were labelled with Cy5 and co-hybridized with a Cy3-labelled cDNA reference pool from a mixture containing equal amounts of RNA from all experimental samples assayed. The use of this reference sample has been widely used in citrus transcriptomic research, as it represents a powerful tool for reducing the number of hybridizations to make all possible pairwise comparisons between samples (Agustí et al., 2008). Microarray hybridization and slide washes were performed by a method modified from that proposed by Forment et al. (2005), as described by Ballester et al. (2011). The cDNA microarrays used were developed in the framework of the CFGP (http://bioinfo.ibmcp.upv.es/genomics/cfgpDB/) and contained 21 081 putative unigenes (20K) isolated from 52 cDNA libraries of citrus generated from a wide range of varieties and developmental and fruit-ripening stages, and from different tissues subjected to biotic and abiotic stress conditions (Martínez-Godoy et al., 2008).
Microarray data acquisition and analysis
Hybridized microarrays were scanned using a GenePix 4000A scanner (Axon Instruments, Sunnyvale, CA, USA) equipped with GenePix Pro 6.0 image acquisition software (Axon Instruments), following the manufacturer’s instructions to adjust the channels intensity ratio to 1.0 and the percentage of saturated spots close to 1%. Non-homogeneous and aberrant spots were discarded. Only spots with a background-subtracted intensity of more than twice the mean of the background intensity were used for normalization and further analysis. In order to compensate labelling differences among samples and other non-biological sources of variability, results were normalized using the print-tip Loess method, included in the Acuity 4.0 software (Axon Instruments), using background-subtracted median values and an intensity-based Loess function within and among microarrays. Thereafter, differentially expressed genes for all possible pairwise comparisons were determined by applying the significant analysis of microarrays program (SAM) (Tusher et al., 2001) from the TM4 Microarray Software Suite (Saeed et al., 2003). Genes that satisfied a statistical threshold (false discovery rate, FDR) of <0.01 were identified as differentially expressed genes. FatiGO+ (Babelomics, http://bioinfo.cipf.es/), developed by Al-Shahrour et al. (2004), was used to identify biological processes significantly under- or over-represented in a particular set of differentially expressed genes relative to a reference group containing all genes present in the microarrays having an Arabidopsis homologue. Gene ontology (GO) analysis for induced and repressed genes was performed independently applying Fisher’s two-tailed test with a P value <0.05. In this analysis, the specificity of the biological process increases with the GO level from 3 to 9. Multivariate analyses such as principal component analysis (PCA) and hierarchical cluster analysis (HCA) (analysis of variance test (ANOVA), Benjamini–Hochberg FDR <0.05) were performed using the MultiExperiment Viewer (MeV) tool of the TM4 Microarray Software Suite (Saeed et al., 2003).
qRT-PCR expression analysis
Reverse transcription followed by quantitative PCR analysis (qRT-PCR) was performed to validate the microarray results and to examine the time-course expression pattern of selected genes throughout fruit storage using a LightCycler 480 Instrument (Roche Diagnostics, Mannheim, Germany) equipped with LightCycler SW 1.5 software. A two-step qRT-PCR assay was designed as suggested by Udvardi et al. (2008). cDNAs were synthesized from all analysed samples using 400 U of SuperScript III RT (Invitrogen, Paisley, UK) in the presence of 0.5 μg of oligo(dT) 20mer (Invitrogen) and 10 U of ribonuclease inhibitor (Invitrogen) according to the manufacturer’s instructions. Gene-specific primers were designed using DNAMAN 4.03 software (Lynnon BioSoft, Quebec, Canada). Both synthesized cDNA and the primer pairs were thereafter incubated with LightCycler 480 SYBR Green I Master (Roche Diagnostics) at 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s, 60 °C for 5 s, and 72 °C for 10 s. Forward (F) and reverse (R) sequences for specific primers and correlation coefficients (r2) between the log2-transformed expression values as measured by microarray and RT-PCR analyses for each gene are shown in Table 1. To rule out non-specific amplified products, melting-curve analysis was performed and the reaction products were sequenced. To transform fluorescent intensity measurements into relative mRNA levels, a 2-fold dilution series of a mixture containing an equal amount of each cDNA sample was used and standard curves were constructed for all studied genes. Reference genes CsACT (F: 5′-TTAACCCCAAGGCCAACAGA-3′, R: 5′-TCCCTCATAGATTGGTACAGTATGAGA-3′), CsEF1α (F: 5′-ATTGACAAGCGTGTGATTGAGC-3′, R: 5′-TCCACAAGGCAATATCAATGGTA-3′), CsGAPDH (F: 5′-CGTCCCTCTGCAAGATGACTCT-3′, R: 5′-GGAAGGTCAAGATCGGAATCAA-3′) and CsTUB (F: 5′-GCATCTTGAACCCGGTAC-3′, R: 5′-ATCAATTCGGCGCCTTCAG-3′), whose constitutive expression throughout fruit storage was confirmed using the geNorm program (Vandesompele et al., 2002), were used for data normalization. Statistical analysis (a pairwise fixed reallocation randomization test) was carried out using the Relative Expression Software Tool (REST: http://rest.gene-quantification.info; Pfaffl, 2001). Each sample was analysed in triplicate and mean ratios ±SE were calculated.
Table 1.
Selected genes and primers used for qRT-PCR analysis and comparison between the Citrus 20K microarray and qRT-PCR gene expression data. Multiple linear regression analysis (r2) was performed for each reported gene including samples from all comparisons and storage periods.
| Gene | Citrus unigene (CFGP DB) | Most similar protein | Homolog in Arabidopsis | Forward/Reverse | Sequence 5′→3′ | r2 |
| CsCOPT2 | aCL7045Contig1 | Copper transporter protein homolog | AT3G46900 | F | GGGGGCCGACCTGAAGAAC | 0.98 |
| R | CGCACTAGCCGCTAGAAAAG | |||||
| CsCOPT5 | aCL1547Contig2 | T1M15_50 protein | AT5G20650 | F | GGAGGACAGGCGCGTCCG | 0.90 |
| R | GCCGAGAATTTCCCGACGAC | |||||
| CsHVA22E | aC31106H02EF_c | Abscisic acid-induced-like protein | AT5G50720 | F | GCGGCATGGCTGGTTCTGC | 0.91 |
| R | GCCTCGTGCTCCCCTTTCTT | |||||
| CsIPS | aC31301D12EF_c | Inositol-3-phosphate synthase | AT2G22240 | F | GGACACAGTGCAACAAGCCA | 0.95 |
| R | CCCATCCTCCAAACACAATG | |||||
| CsMYC | aC04028A10SK_c | MYC transcription factor | AT1G32640 | F | GCCTGAGTCCGGGGAGATAT | 0.92 |
| R | CCCTCTCGAAGTAGGAGATC | |||||
| CsNCED1 | aCL1933Contig1 | 9-cis-epoxicarotenoid dioxygenase | AT3G14440 | F | CCACGATGATAGCTCATCCG | 0.93 |
| R | CCACTTGCTGGTCAGGCACC | |||||
| CsNRAMP1 | aIC0AAA15AB01RM1_c | Metal transporter Nramp1 | AT1G80830 | F | GCCACTGGGCAGCCCCAG | 0.93 |
| R | CAGCTTGTCTTATCGGGCAC | |||||
| CsNRAMP3 | aCL3476Contig1 | Metal transporter Nramp3 | AT2G23150 | F | GGCTCTGAGCTTCTTATTGGC | 0.93 |
| R | GGACACGGCCTTTCTTACTG | |||||
| CsPUB9 | aCL8840Contig1 | F21O3.7 protein | AT3G07360 | F | AGCAAGAGCTGTGCGTGATG | 0.97 |
| R | GCGAAGCATGCAAGAAACTCC | |||||
| CsPUB21 | aC31304F06EF_c | Immediate-early fungal elicitor protein CMPG1 | AT5G37490 | F | AAGATCCGGTGACGACGACT | 0.90 |
| R | GCACCCAACTTGATCCTGTGT | |||||
| CsRD19 | aCL96Contig1 | Cysteine proteinase | AT4G39090 | F | GCACGACCGTAGGTTCACTAT | 0.93 |
| R | GTCCGGCGGAACTCGGCC | |||||
| CsRD21 | aCL23Contig3 | Cysteine protease CP1 | AT1G47128 | F | GCCCTGAGAGCAACACTTGC | 0.90 |
| R | GGGATAGTCATGTGGGCAGC |
ABA analysis
ABA analysis was performed as described by Lafuente et al. (1997). ABA was extracted from 1 g fresh weight frozen flavedo with 80% acetone containing 0.5 g l−1 citric acid and 100 mg l−1 butylated hydroxytoluene. After centrifugation, the supernatant was diluted in three serial dilutions in ice-cold TBS (6.05 g l−1 Tris, 8.8 g l−1 NaCl and 0.2 mg l−1 MgCl2) adjusted to pH 7.8 with 6 M HCl. Three samples for each dilution were analysed by an indirect ELISA method using the ABA–4′-BSA conjugate synthesized as reported previously by Weiler (1980), with some modifications (Norman et al., 1988). The results were the means of three replicate samples ±SE.
Statistics
A mean comparison using the Tukey’s test and Statgraphics.5.1 Software (Manugistics) was performed to determine significant differences at P ≤0.05 in NCPP, fruit weight loss per surface area and ABA levels between samples of Navelate and Pinalate fruits, treated or not with ABA, during fruit storage at 12 °C and 70–75% relative humidity.
Results
Susceptibility of Navelate and the ABA-deficient mutant Pinalate fruit to NCPP and dehydration, and the influence of exogenous ABA
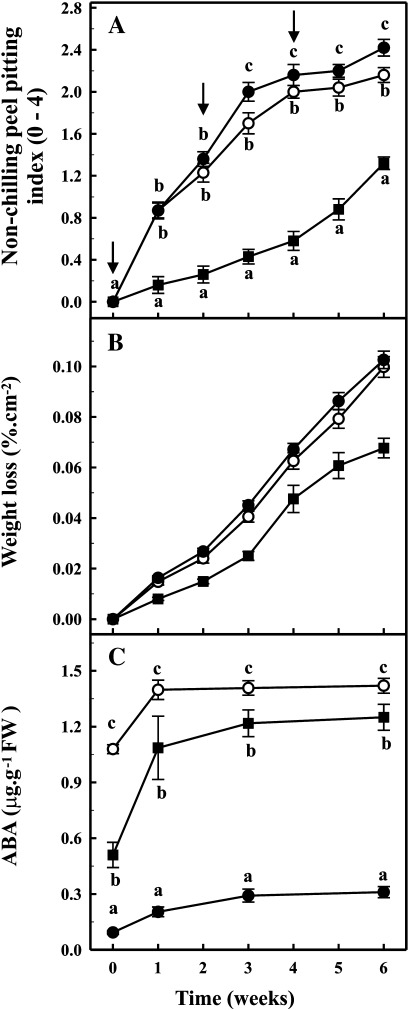
The susceptibility of fruits of the ABA-deficient mutant Pinalate to NCPP was much higher than that of fruits of its parental Navelate (Fig. 1A). Peel pitting was already visible by 1 week in stored Pinalate fruits, while in Navelate fruits the incidence of the disorder was barely detected. This difference between mutant and wild-type fruits was much more evident as storage progressed, reaching the highest difference by 3 weeks, when mutant fruits showed an approximately 5-fold higher NCPP index than the parental fruits (Fig. 1A). By this period, the weight loss per surface area in mutant fruits was twice that of Navelate fruits (Fig. 1B). The ABA level in the flavedo of freshly harvested (FH) Pinalate fruits was about 5-fold lower than in Navelate fruits (Fig. 1C). A rapid increase in the ABA content occurred in Navelate peel by 1 week, while it remained at low levels in Pinalate fruits throughout storage (Fig. 1C). By the end of the experiment (6 weeks), ABA content in parental fruits was about 4-fold higher than in the mutant. In this context, it is noteworthy that ABA-treated Pinalate fruits had slightly higher phytohormone levels than the wild type from the beginning of the experiment (Fig. 1C), but the treatment had little effect on reducing the susceptibility of the mutant to NCPP (Fig. 1A) or its dehydration rate (Fig. 1B). Likewise, exogenous ABA did not significantly modify the severity of NCPP or weight loss per surface area in wild-type fruits (see Supplementary Fig. S1, available in JXB online).
Fig. 1.
NCPP index (A), percentage of fruit weight loss per surface area (B), and ABA content in the flavedo (C) of Navelate (squares) and Pinalate (circles) fruits treated (white) or not (black) with ABA and stored for up to 6 weeks at 12 °C and 70–75% relative humidity. The arrows indicate when ABA was applied. Results are the means of three biological replicates of ten fruits each ±SE. Mean separation was performed by applying Tukey’s test. Significant differences (P ≤0.05) in the NCPP index and ABA content between samples for the same storage period are indicated by different letters. Significant differences (P ≤0.05) in weight loss (B) between Navelate and Pinalate samples, treated or not with ABA, were found from the first week of storage, while no statistical differences were found between control and ABA-treated Pinalate fruits.
Comparative transcriptional profiling during storage conditions inducing moderate water stress
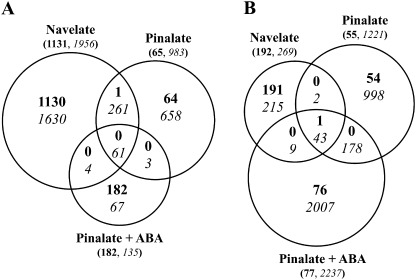
Considering the sharp increase in ABA content in Navelate oranges by 1 week, and also the marked difference in NCPP index between varieties by 3 weeks, both time points were selected for microarray hybridizations to compare changes in transcriptional profiling of both genotypes with respect to FH fruits. The above-described results indicated that applying ABA did not rescue the phenotype of the mutant. In order to determine whether increasing endogenous ABA levels in the mutant might simulate the molecular responses induced by moderate water stress in the wild-type phenotype, ABA-treated Pinalate fruits were also included in the transcriptome analysis. The Venn diagrams in Fig. 2 summarize the number of differentially expressed genes (SAM, FDR <0.01) in fruits stored for 1 (Fig. 2A) or 3 (Fig. 2B) weeks with respect to FH fruits.
Fig. 2.
Venn diagrams showing differentially expressed genes (SAM analysis, FDR <0.01) in the flavedo of Navelate, Pinalate, and ABA-treated Pinalate fruits stored at 12 °C and 70–75% relative humidity for 1 (A) and 3 (B) weeks. Expression levels of upregulated (shown in bold) and downregulated (shown in italics) genes in these fruits were compared with those of FH fruits from each variety. Numbers in brackets are the sum of all induced (bold) or repressed (italics) genes under each particular condition. The sizes of the circles are shown relative to the total number of differentially expressed genes for each condition.
Major changes in the number of differentially expressed genes occurred by 1 week in Navelate fruits (Fig. 2A) and by 3 weeks in Pinalate fruits (Fig. 2B). This effect was even more marked in the ABA-treated fruits (Fig. 2B). It is also noteworthy that repression prevailed in both cultivars throughout the storage period. Major inductions (1131 genes) occurred in parental fruits by 1 week, while a small set of upregulated genes was found in Pinalate fruits, treated or not with ABA (182 and 65 genes, respectively) (Fig. 2A). Likewise, Navelate fruits showed the highest number of downregulated genes by 1 week (1956). The expression of 322 of them also decreased in Pinalate fruits, although this number was reduced (65) when ABA was applied (Fig. 2A). By 3 weeks (Fig. 2B), the number of induced (192) and repressed (269) genes in the flavedo of Navelate fruits was less remarkable. By contrast, a high increase in the number of downregulated genes was observed in Pinalate fruits (1221) and this effect was enhanced by applying ABA (2237) (Fig. 2B).
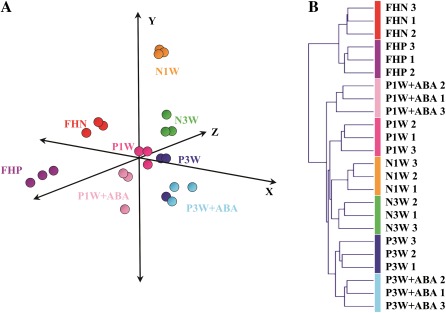
PCA and HCA were performed to validate the repeatability of the microarray data across replications and to cluster samples according to their global gene expression profiles. An ANOVA test revealed that 1471 genes, from a total of 21 081, showed differential expression and were used for PCA and HCA. Under all conditions, the transcriptional profiles of the three separate RNA replicate samples were tightly clustered (Fig. 3A). In contrast, PCA revealed marked differences in gene expression patterns between FH and stored fruits (x-axis, explaining 44% of the total variation), and also between FH fruits of both genotypes (variation on y- and z-axes=18.8%; Fig. 3A). Pinalate fruits stored for 1 week were distributed in the middle of the three axes, close to Pinalate fruits stored for 3 weeks. After storage for 1 week, Navelate fruits were clustered in the upper part of the y-axis and were far from those stored for 3 weeks. ABA-treated Pinalate fruits stored for 3 weeks grouped together, far from both Pinalate fruits stored for 3 weeks and ABA-treated Pinalate fruits stored for 1 week (Fig. 3A). HCA confirmed the results obtained by PCA. Navelate and Pinalate FH fruits were clustered separately in an independent branch from the stored samples, which could be grouped by storage period (Fig. 3B). Interestingly, ABA-treated Pinalate fruits stored for 1 week clustered in an independent group.
Fig. 3.
(A) PCA and (B) HCA of flavedo large-scale transcriptional profiles of Navelate (N), Pinalate (P), and ABA-treated Pinalate (P+ABA) fruits stored for one (1W) and three weeks (3W) at 12 °C and 70–75% relative humidity with respect to FH Navelate (FHN) and Pinalate (FHP) fruits. The colours in PCA for each condition are consistent with those in HCA. The three axes in PCA account for 62.8% of the total variance among varieties and storage periods. Three biological replicates from each condition were used for the two analyses.
Functional categorization of differentially expressed genes
GO analysis identified biological processes significantly under- or over-represented in the sets of differentially expressed genes selected from the SAM analysis. This analysis revealed that repressed genes in Navelate fruits stored for 1 week were enriched in biological processes related to biopolymer, heterocycle, and RNA metabolism and to cellular biosynthesis with respect to FH fruits, while induced genes were enriched in the response to water deprivation and the di-, tri-valent inorganic cation transport processes (Table 2). However, the differentially expressed genes in Navelate fruits stored for 3 weeks were not statistically grouped in any biological process. Likewise, no biological process was over-represented in either Pinalate or ABA-treated Pinalate fruits stored for 1 week. In contrast, the downregulated genes in the mutant fruits stored for 3 weeks, treated or not with ABA, were statistically enriched in the same processes. Among these processes, responses to biotic and abiotic stimulus, including light, temperature, jasmonic acid, and wounding, and to other organisms, as well as processes related to energy derivation and carbohydrate biosynthesis, were identified. Interestingly, the inhibition of protein ubiquitination, associated with protein degradation, was the one unique biological process differentially affected by ABA treatment in mutant fruits (Table 2).
Table 2.
Functional categorization of differentially expressed genes in the flavedo of Navelate, Pinalate, and ABA-treated Pinalate fruits stored at 12 °C and 70–75% relative humidity for 1 and 3 weeks with respect to FH fruits. Arrows indicate enriched biological processes (FatiGO+, P <0.05) in sets of significantly (SAM analysis, FDR <0.01) induced (↑) or repressed (↓) genes into each condition.
| 1 week |
3 weeks |
||||
| GO level | GO code | Biological process | Navelate | Pinalate | Pinalate + ABA |
| 4 | 0043283 | Biopolymer metabolic process | ↓ | ||
| 4 | 0044249 | Cellular biosynthetic process | ↓ | ||
| 4 | 0006091 | Generation of precursor metabolites and energy | ↓ | ↓ | |
| 4 | 0046483 | Heterocycle metabolic process | ↓ | ||
| 4 | 0006800 | Oxygen and reactive oxygen species metabolic process | ↓ | ↓ | |
| 4 | 0048583 | Regulation of response to stimulus | ↓ | ↓ | |
| 4 | 0009753 | Response to jasmonic acid stimulus | ↓ | ↓ | |
| 4 | 0051707 | Response to other organism | ↓ | ↓ | |
| 4 | 0009314 | Response to radiation | ↓ | ↓ | |
| 4 | 0009266 | Response to temperature stimulus | ↓ | ↓ | |
| 4 | 0009415 | Response to water | ↑ | ||
| 4 | 0009611 | Response to wounding | ↓ | ↓ | |
| 5 | 0015980 | Energy derivation by oxidation of organic compounds | ↓ | ↓ | |
| 5 | 0009416 | Response to light stimulus | ↓ | ↓ | |
| 5 | 0009414 | Response to water deprivation | ↑ | ||
| 5 | 0016070 | RNA metabolic process | ↓ | ||
| 7 | 0016051 | Carbohydrate biosynthetic process | ↓ | ↓ | |
| 7 | 0015674 | Di-, tri-valent inorganic cation transport | ↑ | ||
| 9 | 0016567 | Protein ubiquitination | ↓ | ||
Genes belonging to the most relevant and specific biological processes (higher GO levels) are shown in Table 3. Among genes belonging to water deprivation biological process, genes involved in ABA synthesis and perception (NCED1, ZEP, and PP2C), ABA-responsive genes (HVA22E, Lea5, and ADH), and ABA-dependent transcription factors (HB7, NAC4, and ABF4) were found. Furthermore, genes included in this process encoded aquaporins, vacuolar proton pumps, and other proteins playing protective roles against dehydration (Table 3). Within the inorganic cation transport process, iron transporters and chelators, several copper transporters, and two calcium-dependent transporter proteins were identified (Table 3). It is also noteworthy that the most specific process (carbohydrate biosynthesis) repressed in both Pinalate and ABA-treated Pinalate fruits included not only biosynthesis-related genes but also genes related to cell-wall metabolism, a MYC transcription factor, and an inositol-3-phosphate synthase (Table 3). The unique biological process affected by exogenous ABA in Pinalate fruits (protein ubiquitination) included six genes belonging to a superfamily of E3-ubiquitin ligases involved in protein degradation and with high similarity to plant U-box domain-containing proteins (PUBs) of Arabidopsis (Table 3).
Table 3.
Genes differentially expressed in the indicated comparisons and belonging to the most specific and relevant biological processes. N1W>FHN indicates genes induced in Navelate fruits stored for 1 week with respect to FH fruits; P3W<FHP indicates genes repressed in Pinalate fruits stored for 3 weeks with respect to FH fruits; and P3W+ABA<FHP indicates genes repressed in ABA-treated Pinalate fruits stored for 3 weeks with respect to FH fruits. Asterisks indicate genes chosen for multiple linear regression and qRT-PCR analysis.
| Citrus unigene (CFGP DB) | Most similar protein | Homologue in A. thaliana |
| N1W>FHN | Response to water deprivation (GO level 5) | |
| aCL474Contig1 | ABF4: putative ripening-related bZIP protein | AT3G19290 |
| aC18012D10Rv_c | ADH: aldehyde dehydrogenase – putative | AT1G44170 |
| aCL8452Contig1 | AVP1: vacuolar H+-pyrophosphatase | AT1G15690 |
| aCL5941Contig1 | HB7: homeobox-leucine zipper protein | AT2G46680 |
| aCL5217Contig1 | HK3: histidine kinase | AT1G27320 |
| * aC31106H02EF_c | HVA22E: abscisic acid-induced-like protein | AT5G50720 |
| aCL9Contig16 | LEA5: late embryogenesis abundant protein | AT4G02380 |
| aCL35Contig5 | NAC4: NAC domain protein | AT4G27410 |
| * aCL1933Contig1 | NCED1: 9-cis-epoxycarotenoid dioxygenase 1 | AT3G14440 |
| aCL3500Contig1 | PIP1B: plasma membrane aquaporin | AT2G45960 |
| aC31502B11EF_c | PIP1E: aquaporin | AT4G00430 |
| aCL143Contig2 | PP2C: protein phosphatase 2C | AT3G11410 |
| * aCL96Contig1 | RD19: cysteine proteinase | AT4G39090 |
| * aCL23Contig3 | RD21: cysteine protease CP1 | AT1G47128 |
| aCL1551Contig1 | ZEP: zeaxanthin epoxidase | AT5G67030 |
| N1W>FHN | Di-, tri-valent inorganic cation transport (GO level 7) | |
| aC18018E02Rv_c | CNGC1: cyclic nucleotide-gated calmodulin-binding ion channel | AT5G53130 |
| aC01009A02SK_c | COPT1: copper transporter 1 | AT5G59030 |
| * aCL7045Contig1 | COPT2: copper transporter protein homologue | AT3G46900 |
| * aCL1547Contig2 | COPT5: T1M15_50 protein | AT5G20650 |
| aC04013B01SK_c | ECA3: calcium-transporting ATPase3-endoplasmic reticulum-type | AT1G10130 |
| aKN0AAQ10YG21RM1_c | FER4: ferritin | AT2G40300 |
| aC34108F04EF_c | IRT1: root iron transporter protein | AT4G19690 |
| * aIC0AAA15AB01RM1_c | NRAMP1: metal transporter Nramp1 | AT1G80830 |
| * aCL3476Contig1 | NRAMP3: metal transporter Nramp3 | AT2G23150 |
| aCL5880Contig1 | SAG14: NtEIG-A1 protein | AT5G20230 |
| P3W<FHP, P3W+ABA<FHP | Carbohydrate biosynthetic process (GO level 7) | |
| aC31305H08EF_c | ADG1: ADP-glucose pyrophosphorylase small subunit | AT5G48300 |
| aCL5827Contig1 | ADG1: glucose-1-phosphate adenylyltransferase | AT5G48300 |
| aCL6121Contig1 | CALS1: putative callose synthase 1 catalytic subunit | AT1G05570 |
| aCL4673Contig1 | CESA1: cellulose synthase | AT4G32410 |
| aC03001C04Rv_c | CESA2: cellulose synthase | AT4G39350 |
| aCL1466Contig1 | CTL1: T20M3.12 protein | AT1G05850 |
| aCL18Contig7 | CYP79A2: cytochrome P450 79A2 | AT5G05260 |
| aCL60Contig1 | F9L11.8: granule-bound starch synthase 1 | AT1G32900 |
| aCL281Contig3 | GAPB: glyceraldehyde-3-phosphate dehydrogenase B | AT1G42970 |
| aCL3226Contig1 | GATL10: glycosyl transferase-like protein | AT3G28340 |
| aCL1394Contig1 | GMD2: GDP-mannose 4 -6 dehydratase 1 | AT3G51160 |
| aCL381Contig1 | GOLS2: galactinol synthase | AT1G56600 |
| * aC31301D12EF_c | IPS2: inositol-3-phosphate synthase | AT2G22240 |
| aC08005B05SK_c | KAM1: xyloglucan galactosyltransferase KATAMARI 1 | AT2G20370 |
| * aC04028A10SK_c | MYC2: MYC transcription factor | AT1G32640 |
| aCL4197Contig1 | QUA2: putative early-responsive to dehydration stress protein | AT1G78240 |
| aCL2181Contig1 | SIP1: raffinose synthase | AT5G40390 |
| P3W+ABA < FHP | Protein ubiquitination (GO level 9) | |
| * aCL8840Contig1 | PUB9: F21O3.7 protein | AT3G07360 |
| aC34202B10EF_c | PUB17: Avr9/Cf-9 rapidly elicited protein 276 | AT1G29340 |
| * aC31304F06EF_c | PUB21: immediate-early fungal elicitor protein CMPG1 | AT5G37490 |
| aC31801H08EF_c | PUB24: F26K24.13 protein | AT3G11840 |
| aCL270Contig1 | PUB29: photoperiod responsive protein | AT3G18710 |
| aC05134D01SK_c | PUB43: armadillo repeat-containing protein | AT1G76390 |
Expression profiles for selected genes by qRT-PCR analysis
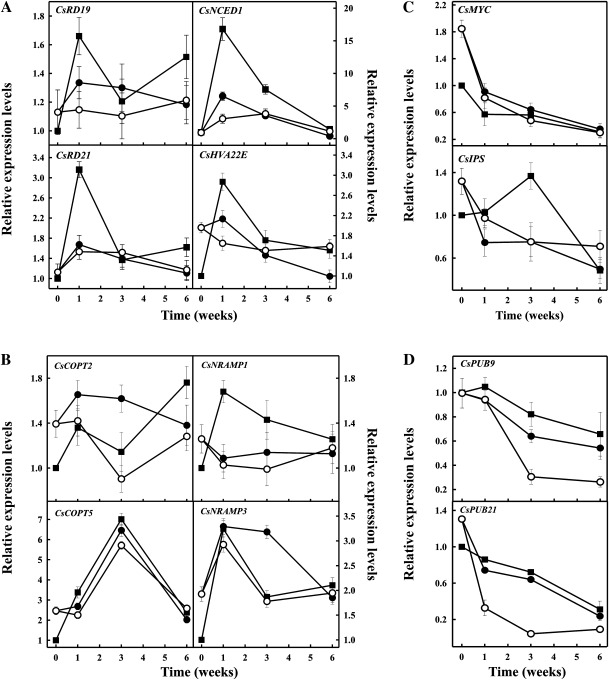
qRT-PCR analysis was conducted to validate the microarray gene expression data and to characterize further the expression patterns of selected genes in fruits exposed to moderate water stress for up to 6 weeks. Comparison between the transcript abundance data obtained by the 20K microarray and by qRT-PCR analysis with gene-specific primers revealed a high correlation for all selected genes with r2 values between 0.90 and 0.98 (Table 1). Among genes belonging to the response to water deprivation biological process, the genes CsRD19 and CsRD21 with homology to dehydration-responsive genes of Arabidopsis (AT4G39090 and AT1G47128, respectively), CsHVA22E homologous to an ABA-inducible gene (AT5G50720), and CsNCED1 (AT3G14440) involved in ABA biosynthesis were selected. A rapid and transient increase in the relative expression levels of these genes was observed by 1 week in parental fruits. Interestingly, the relative expression level of CsNCED1 also increased in the flavedo of Pinalate fruits, but this increase was much lower than that occurring in Navelate fruits. Moreover, such increases were not induced by applying ABA to the mutant (Fig. 4A). Within the di-, tri-valent inorganic cation transport biological process, CsCOPT2 and CsCOPT5 genes, with homology to copper transporters of Arabidopsis (AT3G46900 and AT5G20650, respectively), and CsNRAMP1 and CsNRAMP3, homologous to iron transporter genes (AT1G80830 and AT2G23150, respectively) were selected. The expression levels of all these genes in FH mutant fruits were higher than in the parental fruits (Fig. 4B). However, a higher increase in their expression was detected in wild-type fruits exposed to moderate dehydration for 1 week than in mutant. From these genes, only the expression levels of CsCOPT5 continued increasing in response to dehydration for up to 3 weeks. Accumulation of CsNRAMP1 was, in general, higher during storage in Navelate fruits. In contrast, expression levels of CsCOPT2 and CsNRAMP3 were higher in Pinalate fruits. Interestingly, the expression pattern of these two genes in mutant fruits treated with ABA was more similar to that of parental fruits than to the mutant fruits (Fig. 4B). The citrus unigenes CsIPS and CsMYC, with homology to genes encoding a inositol-3-phosphate synthase (AT2G22240) and a MYC transcription factor (AT1G32640), respectively, were selected as representative genes of the carbohydrate biosynthesis biological process. Both genes were repressed in the ABA-treated and non-treated Pinalate fruits, although their expression levels in FH mutant fruits were higher than in Navelate fruits (Fig. 4C). Expression levels of CsMYC transcription factor also decreased in the parental fruit, while that of CsIPS increased from 1 to 3 weeks of storage. Genes CsPUB9 and CsPUB21 encoding proteins showing homology to E3-ubiquitin ligases of A. thaliana involved in ABA (AT3G07360) and pathogen (AT5G37490) responses, respectively, were selected from genes of the protein ubiquitination biological process (Table 3). The rate of decrease in expression levels of both genes was similar in parental and mutant fruits, but applying ABA had a marked effect, favouring repression (Fig. 4D).
Fig. 4.
Real-time qRT-PCR expression analysis for candidate genes selected from microarray analysis. Relative transcript abundance for selected genes belonging to water deprivation (A), di-, tri-valent inorganic cation transport (B), carbohydrate biosynthesis (C) and protein ubiquitination (D) biological processes differentially regulated in Navelate (squares) and Pinalate (circles) fruits, treated (white) or not (black) with ABA, and stored for up to 6 weeks at 12 °C and 70–75% relative humidity. Transcript levels for all conditions were expressed relative to FH Navelate fruits. Data are the mean values of three biological replicates ±SE.
Discussion
The working hypothesis was that the ABA deficiency may be an important factor for the high susceptibility of Pinalate fruit to dehydration and to NCPP. To test this hypothesis and to understand the molecular mechanisms underlying both processes in citrus fruit, a comparative large-scale transcriptional analysis was performed in harvested Navelate, Pinalate and ABA-treated Pinalate fruits stored under conditions (12 °C and 70–75% relative humidity) causing moderate water stress and peel damage. The higher susceptibility to NCPP (Fig. 1A) and dehydration (Fig. 1B) observed in Pinalate fruit agreed with previous data showing that, under the same storage conditions, fruit weight loss and the decrease in water potential of the flavedo tissue was higher in fruits of the mutant (Alférez et al., 2005).
Differential gene expression analysis (Fig. 2) further revealed the higher ability of Navelate fruit to develop earlier molecular responses to post-harvest dehydration. These responses might contribute to reduce detrimental effects caused by dehydration and hence to the delay in peel damage development with respect to mutant fruit, which showed evident damage by 1 week. Thus, GO analysis revealed that the most specific biological processes induced only in Navelate fruit by 1 week were the response to water deprivation and di-, tri-valent inorganic cation transport (Table 2), which fit into the classical plant responses to water deficit and osmotic adjustment (Shinozaki et al., 1998; Ramanjulu and Bartels, 2002). This result was also in concordance with previous findings showing that transport and abiotic stress-related genes are differentially regulated by dehydration in detached grape berries (Grimplet et al., 2007; Rizzini et al., 2009; Zamboni et al., 2010). As expected, most of the genes belonging to the response to water deprivation biological process (Table 3) were related to ABA. Thus, genes involved in ABA synthesis and perception (NCED1, ZEP, and PP2C), ABA-dependent transcription factors (HB7, NAC4, and ABF4), and also genes encoding ABA-responsive proteins (HVA22E, Lea5, and ADH) were identified, highlighting the fact that the responses of Navelate oranges to dehydration are modulated, at least in part, by this phytohormone. Among the ABA-dependent genes belonging to this process, it is also worth noting those encoding proteins with homology to the plasma membrane PIP1B and PIP1E aquaporins, as they play important roles in adjusting osmotic potential in dehydrated plants (Shinozaki et al., 1998; Shinozaki and Yamaguchi-Shinozaki, 2007). Therefore, and considering the fact that the number of stomata per surface area in fruits of both cultivars is similar (F. Alférez and L. Zacarías, unpublished data), the above results indicated a higher ability of Navelate fruits to synthesize ABA, which controls stomata closure to reduce dehydration, and also to modulate ABA-related genes important for cell homeostasis and viability and hence for the reduction of peel damage. Other genes within this process (e.g. CsRD19 and CsRD21) have not been classified as upregulated by ABA in different plant systems (Koizumi et al., 1993; Coupe et al., 2003). From the results of the present work, it cannot be ruled out that they are ABA-dependent in citrus fruits, as they were not induced by dehydration in the mutant. Nevertheless, genes within other categories such as CsCOPT5 and CsNRAMP3 were induced by dehydration in both Navelate and ABA-deficient Pinalate fruits. In addition, expression of these genes did not increase in Pinalate fruits after ABA treatment. Therefore, these results in citrus fruit might support previous findings suggesting the involvement of ABA-independent genes in the response to dehydration in plants (Riera et al., 2005). In this context, it should be mentioned that the occurrence of alternative dehydration-responsive pathway(s) to minimize water loss in plants under ABA deficiency has been reported (Wilkinson and Davies, 2010). Furthermore, it cannot be excluded that physico-chemical properties of the fruit surface may be altered in the mutant, as ABA may affect epicuticular wax biosynthesis in plants (Islam et al., 2009) and also cuticle permeability, development, and composition in fruits (Curvers et al., 2010). Although the effect of different hormones on the synthesis or morphology of epicuticular waxes have been shown in citrus fruits (El-Otmani et al., 1986; Cajuste et al., 2010), that of ABA has not yet been described. Therefore, the availability of the spontaneous Pinalate ABA-deficient mutant and its high susceptibility to dehydration encourages new investigations aimed at determining how ABA deficiency impacts on cuticle wax composition.
As well as the response to water deprivation process, the inorganic cation transport appeared to be operating in the lower susceptibility of Navelate fruit to dehydration and NCPP. The transport and/or sequestration of ions constitute a plant strategy to prevent water loss from the cytoplasm to the extracellular matrix and the subsequent osmotic stress caused by dehydration (Shinozaki et al., 1998; Ramanjulu and Bartels, 2002). Prevention of water and osmotic stress has been attributed mainly to potassium, chloride, and calcium ions. However, the results obtained in the present work revealed that the di-, tri-valent inorganic cation transport biological process, induced only in Navelate fruit by 1 week, involved calcium (ECA3 and GNC1), iron (FER4, IRT1, NRAMP1, and NRAMP3) and copper chelators and transporters (COPT1, COPT2, COPT5, and SAG14). Copper and iron cations are trace elements and, consequently, their concentration inside the cell should have little effect on cell osmotic pressure. Therefore, an attractive possibility from the present results is that these metal transporters may play a role in the tolerance of citrus fruit to dehydration by modulating ABA-responsive pathways. This would be in concordance with previous findings indicating that these ions may affect the ABA-dependent signal transduction pathway in plants (Sudo et al., 2008). Within the context of this work, it is noteworthy that iron and copper cations are required as co-factors of superoxide dismutases that may contribute to the lower susceptibility of Navelate fruit to developing NCPP (Sala et al., 2005). It is known that an excess of metals may lead to the disruption of cellular processes and finally to cell death, and that the prevention of such harmful effects requires the participation of metal-binding proteins and transporters (Puig et al., 2007). Thus, the higher increase in the expression levels of iron and copper transporters detected in the wild-type fruit (Fig. 4B) suggests that the impaired ability of the ABA-deficient mutant to regulate metal homeostasis could be relevant for its higher susceptibility to dehydration and NCPP.
Most of the differentially expressed genes were downregulated in the mutant by 3 weeks (Fig. 2B) and grouped into numerous biological processes (Table 2), with carbohydrate biosynthesis being the most specific. This is in agreement with previous results showing a higher reduction in soluble sugars and starch in Pinalate with respect to parental fruits during development of NCPP (Holland et al., 2005), and highlights the interplay between ABA and sugars in plants. This process grouped not only genes involved in the metabolism of soluble sugars and starch but also those involved in the metabolism of cell-wall polysaccharides and putative regulatory elements, such as a MYC transcription factor and a gene (CsIPS) involved in regulating the levels of inositol-3-phosphate, which constitutes a node for the crosslink between several signalling pathways (Kaur and Gupta, 2005). The CsMYC transcription factor displays 63% identity with the ABA-responsive AtMYC2, which triggers the slow adaptive response of Arabidopsis to dehydration (Abe et al., 2003; Bartels and Sunkar, 2005) and therefore the CsMYC transcript might be involved in the tolerance of citrus fruit to water stress. Nevertheless, this citrus gene appears not to be a limiting step in this process, as its expression levels continuously decreased in the ABA-deficient mutant but also in the parental fruit. Expression analysis showed that CsIPS transcript levels also decreased in Pinalate fruit for up to 6 weeks but transiently increased in the wild-type phenotype when the highest difference in NCPP between both varieties was observed (Fig. 4C, 3 weeks). This result suggests a higher availability of the second messenger inositol-3-phosphate in the wild type, which might favour putative signalling pathways involved in the protection of fruit against detrimental effects caused by water stress and NCPP, whereas these pathways might be impaired in the ABA-deficient mutant. The above results, together with the high number of downregulated genes belonging to the carbohydrate biosynthesis process in mutant fruit, and the well-known protective roles of sugars against osmotic and water stresses in plants (Bartels and Sunkar, 2005; Seki et al., 2007), suggest that the repression of this biological process is relevant for the susceptibility of citrus fruit to such stresses leading to peel damage. The repression of this process was also associated with the enhancement of NCPP in Navelate fruits exposed to a different stress (Establés-Ortiz et al., 2009), indicating the relevance of carbohydrate metabolism in the convergence of the mechanisms underlying NCPP.
The interpretation of results derived from the application of plant growth regulators to hormone-deficient mutants may be complex, as these treatments may fail to recover the wild-type phenotypes. Different examples can be found in the literature in fruits (Sandhu et al., 2011) and also in seedlings (Mahouachi et al., 2011), in spite of the ability of seedling plants to use foliar- or root-applied hormones and to translocate them to almost all plant parts (Mäkelä et al., 1996). Results from ABA treatment on Navelate fruits suggest that endogenous levels of this phytohormone might be sufficient to trigger cellular processes coping with dehydration and further consequences related to peel damage in the wild-type orange, as the NCPP index and weight loss were not significantly affected by ABA application (see Supplementary Fig. S1). Interestingly, application of ABA increased the hormone content in the flavedo of Pinalate mutant fruit to levels that were always slightly higher than those of the parental, triggered changes in the expression of thousands of genes, and repressed the protein ubiquitination biological process. However, it did not modify either the expression levels of a subset of ABA-regulated genes (Bartels and Sunkar, 2005) (Supplementary Table S1) or rescue the wild-type phenotype, as exogenous ABA slightly affected the incidence of NCPP and did not modify the cumulative weight loss of mutant fruits. Therefore, these results, together with those obtained by multivariate and qRT-PCR analyses (Figs 3 and 4), indicate that exogenous ABA modulates gene expression in Pinalate fruits but is not fully effective in either redirecting the mutant transcriptome towards that of the parental fruit or recovering its phenotype. These results might be unexpected, but there are several examples showing that ABA did not rescue the normal phenotype in ABA-deficient mutants (Busk and Pagès, 1998). In addition, plants may be less sensitive to exogenous ABA under normal conditions than to the stress-induced rises in endogenous ABA (Imay et al., 1995). In agreement with these ideas, Mahouachi et al. (2011) reported that ABA treatment did not stimulate physiological responses of papaya seedlings exposed to drought, whereas treatments favouring the rise of endogenous ABA levels were able to trigger physiological responses coping with dehydration. Taking these ideas together with the fact that Pinalate fruit has reduced ABA levels during the whole period of development and ripening (Rodrigo et al., 2003), the possibility of an altered ABA-perception system in Pinalate fruit, as reported in other hormone-deficient mutants (Guo and Ecker, 2004), or some defect in the ABA signalling transduction pathway that would impair its responses to the ABA treatment cannot be ruled out. Therefore, it would be interesting to investigate further whether there are differences in the regulation of the ABA-signalling components, which have been characterized recently in Arabidopsis (Park et al., 2009; Ma et al., 2009), between mutant and wild-type fruits under water-stress conditions.
In spite of the relevance of plant sensitivity for triggering hormone responses, Hoth et al. (2002) found that treating seedlings of the Arabidopsis ABA-insensitive mutant abi1-1 with ABA induced relevant changes in the expression of genes and processes regulated by the hormone, although, as expected, it did not rescue the typical ABA-insensitive phenotype. Modulation of protein ubiquitination was observed by these authors after ABA treatment. Interestingly, this was the only biological process downregulated by exogenous ABA in Pinalate fruits, which suggests the involvement of protein degradation in the ABA-signalling network in citrus fruits. In this context, it is also worth mentioning different reports associating this biological process with ABA signalling/responses in the model plant Arabidopsis (López-Molina et al., 2003; Zhang et al., 2005; Luo et al., 2006; Ryu et al., 2010). The six citrus genes grouped into the protein ubiquitination biological process encoded PUB domain-containing proteins with E3-ubiquitin ligase activity. Three of them (PUB9, PUB17, and PUB43) have been related to ABA (Samuel et al., 2008; Raab et al., 2009; Ni et al., 2010) and the others (PUB21, PUB24, and PUB29) to cell-death signalling and plant defence responses to biotic stress (Libault et al., 2007). In concordance with this, it was found that rots developed earlier (3 weeks) and with higher incidence during storage in ABA-treated mutant fruits with respect to non-treated mutant or parental fruits (see Supplementary Fig. S2, available in JXB online). Real-time expression analysis of CsPUB9 and CsPUB21 genes further revealed enhanced repression of transcript levels in ABA-treated Pinalate fruit, which further confirmed that the protein ubiquitination process may be negatively regulated by ABA treatment in mutant fruit. Therefore, these results suggest a crosslink between ABA and the modulation of defence responses in citrus fruit through proteins involved in the ubiquitin–proteasome system machinery.
In conclusion, the comparative transcriptional analysis between Navelate and its mutant Pinalate fruits highlights the ability of parental fruit to develop responses to reduce water loss and other detrimental consequences caused by this stress. These responses involve the water deprivation and the di-, tri-valent inorganic cation transport biological processes, which include both ABA-dependent and -independent genes. The alteration of these responses in the mutant fruit suggests their relevance for the prevention of peel damage in citrus fruit. Likewise, repression of the carbohydrate biosynthesis process occurred specifically in Pinalate fruits, which showed higher susceptibility to NCPP. Overall, the results suggest that the sensitivity/response to ABA may be impaired in ABA-deficient mutant fruit, and reveal molecular mechanisms triggering the response to water stress in citrus fruit.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Table S1. Representative set of ABA-regulated genes whose expression did not significantly change (SAM, FDR <0.01) in Pinalate fruits after ABA treatment with respect to FH Pinalate fruits.
Supplementary Fig. S1. NCCP index (A) and percentage of fruit weight loss per surface area (B) of Navelate fruits (squares) treated (white) or not (black) with ABA and stored for up to 6 weeks at 12 °C and 70–75% relative humidity. The arrows indicate when ABA was applied. Results are the means of three biological replicates of ten fruits each ±SE. Mean separation was performed by applying Tukey’s test. No significant differences (P ≤0.05) between samples for the same storage period were found.
Supplementary Fig. S2. Percentage of decay in Navelate (white), Pinalate (grey), and ABA-treated Pinalate (black) fruits stored at 12 °C and 70–75% relative humidity. Different letters for the same storage period indicate significant differences according to Tukey’s test (P ≤0.05).
Acknowledgments
Special thanks to Dr A. C. Adam and Dr B. Establés-Ortiz for their help in microarray hybridization and analysis. The bioinformatics assistance of Dr. J. Gadea (CFGP) is also gratefully acknowledged. We also thank to Dr L. Navarro (IVIA) for allowing us to use the Spanish Citrus Germoplasm Bank. This work was supported by the Spanish Ministry of Science and Technology (research grants AGL2006-09496, AGL2009-11969, and AGL2009-11558) and by the Generalitat Valenciana (grant PROMETEO/2010/010). P.R. was the recipient of a fellowship from the Spanish Ministry of Science and Technology.
Glossary
Abbreviations
- ABA
abscisic acid
- ANOVA
analysis of variance
- CFGP
Citrus Functional Genomic Project
- FDR
false discovery rate
- FH
freshly harvested
- GO
gene ontology
- HCA
hierarchical cluster analysis
- NCPP
non-chilling peel pitting
- PCA
principal component analysis
- SAM
significant analysis of microarrays
References
- Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell. 2003;15:63–78. doi: 10.1105/tpc.006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agustí J, Merelo P, Cercós M, Tadeo FR, Talón M. Ethylene-induced differential gene expression during abscission of citrus leaves. Journal of Experimental Botany. 2008;59:2717–2733. doi: 10.1093/jxb/ern138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agustí M, Almela V, Juan M, Alférez F, Tadeo FR, Zacarías L. Histological and physiological characterization of rind breakdown of ‘Navelate’ sweet orange. Annals of Botany. 2001;88:415–422. [Google Scholar]
- Al-Shahrour F, Díaz-Uriarte R, Dopazo J. FatiGO: a web tool for finding significant associations of Gene Ontology terms with groups of genes. Bioinformatics. 2004;20:578–580. doi: 10.1093/bioinformatics/btg455. [DOI] [PubMed] [Google Scholar]
- Alférez F, Agustí M, Zacarías L. Postharvest rind staining in Navel oranges is aggravated by changes in storage relative humidity: effect on respiration, ethylene production and water potential. Postharvest Biology and Technology. 2003;28:143–152. [Google Scholar]
- Alférez F, Lluch Y, Burns JK. Phospholipase A2 and postharvest peel pitting in citrus fruit. Postharvest Biology and Technology. 2008;49:69–76. [Google Scholar]
- Alférez F, Sala JM, Sánchez-Ballesta MT, Mulas M, Lafuente MT, Zacarías L. A comparative study of the postharvest performance of an ABA-deficient mutant of oranges: I. Physiological and quality aspects. Postharvest Biology and Technology. 2005;37:222–231. [Google Scholar]
- Alós E, Roca M, Iglesias DJ, et al. An evaluation of the basis and consequences of a stay-green mutation in the navel negra citrus mutant using transcriptomic and proteomic profiling and metabolite analysis. Plant Physiology. 2008;147:1300–1315. doi: 10.1104/pp.108.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong F, Leung J, Grabov A, Brearley J, Giraudat J, Blatt MR. Sensitivity to abscisic acid of guard-cell K+ channels is suppressed by abi1-1, a mutant Arabidopsis gene encoding a putative protein phosphatase. Proceedings of the National Academy of Sciences USA. 1995;92:9520–9524. doi: 10.1073/pnas.92.21.9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester AR, Lafuente MT, Forment J, Gadea J, de Vos RCH, Bovy AG, Gonzalez-Candelas L. Transcriptomic profiling of citrus fruit peel tissues reveals fundamental effects of phenylpropanoids and ethylene on induced resistance. Molecular Plant Pathology. 2011;12:879–897. doi: 10.1111/j.1364-3703.2011.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester AR, Lafuente MT, González-Candelas L. Spatial study of antioxidant enzymes, peroxidase and phenylalanine ammonia-lyase in the citrus fruit–Penicillium digitatum interaction. Postharvest Biology and Technology. 2006;39:115–124. [Google Scholar]
- Bartels D, Sunkar R. Drought and salt tolerance in plants. Critical Reviews in Plant Sciences. 2005;24:23–58. [Google Scholar]
- Bray EA, Bayley-Serres J, Weretilnyk E. Response to abiotic stresses. In: Gruissem W, Buchannan B, Jones R, editors. Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists: Rockville, MD; 2000. pp. 1158–1249. [Google Scholar]
- Brumós J, Colmenero-Flores JM, Conesa A, et al. Membrane transporters and carbon metabolism implicated in chloride homeostasis differentiate salt stress responses in tolerant and sensitive Citrus rootstocks. Functional & Integrative Genomics. 2009;9:293–309. doi: 10.1007/s10142-008-0107-6. [DOI] [PubMed] [Google Scholar]
- Burbidge A, Grieve TM, Jackson A, Thompson A, McCarty DR, Taylor IB. Characterization of the ABA-deficient tomato mutant notabilis and its relationship with maize Vp14. The Plant Journal. 1999;17:427–431. doi: 10.1046/j.1365-313x.1999.00386.x. [DOI] [PubMed] [Google Scholar]
- Busk PK, Pagès M. Regulation of abscisic acid-induced transcription. Plant Molecular Biology. 1998;37:425–435. doi: 10.1023/a:1006058700720. [DOI] [PubMed] [Google Scholar]
- Cajuste JF, González-Candelas L, Veyrat A, García-Breijo FJ, Reig-Armiñana J, Lafuente MT. Epicuticular wax content and morphology as related to ethylene and storage performance ‘Navelate’ orange fruit. Postharvest Biology and Technology. 2010;55:29–35. [Google Scholar]
- Cercós M, Soler G, Iglesias DJ, Gadea J, Forment J, Talón M. Global analysis of gene expression during development and ripening of citrus fruit flesh. A proposed mechanism for citric acid utilization. Plant Molecular Biology. 2006;62:513–527. doi: 10.1007/s11103-006-9037-7. [DOI] [PubMed] [Google Scholar]
- Coupe SA, Sinclair BK, Watson LM, Heyes JA, Eason JR. Identification of dehydration−responsive cysteine proteases during post−harvest senescence of broccoli florets. Journal of Experimental Botany. 2003;54:1045–1056. doi: 10.1093/jxb/erg105. [DOI] [PubMed] [Google Scholar]
- Curvers K, Seifi H, Mouille G, et al. Abscisic acid deficiency causes changes in cuticle permeability and pectin composition that influence tomato resistance to Botrytis cinerea. Plant Physiology. 2010;154:847–860. doi: 10.1104/pp.110.158972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluc L, Quilici D, Decendit A, et al. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genomics. 2009;10:212. doi: 10.1186/1471-2164-10-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Otmani M, Coggins VW, Jr, Eaks IL. Fruit age and gibberellic acid effect on epicuticularwax accumulation, respiration, and internal atmosphere of Navel orange fruit. Journal of the American Society for Horticultural Science. 1986;111:228–232. [Google Scholar]
- Establés-Ortiz B, Lafuente MT, González-Candelas L, Forment J, Gadea J. Transcriptomic analysis of ethylene-induced tolerance to non-chilling peel pitting in citrus fruit. Acta Horticulturae. 2009;839:555–560. [Google Scholar]
- Forment J, Gadea J, Huerta L, et al. Development of a citrus genome-wide EST collection and cDNA microarray as resources for genomic studies. Plant Molecular Biology. 2005;57:375–391. doi: 10.1007/s11103-004-7926-1. [DOI] [PubMed] [Google Scholar]
- Gandía M, Conesa A, Ancillo G, et al. Transcriptional response of Citrus aurantifolia to infection by Citrus tristeza virus. Virology. 2007;367:298–306. doi: 10.1016/j.virol.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Gimeno J, Gadea J, Forment J, et al. Shared and novel molecular responses of mandarin to drought. Plant Molecular Biology. 2009;70:403–420. doi: 10.1007/s11103-009-9481-2. [DOI] [PubMed] [Google Scholar]
- Grimplet J, Deluc L, Tillett R, Wheatley M, Schlauch K, Cramer G, Cushman J. Tissue-specific mRNA expression profiling in grape berry tissues. BMC Genomics. 2007;8:187. doi: 10.1186/1471-2164-8-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot SPC, Karssen CM. Dormancy and germination of abscisic acid-deficient tomato seeds: studies with the sitiens mutant. Plant Physiology. 1992;99:952–958. doi: 10.1104/pp.99.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ecker JR. The ethylene signaling pathway: new insights. Current Opinion in Plant Biology. 2004;7:40–49. doi: 10.1016/j.pbi.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Holland N, Menezes HC, Lafuente MT. Carbohydrate metabolism as related to high-temperature conditioning and peel disorders occurring during storage of citrus fruit. Journal of Agricultural and Food Chemistry. 2005;53:8790–8796. doi: 10.1021/jf051293o. [DOI] [PubMed] [Google Scholar]
- Hoth S, Morgante M, Sanchez JP, Hanafey MK, Tingey SV, Chua NH. Genome-wide gene expression profiling in Arabidopsis thaliana reveals new targets of abscisic acid and largely impaired gene regulation in the abi1-1 mutant. Journal of Cell Science. 2002;115:4891–4900. doi: 10.1242/jcs.00175. [DOI] [PubMed] [Google Scholar]
- Huerta L, Forment J, Gadea J, Fagoaga C, Peña L, Pérez-Amador MA, García-Martínez JL. Gene expression analysis in citrus reveals the role of gibberellins on photosynthesis and stress. Plant, Cell and Environment. 2008;61:1620–1633. doi: 10.1111/j.1365-3040.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- Imay R, Moses MS, Bray EA. Expression of an ABA-induced gene of tomato in transgenic tobacco during periods of water deficit. Journal of Experimental Botany. 1995;46:1077–1084. [Google Scholar]
- Islam M, Du H, Ning J, Ye H, Xiong L. Characterization of Glossy1-homologous genes in rice involved in leaf wax accumulation and drought resistance. Plant Molecular Biology. 2009;70:443–456. doi: 10.1007/s11103-009-9483-0. [DOI] [PubMed] [Google Scholar]
- Kaur N, Gupta AK. Signal transduction pathways under abiotic stresses in plants. Current Science. 2005;88:1771–1780. [Google Scholar]
- Koizumi M, Yamaguchi-Shinozaki K, Tsuji H, Shinozaki K. Structure and expression of two genes that encode distinct drought-inducible cysteine proteinases in Arabidopsis thaliana. Gene. 1993;129:175–182. doi: 10.1016/0378-1119(93)90266-6. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Alonso-Blanco C, Vreugdenhil D. Naturally occurring genetic variation in Arabidopsis thaliana. Annual Review of Plant Biology. 2004;55:141–172. doi: 10.1146/annurev.arplant.55.031903.141605. [DOI] [PubMed] [Google Scholar]
- Lafuente MT, Martínez-Téllez MA, Zacarías L. Abscisic acid in the response of ‘Fortune’ mandarins to chilling. Effect of maturity and high-temperature conditioning. Journal of the Science of Food Agriculture. 1997;73:494–502. [Google Scholar]
- Lafuente MT, Sala JM. Abscisic acid levels and the influence of ethylene, humidity and storage temperature on the incidence of postharvest rindstaning of ‘Navelina’ orange (Citrus sinensis L. Osbeck) fruit. Postharvest Biology and Technology. 2002;25:49–57. [Google Scholar]
- Lafuente MT, Zacarías L. Postharvest physiological disorders in citrus fruit. Stewart Postharvest Review. 2006;1:1–9. [Google Scholar]
- Libault M, Wan J, Czechowski T, Udvardi M, Stacey G. Identification of 118 Arabidopsis transcription factor and 30 ubiquitin-ligase genes responding to chitin, a plant-defense elicitor. Molecular Plant-Microbe Interactions. 2007;20:900–911. doi: 10.1094/MPMI-20-8-0900. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhu A, Chai L, et al. Transcriptome analysis of a spontaneous mutant in sweet orange (Citrus sinensis L. Osbeck) during fruit development. Journal of Experimental Botany. 2009;60:801–813. doi: 10.1093/jxb/ern329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Molina L, Mongrand S, Kinoshita N, Chua NH. AFP is a novel negative regulator of ABA signaling that promotes ABI5 protein degradation. Genes & Development. 2003;17:410–418. doi: 10.1101/gad.1055803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Shen G, Yan J, He C, Zhang H. AtCHIP functions as an E3 ubiquitin ligase of protein phosphatase 2A subunits and alters plant response to abscisic acid treatment. The Plant Journal. 2006;46:649–657. doi: 10.1111/j.1365-313X.2006.02730.x. [DOI] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Alexander C, Grill E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- Mahouachi J, Argamasilla R, Gómez-Cadenas A. Influence of exogenous glycine betaine and abscisic acid on papaya in responses to water-deficit stress. Journal of Plant Growth Regulation. 2011:1–10. [Google Scholar]
- Mäkelä P, Peltonen-Sainio P, Jokinen K, Pehu E, Setälä H, Hinkkanen R, Somersalo S. Uptake and translocation of foliar-applied glycinebetaine in crop plants. Plant Science. 1996;121:221–230. [Google Scholar]
- Martínez-Godoy MA, Mauri N, Juarez J, Marques MC, Santiago J, Forment J, Gadea J. A genome-wide 20 K citrus microarray for gene expression analysis. BMC Genomics. 2008;9:318. doi: 10.1186/1471-2164-9-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul P, McCollum GT, Popp M, Guy CL, Porat R. Transcriptome profiling of grapefruit flavedo following exposure to low temperature and conditioning treatments uncovers principal molecular components involved in chilling tolerance and susceptibility. Plant, Cell and Environment. 2008;31:752–768. doi: 10.1111/j.1365-3040.2008.01793.x. [DOI] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell and Environment. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- Ni X, Tian Z, Liu J, Song B, Li J, Shi X, Xie C. StPUB17, a novel potato UND/PUB/ARM repeat type gene, is associated with late blight resistance and NaCl stress. Plant Science. 2010;178:158–169. [Google Scholar]
- Norman SM, Poling SM, Maier VP. An indirect enzyme-linked immunosorbent assay for (+)-abscisic acid in Citrus, Ricinus, and Xanthium leaves. Journal of Agricultural and Food Chemistry. 1988;36:225–231. [Google Scholar]
- Park SY, Fung P, Nishimura N, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Cortes H, Sanchez-Serrano JJ, Mertens R, Willmitzer L, Prat S. Abscisic acid is involved in the wound-induced expression of the proteinase inhibitor II gene in potato and tomato. Proceedings of the National Academy of Sciences USA. 1989;86:9851–9855. doi: 10.1073/pnas.86.24.9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT−PCR. Nucleic Acids Research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig S, Andrés-Colás N, García-Molina A, Peñarrubia L. Copper and iron homeostasis in Arabidopsis: responses to metal deficiencies, interactions and biotechnological applications. Plant, Cell and Environment. 2007;30:271–290. doi: 10.1111/j.1365-3040.2007.01642.x. [DOI] [PubMed] [Google Scholar]
- Raab S, Drechsel G, Zarepour M, Hartung W, Koshiba T, Bittner F, Hoth S. Identification of a novel E3 ubiquitin ligase that is required for suppression of premature senescence in Arabidopsis. The Plant Journal. 2009;59:39–51. doi: 10.1111/j.1365-313X.2009.03846.x. [DOI] [PubMed] [Google Scholar]
- Ramanjulu S, Bartels D. Drought- and desiccation-induced modulation of gene expression in plants. Plant, Cell and Environment. 2002;25:141–151. doi: 10.1046/j.0016-8025.2001.00764.x. [DOI] [PubMed] [Google Scholar]
- Riera M, Valon C, Fenzi F, Giraudat J, Leung J. The genetics of adaptive responses to drought stress: abscisic acid-dependent and abscisic acid-independent signalling components. Physiologia Plantarum. 2005;123:111–119. [Google Scholar]
- Rizzini FM, Bonghi C, Tonutti P. Postharvest water loss induces marked changes in transcript profiling in skins of wine grape berries. Postharvest Biology and Technology. 2009;52:247–253. [Google Scholar]
- Rodrigo MJ, Marcos JF, Alférez F, Mallent MD, Zacarías L. Characterization of ‘Pinalate’, a novel Citrus sinensis mutant with a fruit-specific alteration that results in yellow pigmentation and decreased ABA content. Journal of Experimental Botany. 2003;54:727–738. doi: 10.1093/jxb/erg083. [DOI] [PubMed] [Google Scholar]
- Rodrigo MJ, Marcos JF, Zacarías L. Biochemical and molecular analysis of carotenoid biosynthesis in flavedo of orange (Citrus sinensis L.) during fruit development and maturation. Journal of Agricultural and Food Chemistry. 2004;52:6724–6731. doi: 10.1021/jf049607f. [DOI] [PubMed] [Google Scholar]
- Ryu MY, Cho SK, Kim WT. The Arabidopsis C3H2C3-type RING E3 ubiquitin ligase AtAIRP1 is a positive regulator of an abscisic acid-dependent response to drought stress. Plant Physiology. 2010;154:1983–1997. doi: 10.1104/pp.110.164749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed AI, Sharov V, White J, et al. TM4: a free, open-source system for microarray data management and analysis. BioTechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- Sala JM, Sánchez-Ballesta MT, Alférez F, Mulas M, Zacarías L, Lafuente MT. A comparative study of the postharvest performance of an ABA-deficient mutant of oranges: II. Antioxidant enzymatic system and phenylalanine ammonia-lyase in non-chilling and chilling peel disorders of citrus fruit. Postharvest Biology and Technology. 2005;37:232–240. [Google Scholar]
- Samuel MA, Mudgil Y, Salt JN, Delmas F, Ramachandran S, Chilelli A, Goring DR. Interactions between the S-domain receptor kinases and AtPUB-ARM E3 ubiquitin ligases suggest a conserved signaling pathway in Arabidopsis. Plant Physiology. 2008;147:2084–2095. doi: 10.1104/pp.108.123380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu AK, Gray DJ, Lu J, Gu L. Effects of exogenous abscisic acid on antioxidant capacities, anthocyanins, and flavonol contents of muscadine grape (Vitis rotundifolia) skins. Food Chemistry. 2011;126:982–988. [Google Scholar]
- Schwartz SH, Tan BC, Gage DA, Zeevaart JAD, McCarty DR. Specific oxidative cleavage of carotenoids by VP14 of maize. Science. 1997;276:1872–1874. doi: 10.1126/science.276.5320.1872. [DOI] [PubMed] [Google Scholar]
- Seki M, Umezawa T, Urano K, Shinozaki K. Regulatory metabolic networks in drought stress responses. Current Opinion in Plant Biology. 2007;10:296–302. doi: 10.1016/j.pbi.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. Journal of Experimental Botany. 2007;58:221–227. doi: 10.1093/jxb/erl164. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K, Mizoguchi T, et al. Molecular responses to water stress in Arabidopsis thaliana. Journal of Plant Research. 1998;111:345–351. [Google Scholar]
- Sudo E, Itouga M, Yoshida-Hatanaka K, Ono Y, Sakakibara H. Gene expression and sensitivity in response to copper stress in rice leaves. Journal of Experimental Botany. 2008;59:3465–3474. doi: 10.1093/jxb/ern196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrell FM. Tables of Surfaces and Volumes of Spheres and of Prolate and Oblate Spheroids, and Spheroidal Coefficients. University of California Press: Berkley, CA; 1946. [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proceedings of the National Academy of Sciences USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvardi MK, Czechowski T, Scheible WR. Eleven golden rules of quantitative RT-PCR. Plant Cell. 2008;20:1736–1737. doi: 10.1105/tpc.108.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano K, Kurihara Y, Seki M, Shinozaki K. ‘Omics’ analyses of regulatory networks in plant abiotic stress responses. Current Opinion in Plant Biology. 2010;13:132–138. doi: 10.1016/j.pbi.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, de Preter K, Pattyn F, Poppe B, Van Roy N, de Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034-RESEARCH0034.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vij S, Tyagi AK. Emerging trends in the functional genomics of the abiotic stress response in crop plants: review article. Plant Biotechnology Journal. 2007;5:361–380. doi: 10.1111/j.1467-7652.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- Weiler EW. Radioimmunoassays for the differential and direct analysis of free and conjugated abscisic acid in plant extracts. Planta. 1980;148:262–272. doi: 10.1007/BF00380037. [DOI] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ. Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant, Cell and Environment. 2010;33:510–525. doi: 10.1111/j.1365-3040.2009.02052.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stress. Annual Review of Plant Biology. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- Zamboni A, Di Carli M, Guzzo F, et al. Identification of putative stage-specific grapevine berry biomarkers and omics data integration into networks. Plant Physiology. 2010;154:1439–1459. doi: 10.1104/pp.110.160275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Garreton V, Chua NH. The AIP2 E3 ligase acts as a novel negative regulator of ABA signaling by promoting ABI3 degradation. Genes & Development. 2005;19:1532–1543. doi: 10.1101/gad.1318705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziliotto F, Begheldo M, Rasori A, Bonghi C, Tonutti P. Transcriptome profiling of ripening nectarine (Prunus persica L. Batsch) fruit treated with 1-MCP. Journal of Experimental Botany. 2008;59:2781–2791. doi: 10.1093/jxb/ern136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.