Abstract
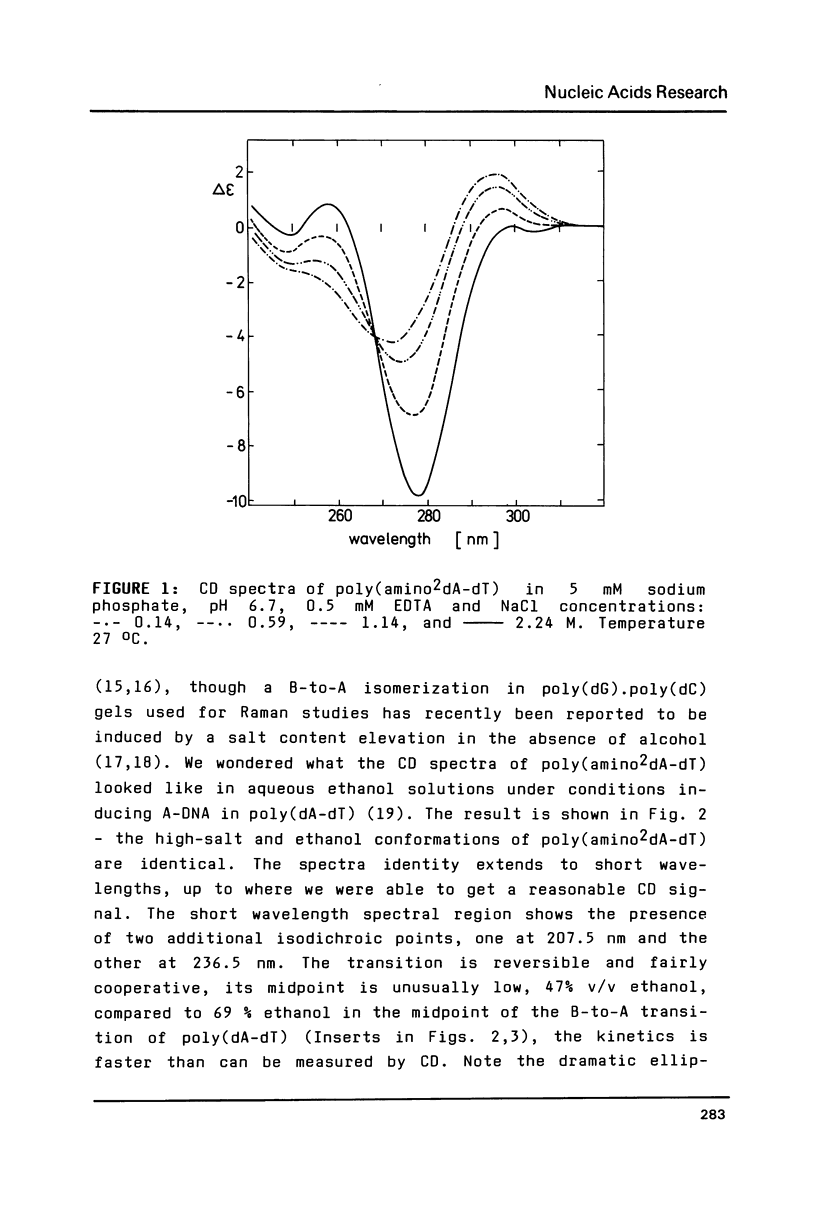
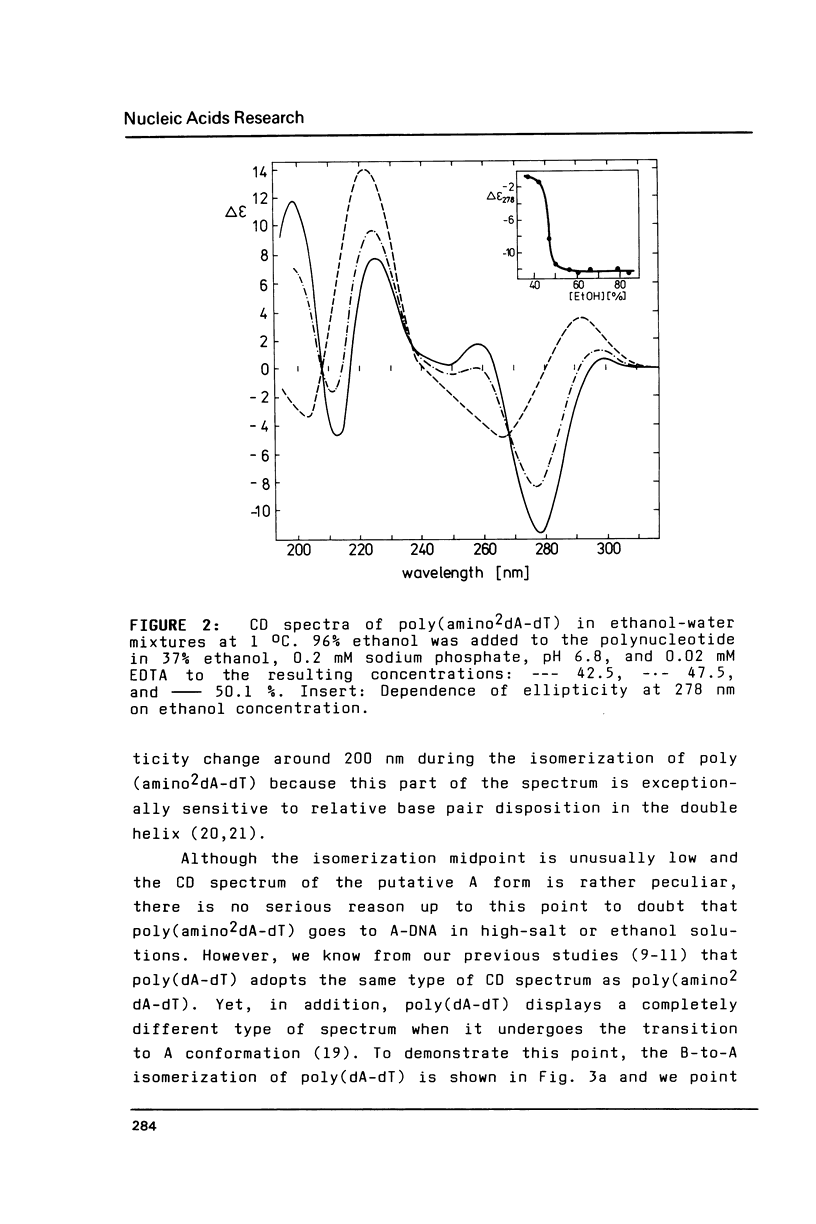
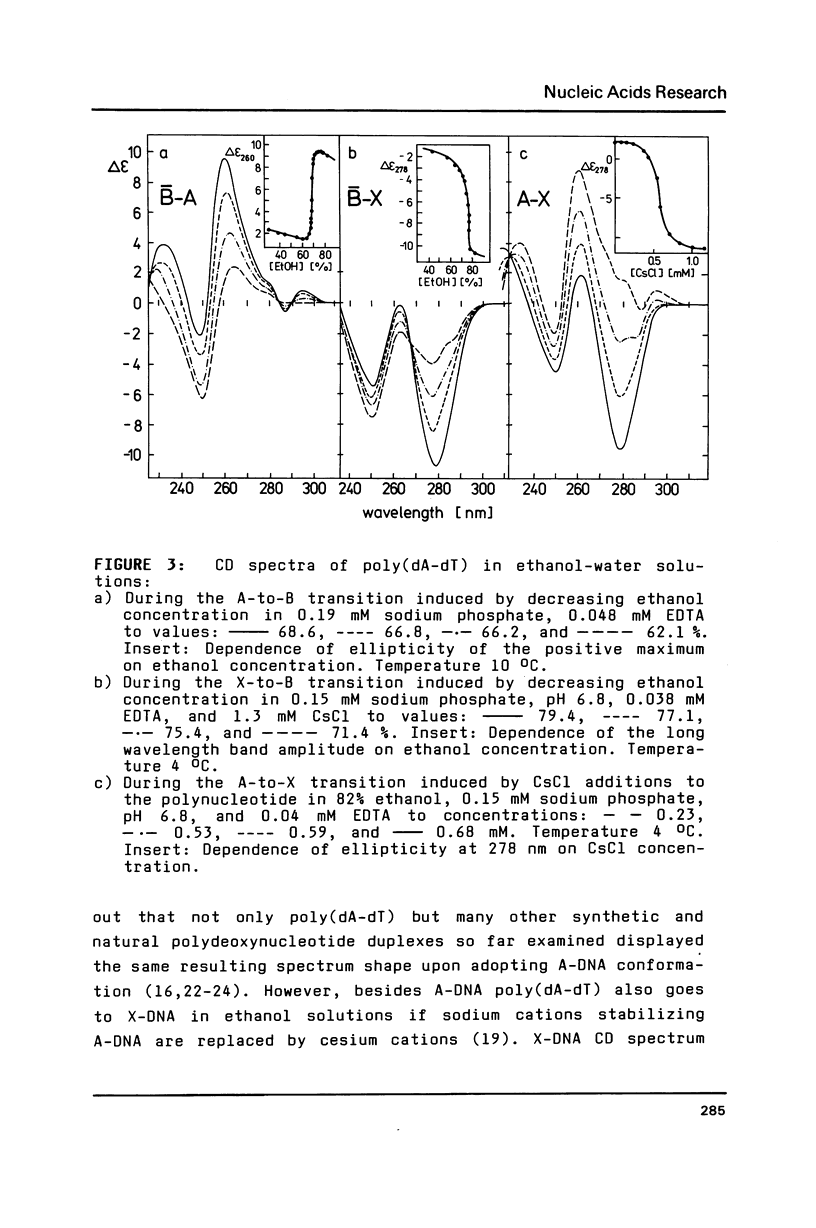
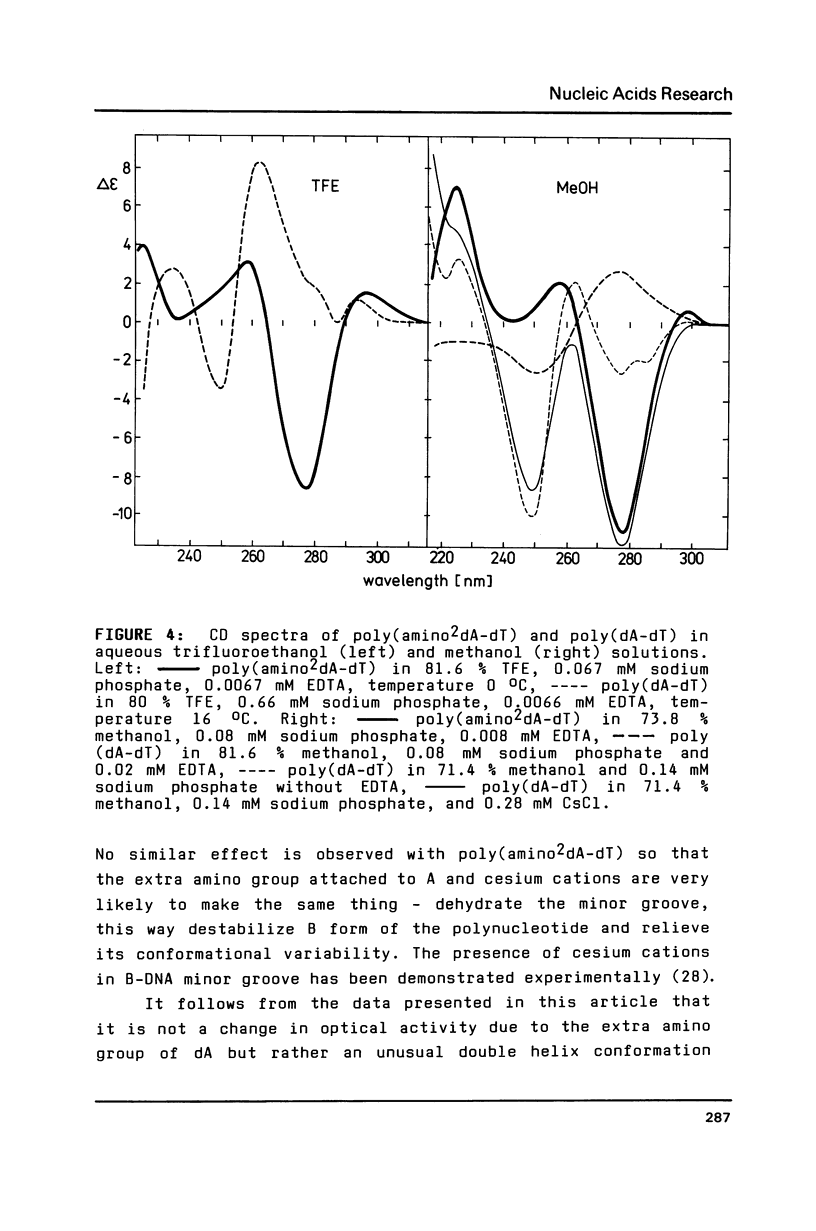
It has previously been demonstrated by other workers that the duplex of a synthetic DNA poly(amino2dA-dT) undergoes a salt-induced conformational isomerization. We show in the present work using circular dichroism that the same isomerization is induced in poly(amino2dA-dT) by various alcohols. The isomerization was originally identified as the B-to-Z and then B-to-A conformational transition of DNA but we demonstrate that the high-salt or alcohol conformation of poly (amino2dA-dT) is the non Z-DNA zig-zag double helix we have previously observed with poly(dA-dT) and called X-DNA. X-DNA is a cesium cation specific conformation of poly(dA-dT) while no similar cation specificity is observed with poly(amino2dA-dT). Thus it appears that the extra amino group attached to A and cesium cations make the same thing; they probably dehydrate the double helix minor groove and relieve its conformational variability. Poly(amino2dA-dT) is exceptionally stable in X-DNA and conditions inducing it are mild, which opens the door to assess its molecular structure.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAHMS J., MOMMAERTS W. F. A STUDY OF CONFORMATION OF NUCLEIC ACIDS IN SOLUTION BY MEANS OF CIRCULAR DICHROISM. J Mol Biol. 1964 Oct;10:73–88. doi: 10.1016/s0022-2836(64)80029-2. [DOI] [PubMed] [Google Scholar]
- Benevides J. M., Wang A. H., Rich A., Kyogoku Y., van der Marel G. A., van Boom J. H., Thomas G. J., Jr Raman spectra of single crystals of r(GCG)d(CGC) and d(CCCCGGGG) as models for A DNA, their structure transitions in aqueous solution, and comparison with double-helical poly(dG).poly(dC). Biochemistry. 1986 Jan 14;25(1):41–50. doi: 10.1021/bi00349a007. [DOI] [PubMed] [Google Scholar]
- Borah B., Cohen J. S., Howard F. B., Miles H. T. Poly(d2NH2A-dT): two-dimensional NMR shows a B to A conversion in high salt. Biochemistry. 1985 Dec 3;24(25):7456–7462. doi: 10.1021/bi00346a064. [DOI] [PubMed] [Google Scholar]
- Borah B., Howard F. B., Miles H. T., Cohen J. S. Conversions of poly(2-aminodeoxyadenylate-5-halodeoxyuridylate) from B to A forms in high salt. An NMR and circular dichroism study. Biochemistry. 1986 Nov 18;25(23):7464–7470. doi: 10.1021/bi00371a031. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E. Structure of a B-DNA dodecamer. III. Geometry of hydration. J Mol Biol. 1981 Sep 25;151(3):535–556. doi: 10.1016/0022-2836(81)90009-7. [DOI] [PubMed] [Google Scholar]
- Gaffney B. L., Marky L. A., Jones R. A. The influence of the purine 2-amino group on DNA conformation and stability. Synthesis and conformational analysis of d[T(2-aminoA)]3. Nucleic Acids Res. 1982 Jul 24;10(14):4351–4361. doi: 10.1093/nar/10.14.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. M., Ratliff R. L. Circular dichroism spectra of poly[d(AC):d(GT)], poly[r(AC):r(GU)], and hybrids poly[d(AC):r(GU)] and poly[r(AC):d(GT)] in the presence of ethanol. Biopolymers. 1975 Mar;14(3):487–498. doi: 10.1002/bip.1975.360140305. [DOI] [PubMed] [Google Scholar]
- Howard F. B., Chen C. W., Cohen J. S., Miles H. T. Poly(d2NH2A-dT): effect of 2-amino substituent on the B to Z transition. Biochem Biophys Res Commun. 1984 Feb 14;118(3):848–853. doi: 10.1016/0006-291x(84)91472-4. [DOI] [PubMed] [Google Scholar]
- Howard F. B., Miles H. T. 2NH2A X T helices in the ribo- and deoxypolynucleotide series. Structural and energetic consequences of 2NH2A substitution. Biochemistry. 1984 Dec 18;23(26):6723–6732. doi: 10.1021/bi00321a068. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Krylov DYu, Minyat E. E. Three-state diagram for DNA. J Biomol Struct Dyn. 1985 Aug;3(1):43–55. doi: 10.1080/07391102.1985.10508397. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Minyat E. E., Frank-Kamenetskii M. D., Schyolkina A. K. The B to A transition of DNA in solution. J Mol Biol. 1974 Aug 25;87(4):817–833. doi: 10.1016/0022-2836(74)90086-2. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minyat E. E. The transitions between left- and right-handed forms of poly(dG-dC). Nucleic Acids Res. 1981 Sep 25;9(18):4783–4798. doi: 10.1093/nar/9.18.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovin T. M., McIntosh L. P., Arndt-Jovin D. J., Zarling D. A., Robert-Nicoud M., van de Sande J. H., Jorgenson K. F., Eckstein F. Left-handed DNA: from synthetic polymers to chromosomes. J Biomol Struct Dyn. 1983 Oct;1(1):21–57. doi: 10.1080/07391102.1983.10507425. [DOI] [PubMed] [Google Scholar]
- Kypr J., Vorlícková M. Conformations of alternating purine-pyrimidine DNAs in high-CsF solutions and their reversal by dipyrandium, ethidium and high temperature. Biochim Biophys Acta. 1985 Feb 15;838(2):244–251. doi: 10.1016/0304-4165(85)90085-6. [DOI] [PubMed] [Google Scholar]
- Leslie A. G., Arnott S., Chandrasekaran R., Ratliff R. L. Polymorphism of DNA double helices. J Mol Biol. 1980 Oct 15;143(1):49–72. doi: 10.1016/0022-2836(80)90124-2. [DOI] [PubMed] [Google Scholar]
- Malenkov G., Minchenkova L., Minyat E., Schyolkina A., Ivanov V. The nature of the B-A transition of DNA in solution. FEBS Lett. 1975 Mar 1;51(1):38–42. doi: 10.1016/0014-5793(75)80850-7. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Torigoe C., Tsuboi M. Salt induced B----A transition of poly(dG).poly(dC) and the stabilization of A form by its methylation. Nucleic Acids Res. 1986 Mar 25;14(6):2737–2748. doi: 10.1093/nar/14.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Sprecher C. A., Baase W. A., Johnson W. C., Jr Conformation and circular dichroism of DNA. Biopolymers. 1979 Apr;18(4):1009–1019. doi: 10.1002/bip.1979.360180418. [DOI] [PubMed] [Google Scholar]
- Sutherland J. C., Griffin K. P., Keck P. C., Takacs P. Z. Z-DNA: vacuum ultraviolet circular dichroism. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4801–4804. doi: 10.1073/pnas.78.8.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sági J., Szabolcs A., Szemź A., Otvös L. Modified polynucleotides. VII. Impaired integrity of a synthetic DNA containing the antiherpetic agent 5-isopropyl-2'-deoxyuridine. Nucleic Acids Res. 1986 Apr 25;14(8):3449–3462. doi: 10.1093/nar/14.8.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unis M. J., Hearst J. E. On the hydration of DNA. II. Base composition dependence of the net hydration of DNA. Biopolymers. 1968;6(9):1345–1353. doi: 10.1002/bip.1968.360060909. [DOI] [PubMed] [Google Scholar]
- Vorlícková M., Kypr J. Conformational variability of poly(dA-dT).poly(dA-dT) and some other deoxyribonucleic acids includes a novel type of double helix. J Biomol Struct Dyn. 1985 Aug;3(1):67–83. doi: 10.1080/07391102.1985.10508399. [DOI] [PubMed] [Google Scholar]
- Vorlícková M., Kypr J., Sklenár V. Salt-induced conformational transition of poly[d(A-T)] X poly[d(A-T)]. J Mol Biol. 1983 May 5;166(1):85–92. doi: 10.1016/s0022-2836(83)80052-7. [DOI] [PubMed] [Google Scholar]
- Vorlícková M., Minyat E. E., Kypr J. Cooperative changes in the chiroptical properties of DNA induced by methanol. Biopolymers. 1984 Jan;23(1):1–4. doi: 10.1002/bip.360230102. [DOI] [PubMed] [Google Scholar]
- Vorlícková M., Sedlácek P., Kypr J., Sponar J. Conformational transitions of poly(dA-dT)poly(dA-dT) in ethanolic solutions. Nucleic Acids Res. 1982 Nov 11;10(21):6969–6979. doi: 10.1093/nar/10.21.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C., Tymen S., Marck C., Guschlbauer W. Conformational transitions of poly(dA-dC).poly(dG-dT) induced by high salt or in ethanolic solution. Nucleic Acids Res. 1982 Feb 11;10(3):1081–1091. doi: 10.1093/nar/10.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]


