Abstract
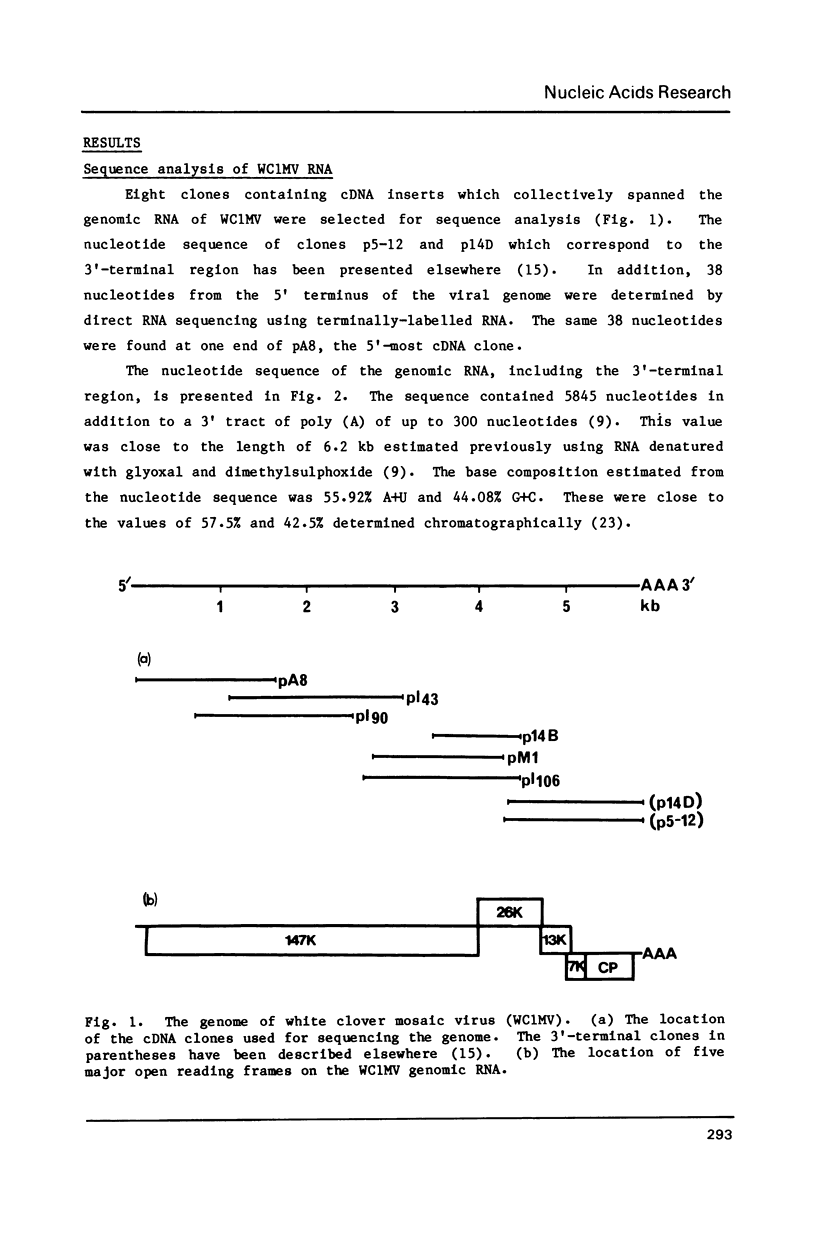
The complete nucleotide sequence (5845 nucleotides) of the genomic RNA of the potexvirus white clover mosaic virus (WC1MV) has been determined from a set of overlapping cDNA clones. Forty of the most 5'-terminal nucleotides of WC1MV showed homology to the 5' sequences of other potexviruses. The genome contained five open reading frames which coded for proteins of Mr 147, 417, Mr 26,356, Mr 12,989, Mr 7,219 and Mr 20,684 (the coat protein). The Mr 147,417 protein had domains of amino acid sequence homology with putative polymerases of other RNA viruses. The Mr 26,356 and Mr 12,989 proteins had homology with proteins of the hordeivirus barley stripe mosaic virus RNA beta and the furovirus beet necrotic yellow vein virus (BNYVV) RNA-2. A portion of the Mr 26,356 protein was also conserved in the cylindrical inclusion proteins of two potyviruses. The Mr 7,219 protein had homology with the 25K putative fungal transmission factor of BNYVV RNA-3.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Shih D. S., Zimmern D., Kaesberg P. Two-step binding of eukaryotic ribosomes to brome mosaic virus RNA3. Nature. 1979 Sep 27;281(5729):277–282. doi: 10.1038/281277a0. [DOI] [PubMed] [Google Scholar]
- Ahlquist P., Strauss E. G., Rice C. M., Strauss J. H., Haseloff J., Zimmern D. Sindbis virus proteins nsP1 and nsP2 contain homology to nonstructural proteins from several RNA plant viruses. J Virol. 1985 Feb;53(2):536–542. doi: 10.1128/jvi.53.2.536-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Cowie A., Tyndall C., Kamen R. Sequences at the capped 5'-ends of polyoma virus late region mRNAs: an example of extreme terminal heterogeneity. Nucleic Acids Res. 1981 Dec 11;9(23):6305–6322. doi: 10.1093/nar/9.23.6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domier L. L., Franklin K. M., Shahabuddin M., Hellmann G. M., Overmeyer J. H., Hiremath S. T., Siaw M. F., Lomonossoff G. P., Shaw J. G., Rhoads R. E. The nucleotide sequence of tobacco vein mottling virus RNA. Nucleic Acids Res. 1986 Jul 11;14(13):5417–5430. doi: 10.1093/nar/14.13.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach R. Genome similarities between plant and animal RNA viruses. Microbiol Sci. 1987 Jul;4(7):197–202. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Guilley H., Carrington J. C., Balàzs E., Jonard G., Richards K., Morris T. J. Nucleotide sequence and genome organization of carnation mottle virus RNA. Nucleic Acids Res. 1985 Sep 25;13(18):6663–6677. doi: 10.1093/nar/13.18.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson G., Armour S. L. The complete nucleotide sequence of RNA beta from the type strain of barley stripe mosaic virus. Nucleic Acids Res. 1986 May 12;14(9):3895–3909. doi: 10.1093/nar/14.9.3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Hiles I. D., Salmond G. P., Gill D. R., Downie J. A., Evans I. J., Holland I. B., Gray L., Buckel S. D., Bell A. W. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986 Oct 2;323(6087):448–450. doi: 10.1038/323448a0. [DOI] [PubMed] [Google Scholar]
- Kamer G., Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984 Sep 25;12(18):7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig R. Nucleic acids in the potato virus X group and in some other plant viruses: comparison of the molecular weights by electrophoresis in acrylamide-agarose composite gels. J Gen Virol. 1971 Jan;10(1):111–114. doi: 10.1099/0022-1317-10-1-111. [DOI] [PubMed] [Google Scholar]
- Short M. N., Davies J. W. Narcissus mosaic virus: a potexvirus with an encapsidated subgenomic messenger RNA for coat protein. Biosci Rep. 1983 Sep;3(9):837–846. doi: 10.1007/BF01133782. [DOI] [PubMed] [Google Scholar]
- Sonenberg N., Shatkin A. J., Ricciardi R. P., Rubin M., Goodman R. M. Analysis of terminal structures of RNA from potato virus X. Nucleic Acids Res. 1978 Jul;5(7):2501–2512. doi: 10.1093/nar/5.7.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma A., Gibbs A. J., Woods R. D. A comparative study of red clover vein mosaic virus and some other plant viruses. J Gen Virol. 1970 Jul;8(1):21–32. doi: 10.1099/0022-1317-8-1-21. [DOI] [PubMed] [Google Scholar]
- Wodnar-Filipowicz A., Skrzeczkowski L. J., Filipowicz W. Translation of potato virus X RNA into high molecular weight proteins. FEBS Lett. 1980 Jan 1;109(1):151–155. doi: 10.1016/0014-5793(80)81331-7. [DOI] [PubMed] [Google Scholar]


