Abstract
Although priming with replicating adenovirus type 5 host range mutant (Ad5hr)-human immunodeficiency virus (HIV)/simian immunodeficiency virus (SIV) recombinants, followed by HIV/SIV envelope boosting, has proven highly immunogenic, resulting in protection from SIV/simian-human immunodeficiency virus (SHIV) challenges, Ad5hr recombinant distribution, replication, and persistence have not been examined comprehensively in nonhuman primates. We utilized Ad5hr-green fluorescent protein and Ad5hr-SIV recombinants to track biodistribution and immunogenicity following mucosal priming of rhesus macaques by the intranasal/intratracheal, sublingual, vaginal, or rectal route. Ad recombinants administered by all routes initially targeted macrophages in bronchoalveolar lavage (BAL) fluid and rectal tissue, later extending to myeloid dendritic cells in BAL fluid with persistent expression in rectal mucosa 25 weeks after the last Ad immunization. Comparable SIV-specific immunity, including cellular responses, serum binding antibody, and mucosal secretory IgA, was elicited among all groups. The ability of the vector to replicate in multiple mucosal sites irrespective of delivery route, together with the targeting of macrophages and professional antigen-presenting cells, which provide potent immunogenicity at localized sites of virus entry, warrants continued use of replicating Ad vectors.
INTRODUCTION
Mucosally administered, replication-competent adenovirus type 5 host range mutant (Ad5hr)-human immunodeficiency virus (HIV)/simian immunodeficiency virus (SIV) recombinants, coupled with HIV/SIV envelope boosting, mimic live viral vaccines by engaging all components of the immune system, eliciting cellular, humoral, innate, and mucosal immune responses. This replicating Ad recombinant prime-boost approach elicits potent T cell immunity (35) and functional systemic and mucosal antibodies mediating neutralization (2, 43), antibody-dependent cellular cytotoxicity, antibody-dependent cell-mediated viral inhibition, and transcytosis inhibition (13, 15, 16, 20, 40). Memory B cells that recall functional antibody activity develop (3) along with increased antibody avidity (40), indicative of maturation. The vaccine strategy elicits strong protective efficacy in nonhuman primates (33, 35).
Despite this history of immunogenicity and protective efficacy, we know little about Ad5hr biodistribution and replication in mucosally immunized nonhuman primates. In chimpanzees, following intranasal (IN) priming, human Ad is shed from the gut for 8 to 13 days, suggesting active replication, while less shedding into nasal or pharyngeal secretions occurs (28, 29). In contrast, IN/oral priming of rhesus macaques with Ad5hr recombinants results in greater shedding into nasal secretions (mean, 30 days) compared to that in the gut (4 to 8 days) (5, 34). We postulate that this persistent virus expression primes the immune system efficiently and works in concert with protein boosting to broaden protective immunity.
Our vaccine regimen, using sequential IN plus oral and then intratracheal (IT) priming, is based on the preferential replication of Ad5hr in the upper respiratory tract (URT). IN immunization can induce T and B cell immunity in the genital tract, a key site of HIV entry (6, 11). However, other mucosal routes of replicating Ad delivery may lead to broader in vivo distribution and enhanced local immunity. An alternative to nasal administration, the sublingual (SL) route, is as effective as IN administration in inducing mucosal and systemic T cell responses and antibodies to cholera toxin in mice (9). Vaccine, administered under the tongue in a small volume, becomes available to a dense network of dendritic cells in the SL mucosa. SL delivery is also as effective in inducing cytotoxic T lymphocytes and antibody-secreting cells in the genital mucosa as IN or intravaginal (IVag) immunization and is better than intragastric delivery (8). Moreover, SL immunization with human papillomavirus (HPV)-like particles in cholera toxin adjuvant resulted in protection of mice from HPV challenge (8). Further, mice administered tetanus toxoid in mucosal adjuvant (32) or HIV gp41 and reverse transcriptase peptide coupled to cholera B subunit (18) by the SL route developed antibodies and cytotoxic T cells in the female genital tract and systemic compartments.
Although HIV is transmitted principally across rectal/genital mucosae, few studies have investigated IVag or intrarectal (IR) delivery of HIV vaccines. Drawbacks to IVag immunization include effects of hormonal fluctuations and the menstrual cycle on induction of reproducible immunity. Moreover, IR immunization may not be readily or widely acceptable. Nevertheless, vaccination at both sites might elicit strong local immunity. HPV pseudovirions encapsidating a respiratory syncytial virus (RSV) DNA vaccine induced RSV-specific systemic and mucosal immunity in mice after IVag vaccination (17). Further, a trimeric HIV gp140 protein delivered vaginally in a stabilizing polymeric gel to guinea pigs elicited genital tract IgG and IgA and serum IgG (10). IR priming of rhesus macaques with a simian-human immunodeficiency virus (SHIV) DNA followed by vector and envelope boosting elicited transient SIV-specific IgA in rectal secretions and systemic cellular and humoral immunity, although protection against SHIV89.6P acquisition was not obtained (38).
Here we compared the biodistribution and persistence of replication-competent Ad5hr recombinants delivered by i.n./IT, SL, IVag, and IR routes. Additionally, we compared the systemic and mucosal immunity elicited by the four priming regimens followed by boosts with SIV envelope protein. We show that unlike replication-defective vectors which maintain localized anatomic distribution (22), replication-competent Ads are distributed throughout the macaque regardless of immunization route, target tissue macrophages and myeloid dendritic cells (mDCs), and persist in rectal tissue macrophages. Consequently, cellular and humoral immune responses are comparable across immunization routes. Our results strengthen the utility of replicating vectors for vaccine design, showing that they are distributed throughout the body, overcoming restrictions on immunization route. Further, their targeting of antigen-presenting cells (APCs) and persistence at rectal sites allow long-term induction of mucosal antibody, known to be capable of blocking HIV/SIV transmission in animal models.
MATERIALS AND METHODS
Immunogens and study design.
An Ad5hr recombinant expressing green fluorescent protein (GFP) was constructed by subcloning the GFP gene from the Stratagene pShuttle-IRES-hrGFP-1 vector along with a downstream bovine growth hormone poly(A) (BGHpA) signal sequence (pCDNA3.1 plasmid; Invitrogen) into a plasmid containing the Ad5 tripartite leader (pAd5tpl-18RD2; Wyeth Lederle Vaccines). The resultant plasmid, pAd5tpl-GFP-BGHpA, was digested with XbaI, and the sequence was inserted into the Ad5 shuttle vector with an E3 deletion as described previously (19). Replication-competent virus was generated by homologous recombination. After 4 rounds of plaque purification, the insert was confirmed by DNA sequencing, and GFP expression was confirmed by fluorescence microscopy and flow cytometry of Ad-GFP-transduced 293 cells.
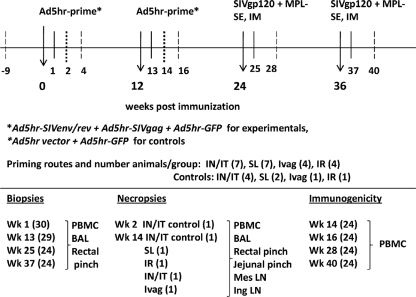
Ten female and 20 male rhesus macaques, negative for SIV, simian retrovirus type D, and simian T cell leukemia virus, were housed according to NIH animal care guidelines at Bioqual, Inc., Rockville, MD. Priming immunizations (Fig. 1) for experimental macaques included Ad5hr-GFP, Ad5hr-SIVSMH4 env/rev, and Ad5hr-SIV239 gag (42) (5 × 108 PFU of each recombinant; total, 1.5 × 109 PFU/immunization). Control macaques received Ad5-GFP (5 × 108 PFU) plus Ad5hr empty vector (1 × 109 PFU). Ad recombinant mixtures in phosphate-buffered saline (PBS) were administered SL under the tongue in 0.25 ml; IN (0.25 ml/nostril); and IT, IVag, and IR in 0.5 ml. IVag vaccinations were administered at midfollicular phase, avoiding menstruation and the postovulation period, when innate and adaptive immunity are depressed in preparation for pregnancy (39). SIVmac251 gp120 protein, 100 μg/macaque, was given intramuscularly in a 1:10 final dilution of monophosphoryl lipid A-stable emulsion (MPL-SE; Corixa).
Fig 1.
Immunization, sampling, and study design. Two priming immunizations with an equal mixture of the 3 separate Ad5hr recombinants listed were given to the experimental macaque groups at weeks 0 and 12. Groups were defined by the route of Ad immunizations, either intranasal at week 0/intratracheal at week 12 (i.n./IT) or SL, Ivag, or IR at both weeks 0 and 12. The total number of animals in each group is shown in parentheses. Control animals receiving empty vector plus Ad5hr-GFP priming were divided among all four route groups. Experimental macaques received SIV gp120 in MPL-SE intramuscularly (IM) at weeks 24 and 36. Controls received adjuvant only. Biopsy, necropsy, and immunogenicity time points are listed, with the total number of animals sampled indicated in parentheses. Tissues sampled for each time point are listed. Arrows, time of immunizations; solid lines, time of biopsies; heavy dashed lines, time of necropsies; long dashed lines, time of immunogenicity measurements; Mes LN, mesenteric lymph node; Ing LN, inguinal lymph node.
Tissue processing and cell isolation.
Mononuclear lymphocytes were isolated from EDTA blood by centrifugation over Ficoll-Paque Plus (GE) as described previously (20, 33). Viable cells in 40 to 50 ml of bronchoalveolar lavage (BAL) fluid were isolated after centrifugation over 35%/65% Percoll gradients. The middle whitish layer was directly stained for flow analysis. Rectal and jejunal tissues (biopsy specimens, 10 to 12 pinches; necropsy specimens, 30 to 40 pinches) were teased apart with 23-gauge needles and then digested with shaking at 37°C with RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, and penicillin-streptomycin (R10) plus collagenase II (1 mg/ml; Sigma) for 1 h, changing to fresh R10 plus collagenase after 30 min. Digested tissue was pressed through a 70-μm-pore-size filter. The cells in the resultant single-cell suspension were counted in trypan blue and stained for flow cytometry (1 × 106 to 2 × 106 cells/tube).
GFP expression.
GFP expression among CD4+ and CD8+ T cells, monocytes/macrophages, mDCs, plasmacytoid dendritic cells (pDCs), B cells, and epithelial cells was assessed for each tissue and each macaque. Viable cells were washed twice with PBS and stained with 5 μl Aqua live/dead amine-reactive dye (Invitrogen) at a 1:40 dilution in PBS. After 10 min, cells were washed once in PBS, resuspended in fluorescence-activated cell sorter (FACS) buffer (PBS plus 1% FBS), and surface stained with antibodies (all from BD BioSciences, unless noted) to CD4 (Qdot 605, custom conjugation; Invitrogen), CD8 (Qdot 655, custom conjugation; Invitrogen), CD14 (Texas Red-phycoerythrin [TR-PE]; Caltag, Invitrogen), CD123 (peridinin chlorophyll protein [PerCP]-Cy5.5), CD20 (PE-Cy7; Biolegend), CD11c (allophycocyanin [APC]), and HLA-DR (APC-Cy7) for 20 min at room temperature. Cells were washed twice with FACS buffer, and 250 μl of fix/perm solution (BD) was added for 20 min at 4°C. After 2 washes with 1× BD wash buffer, intracellular staining with antibodies to CD3 (Alexa-700) and cytokeratin (Pan-reactive, PE; Abcam) was performed for 20 min at 4°C, followed by acquisition on an LSRII flow cytometer (BD Biosciences), compensated with similarly stained beads using FACS DIVA (version 6.0) software. Data were analyzed using FlowJo software (TreeStar).
Ad5-specific T cell activation.
Fresh peripheral blood mononuclear cells (PBMCs; 1 × 106 to 2 × 106) were stimulated for 6 h with an Ad5 fiber peptide pool (142 15-mers overlapping by 11 amino acids; final concentration, 2 μg/ml) and stained with fluorescent antibodies (all from BD Biosciences, unless noted), including CD3 (Alexa-700), CD4 (PerCP-Cy5.5), CD8 (APC-Cy7), CD28 (TR-PE; Invitrogen), CD95 (APC), CCR7 (PE-Cy7), CCR5 (fluorescein isothiocyanate), and Ki67 (PE). Samples were acquired on an LSRII flow cytometer, and data were analyzed using FACS DIVA software.
In situ hybridization.
GFP RNA was detected in formalin-fixed and paraffin-embedded tissues as described previously (25). Briefly, 6- to 8-mm sections were cut and adhered to silanized slides. After deparaffinization in xylene, rehydration in PBS, and permeabilization by treating the sections with HCl, digitonin, and proteinase K, the sections were acetylated and hybridized to 35S-labeled SIV-specific riboprobes. After washing and digestion with RNases, sections were coated with nuclear track emulsion, exposed, and developed.
Ad5 neutralization assay.
Ad5-specific serum neutralization titers were measured as described previously (23). Titer was defined as the reciprocal of the serum dilution at which a 50% reduction in luciferase activity was obtained relative to that of preimmunization serum diluted 1:20.
ELISPOT and CFSE proliferation assays.
SIV-specific gamma interferon (IFN-γ)-secreting cells were measured in fresh PBMCs after stimulation with pools of SIV239 Gag or SIVsmH4 Env peptides (15-mers overlapping by 11 amino acids) using enzyme-linked immunosorbent spot (ELISPOT) assay kits as described previously (30). Gag peptides (total, 125) were obtained from the NIH AIDS Research and Reference Reagent Program, and Env peptides (total, 214) were obtained from Advanced BioScience Laboratories, Inc. (ABL), Gaithersburg, MD. Assays were carried out in triplicate. After subtraction of spots in medium-only wells, the mean numbers of spot-forming cells (SFC) per million PBMCs were recorded.
T cell proliferative responses were assessed by carboxyfluorescein diacetate succinimidyl ester (CFSE) assay as described previously (31). Briefly, fresh PBMCs (1 × 106/ml) were labeled with 1 μM CFSE (Invitrogen) for 10 min in the dark, washed three times with PBS, and cultured for 6 days in medium alone or with native SIVmac251 gp120 (21) or p27 protein (both from ABL) at a 2-μg/ml final concentration. Cells were stained with anti-CD3 PE, anti-CD4 APC, and anti-CD8 PE-Cy7 (all BD antibodies), fixed in 1% paraformaldehyde, and acquired and analyzed using FACSCalibur flow cytometry and CellQuest-Pro software (BD Biosciences). Antigen-specific proliferation was calculated by subtracting the proportion of proliferating cells in unstimulated samples from the proliferating fraction in stimulated samples.
Antibody responses.
SIV gp120-specific binding antibody titers in sera were assayed by enzyme-linked immunosorbent assay (ELISA) (5). Titer was defined as the reciprocal of the serum dilution at which absorbance was equivalent to twice the absorbance of normal rhesus macaque serum diluted 1:50.
SIV gp120-specific secretory IgA (sIgA) titers were determined by ELISA using anti-monkey secretory component for detection. Briefly, mucosal samples were 2-fold serially diluted, applied to a half-area 96-well plate (Greiner Bio-One) coated with 1 μg/ml SIVmac251 gp120 protein, and incubated at 4°C overnight. Horseradish peroxidase (HRP)-conjugated goat anti-monkey secretory component (Nordic) and tetramethylbenzidine substrate were used sequentially, followed by reading of the absorbance at 450 nm. Endpoint titer was defined as the reciprocal of the dilution at which the absorbance of the test sample was equal to twice the mean background.
Ad5hr recombinant shedding.
Ad5hr recombinant shedding in rectal and nasal swabs and in saliva was evaluated 4 and 10 weeks after the first and second Ad5hr-SIV recombinant priming immunizations. Secretions were clarified by low-speed centrifugation and were scored positive or negative using a nested PCR assay with primers specific for the Ad5 fiber gene (5).
Statistical analysis.
Comparison of Ad-GFP expression and antibody titers between immunization groups used the exact Kruskal-Wallis test. Comparison of Ad-GFP expression between different time points made use of the Wilcoxon signed-rank test. Frequency differences seen among tissues were analyzed by the Mann-Whitney U test.
RESULTS
Ad-GFP expression after Ad priming.
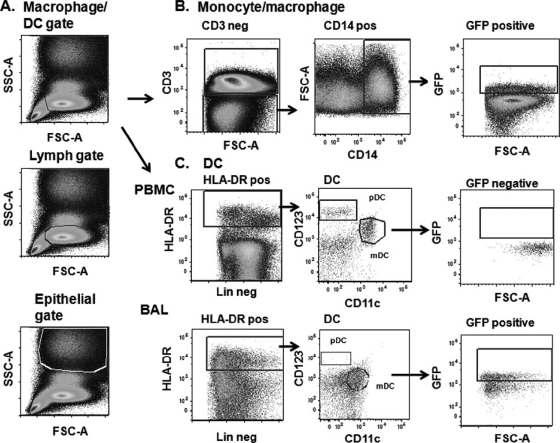
Our primary goals were to examine Ad-GFP biodistribution and SIV Env- and Gag-specific immune responses in the i.n./IT and SL immunization groups (7 macaques/group; Fig. 1). IVag and IR priming immunizations (4 macaques/group) were included to determine if i.n./IT and SL delivery would target rectal/genital sites as readily as local administration and elicit immune responses as potent. Ad-GFP expression was evaluated (Fig. 2). After singlet gating and exclusion of dead cells, forward scatter (FSC) versus side scatter (SSC) distinguished cells on the basis of size and granularity (Fig. 2A). A tight gate was applied to analyze lymphocytes. A slightly larger one was used for monocytes/macrophages and DCs. Larger epithelial cells, discerned using the epithelial BSC-1 cell line, appeared in the top half of the graph. Representative GFP expression among CD3− CD14+ monocytes/macrophages in PBMCs is shown in Fig. 2B. For DC gating, lineage-negative CD3−, CD14−, and CD20− cells, followed by HLA-DR-positive cells, were selected. Subsequently, CD123 versus CD11c distinguished mDCs (CD11c+) from pDCs (CD123+) in both PBMCs (Fig. 2C, top) and BAL fluid (Fig. 2C, bottom). GFP-positive epithelial cells were detected with a pancytokeratin antibody.
Fig 2.
General gating strategy. (A) Representative initial cell size gating, including DCs/macrophages, lymphocytes, and epithelial cells, used for all tissues, shown here for a PBMC sample. (B) Determination of GFP expression in the monocyte/macrophage gate within a representative PBMC sample. (C) Gating for GFP-positive cells in the dendritic cell gate in PBMCs (top) and BAL fluid (bottom). Lin, lineage. Final positive GFP expression only among gated mDCs in BAL fluid is shown, since no positive GFP expression was observed in PBMCs.
Ad-GFP expression in biopsy and necropsy tissue specimens was assessed 1 and 2 weeks, respectively, after each Ad immunization. Although Ad shedding in macaques can last as long as 30 days (5, 34), peak replication occurs within the first week (37). Here, Ad-GFP expression in biopsy samples was seen after each Ad immunization, with lesser or no expression seen in necropsy tissue specimens (data not shown). Only results from the biopsy samples are reported below.
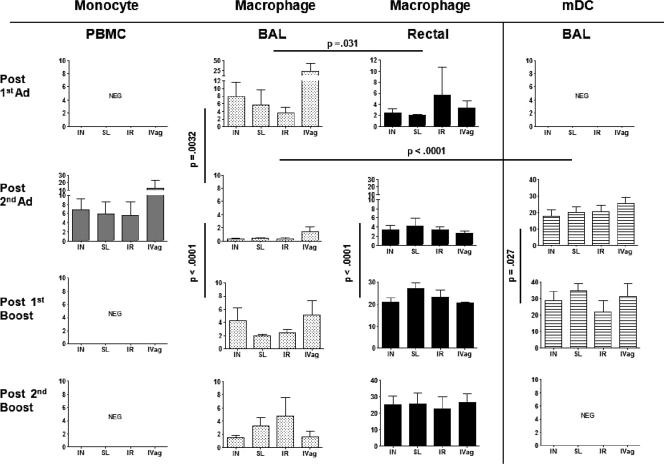
One week after the first Ad immunization, Ad-GFP expression was detectable only in BAL fluid and rectal tissue macrophages of each immunization group (Fig. 3), with no differences in expression frequency detected between groups. Overall, expression frequency was higher in BAL fluid (4 to >20% of macrophages) than rectal tissue (2 to 6%) (P = 0.031).
Fig 3.
Frequency of GFP expression among total live cells in multiple tissues over time. For PBMCs and BAL fluid, average expression ± SEM for all macaques is shown for each group at biopsy time points after the 1st Ad immunization (n = 18), after the 2nd Ad immunization (n = 28), and after the 1st and 2nd boosts (n = 24). At some time points, fewer rectal tissue biopsy specimens were obtained. Shown are results for after the 1st (n = 8) and 2nd (n = 28) Ad immunizations and after the 1st (n = 16) and 2nd (n = 24) protein boosts. NEG, negative for GFP expression (<0.1% GFP positivity). Significant differences between BAL fluid and rectal tissue macrophages after the first Ad immunization, between BAL fluid macrophages after the first and second Ad immunizations, and between BAL fluid macrophages and mDCs after the second Ad immunization were obtained as shown across all immunization groups. Significant differences across all immunization groups were seen for BAL fluid and rectal tissue macrophages and BAL fluid mDCs between the second Ad immunization and first protein boost.
One week after the second Ad immunization, Ad-GFP expression exhibited an expanded distribution, including PBMC monocytes and BAL fluid mDCs (Fig. 3). Expression frequency in PBMCs was low (∼5% of total monocytes), with no differences seen between groups. The detection of GFP-positive peripheral blood monocytes suggested the potential for continued Ad spread in vivo. GFP expression in BAL fluid macrophages across the 4 groups (<2%) was significantly lower than that in BAL fluid macrophages after the first Ad immunization (P = 0.0032) and that in BAL fluid mDCs (15 to 25%) after the second Ad immunization (P = <0.0001). The first Ad priming of BAL fluid macrophages may have facilitated the expanded distribution to BAL fluid mDCs due to persistent virus. In the URT, uptake of virus by professional APCs such as mDCs might enhance immune system priming. GFP expression in mDCs was seen only in BAL fluid samples over the course of immunization, irrespective of Ad immunization route. Notably, Ad-GFP expression persisted in rectal tissue macrophages of all groups at the same frequency seen after the first Ad administration (Fig. 3).
As Ad infects epithelial cells lining mucosal surfaces, we expected that they would exhibit a high frequency of Ad-GFP expression. However, in 24 of 27 animals, <2% of epithelial cells expressed GFP, with no differences between routes. However, epithelial cell viability was low, suggesting that the isolation and preservation methods may not have been optimal. Further, the window of peak Ad-GFP expression was perhaps missed.
Ad-GFP expression in cells and tissues after envelope boosting.
One week after the first protein boost, GFP expression in BAL fluid and rectal tissue macrophages persisted, with no differences among immunization routes. The expression frequency in BAL fluid across all groups was higher than that seen after the second Ad immunization (P < 0.0001), possibly due to diminished anti-Ad cellular immunity, which declines over time (36). Compared to BAL fluid, GFP expression frequency in rectal tissue macrophages was significantly elevated (20 to 25%; P < 0.0001) (Fig. 3). Ongoing Ad expansion and persistence were noted in BAL fluid mDCs, with higher GFP expression compared to that for the time point after the second Ad immunization (P = 0.027) (Fig. 3). Overall, the mean fluorescent intensity (MFI) of GFP expression on both BAL fluid and rectal tissue macrophages was elevated in all immunization groups (20,000 to 25,000) at all time points tested (data not shown) compared to that on PBMC monocytes (mean MFI, 1,000) after the second Ad immunization.
One week after the second protein boost, 25 weeks after the second Ad immunization, high, persistent GFP expression was again observed in 20 to 25% of rectal tissue macrophages and continued low-level expression was observed in BAL fluid (Fig. 3), with no differences in frequency or MFI seen among immunization groups.
GFP expression by in situ hybridization.
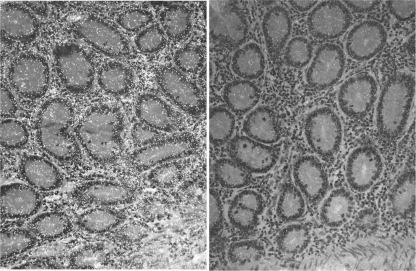
Multiple necropsy tissue specimens were collected 2 weeks after the second Ad immunization for in situ hybridization. Consistent with the failure to detect Ad-GFP expression by flow cytometry in necropsy samples obtained after the first immunization, GFP-positive cells were not readily detected. Ad replication was likely declining, and the tissue sections examined may have missed small foci of Ad-infected cells. GFP-positive mRNA staining was detected in only one jejunal tissue specimen (Fig. 4). The positive cells are most likely macrophages, given their distribution, although macrophage staining was not performed.
Fig 4.
In situ hybridization for GFP in jejunal tissue 2 weeks after the 2nd Ad immunization. (Left) GFP RNA-positive signals appear as whitish dots in transmitted light, located mostly in lamina propria. (Right) Negative-control staining.
Ad recombinant replication.
To evaluate Ad recombinant replication and persistence, we monitored viral shedding in various secretions. Ad was detected by nested PCR most readily in nasal secretions (Table 1). The majority of i.n./IT-immunized macaques shed Ad into nasal secretions following each administration. However, Ad was also regularly detected at this site in the other immunization groups, indicating the wide biodistribution of the replicating virus. Ad was detected with much less frequency in rectal swabs and saliva, suggestive of the preference for Ad to replicate in the URT. Whereas Ad can be detected in stool samples, here we collected rectal secretions rather than stool for immunologic studies. This may explain the lower frequency of viral detection at this site. Further, since secretions were routinely collected at 4 and 10 weeks postimmunization, peak virus replication most likely was missed.
Table 1.
Ad5hr recombinant replication and specific neutralizing antibody responsea
| Group | Ad5hr shedding (no. positive/no. tested) |
Geometric mean Ad5 neutralizing antibody titer |
||||||
|---|---|---|---|---|---|---|---|---|
| Nasal secretion |
Rectal swab |
Saliva |
||||||
| After 1st Ad | After 2nd Ad | After 1st Ad | After 2nd Ad | After 1st Ad | After 2nd Ad | After 1st Ad | After 2nd Ad | |
| SL | 4/9 | 3/8 | 0/9 | 1/8 | 1/9 | 0/8 | 19 | 26 |
| i.n./IT | 9/10 | 7/8 | 0/10 | 0/8 | 3/10 | 0/8 | 150 | 3444 |
| IVag | 3/5 | 1/4 | 1/5 | 1/4 | 0/5 | 0/4 | 28 | 256 |
| IR | 1/5 | 1/4 | 1/5 | 0/4 | 1/5 | 0/4 | 13 | 36 |
Ad5hr shedding was assessed at weeks 4 and 10 after the first and second Ad immunizations. Results reflect shedding at either time point. Ad5 neutralizing antibody titers in serum were determined 4 weeks after each Ad administration.
We also evaluated Ad5-specific neutralizing antibody titers as an indirect measure of Ad expression (Table 1). The highest titers were in sera of i.n./IT-immunized macaques, implicating expanded viral replication in the URT. However, neutralizing antibodies developed in all immunization groups, indicating that the viral load was sufficient to elicit a systemic antivector response.
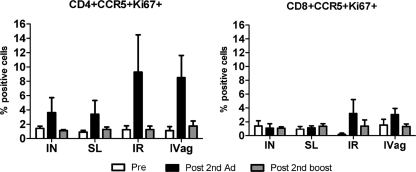
Finally, we investigated Ad-specific T cell activation in PBMCs (Fig. 5). Compared to preimmunization levels, after the second Ad immunization CD4+ CCR5+ Ki67+ T cells were elevated in all immunization groups and CD8+ CCR5+ Ki67+ cells were elevated to a lesser extent in the IVag and IR groups. Both declined to baseline levels after the second protein boost.
Fig 5.
Ad5-specific T cell activation among PBMCs during immunization. PBMCs obtained preimmunization, after the 2nd Ad prime (week 20), and after the 2nd boost (week 40) were stimulated with Ad5 fiber peptides. The percentage of total CD4+ and CD8+ cells expressing CCR5+ Ki67+ staining is shown for each immunization group. Mean values ± SEMs are shown.
SIV-specific cellular immune responses.
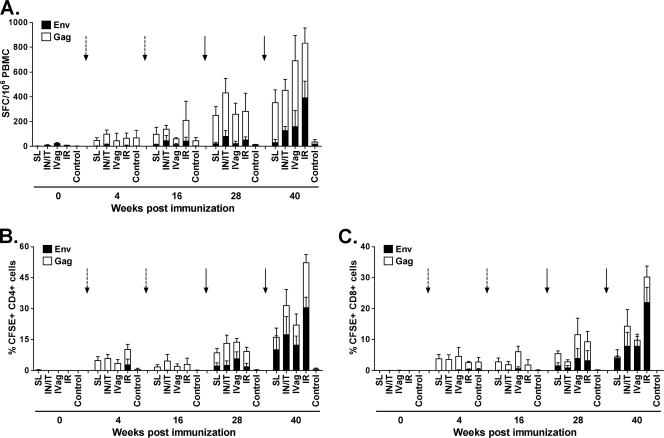
To determine if the immunization routes impacted development of cellular immunity, we examined PBMCs for SIV-specific IFN-γ-secreting cells by ELISPOT assay. Low-level responses to Gag were detected after the first Ad immunization and were enhanced over the immunization course (Fig. 6A). Env-specific responses were low or undetectable until after the second envelope boost. SL priming elicited the lowest responses, but overall, there were no significant differences in ELISPOT assay levels across experimental groups.
Fig 6.
Induction of SIV-specific cellular immunity by the prime-boost immunization regimens. (A) IFN-γ ELISPOT assay responses after peptide stimulation of PBMCs with SIV239 Gag or SIVSMH4 envelope peptide pools over the immunization course for each immunization group. Arrows indicate priming (dotted) or boosting (solid) time points. Means ± SEMs for each stimulation condition are shown. (B and C) Proliferative responses (mean ± SEM) among CD4 and CD8 T cells, respectively, after stimulation with SIVmac251 p27 (open boxes) or SIVmac251 gp120 (solid boxes) protein at the same time points as in panel A.
SIV Gag- and Env-specific CD4+ and CD8+ T cell proliferative response patterns resembled the ELISPOT assay results (Fig. 6B and C). The predominant responses were to SIV Gag and were enhanced over the course of immunization. SIV Env-specific proliferation was clearly enhanced by the gp120 boosts. By week 40, there were no significant differences in levels of CD4 or CD8 proliferative T cell responses across the immunization groups.
SIV-specific humoral immune responses.
We also investigated induction of SIV-specific antibodies. Following the second protein boost, all macaques showed high titers of SIV gp120-specific binding antibody (Table 2). The titers were not equivalent across groups (P = 0.037) due to lower titers in the SL group and higher titers in the IR group. SIV Env-specific IgA was observed in BAL fluid, and again, the levels were not equivalent over the immunization groups (P = 0.011) due to significantly lower levels in the IVag group (P = 0.0098). Env-specific sIgA was elicited in rectal and vaginal secretions in all immunization groups. SIV-specific sIgA levels in rectal secretions exhibited no differences across immunization groups. Statistical analysis of vaginal sIgA was not possible due to the small number of female macaques studied.
Table 2.
SIV Env-specific antibody responses following two envelope protein boosts
| Immunization route | Serum binding titer (geometric mean) | gp120-specific IgA/total IgA in BAL fluid (fold increase [ng/μg] over control) | Fold increase in gp120-specific sIgA titer over controla |
|
|---|---|---|---|---|
| Rectal secretion | Vaginal secretionb | |||
| SL (n = 6) | 128,000 | 3.3 | 3.0 | 2.0 |
| i.n./IT (n = 6) | 406,375 | 4.6 | 5.1 | 4.0 |
| IVag (n = 3) | 512,000 | 1.4 | 2.2 | 4.7 |
| IR (n = 3) | 812,749 | 2.9 | 6.2 | 128.0 |
| Control (n = 6) | <50 | 1.0 | 1.0 | 1.0 |
Reported as fold increase in mean titer over that of the control group. Actual sIgA titers in rectal and vaginal secretions from macaques in the 4 immunization groups and the control group are reported by Xiao et al. (40a).
There were 7 female macaques in total. Secretions were obtained from 3 in the IVag group and from 1 each in the other groups. Therefore, statistical analysis was not done.
DISCUSSION
The potent immunogenicity and protective efficacy of replicating Ad5hr-HIV/SIV prime-protein boost regimens in nonhuman primates are well documented (2, 3, 13, 20, 28, 33–35, 40). Despite this history, we know little about the replication kinetics, distribution, and persistence of replicating Ad5hr vectors in rhesus macaques. The ability of Ad5hr to prime durable immunity may be due to properties different from those of nonreplicating viral or DNA vaccines. As serial sacrifice of many macaques was not feasible, we simultaneously examined biodistribution and immunogenicity after Ad5hr recombinant administration by various mucosal routes.
Regardless of immunization route, Ad-GFP expression was detected at all sites in the mucosa but was mainly detected in BAL fluid and rectal tissue, whereas expression in peripheral blood was observed only transiently. Ad did not infect T or B cells but targeted monocytes/macrophages and, in the lung, mDCs. Macrophages, due to their phagocytic potential, repeatedly exhibited a high GFP expression frequency which persisted up to 25 weeks after immunization with replicating Ad recombinants, hinting at a potential reservoir and source of continual antigen presentation. Analysis of total cell (not just GFP-expressing cell) numbers after Ad administration showed significant increases in the percentage of macrophages in rectal tissue in all animals after the 1st (P = 0.021) and 2nd (P = 0.011) Ad immunizations, with a concomitant decrease in mDCs after the 1st one (P = 0.011) (data not shown). The influx of macrophages into rectal mucosa, the primary site of HIV/SIV exposure, is potentially important. A cause for the decreased mDC numbers might be localized mDC death. More interestingly, if mDCs migrate out of rectal tissue, they may traffic back to draining lymph nodes, present antigen, and contribute to effective priming.
mDC are highly efficient APCs and are necessary for CD4+ T cell activation at mucosal sites, which contributes to induction of mucosal IgA (12). mDCs also stimulate CD8+ T cell responses following immunization with nonreplicating Ad5 vectors (26). Replication-defective Ad5 and Ad35 vectors encoding cytomegalovirus (CMV) pp65 preferentially infect mDCs, induce differentiation, and activate CMV-specific polyfunctional CD4/8 T cell responses (27). Here, replicating Ad recombinants effectively primed mDCs in the lung, illustrating the targeting of critical APCs.
With induction of Ad5-specific neutralizing antibodies, Ad shedding, a surrogate for replication, was decreased after a second Ad immunization. However, no diminution of Ad-GFP expression frequency was observed in rectal tissue macrophages. If Ad is sequestered within tissue macrophages and is spread only locally, Ad-specific serum antibodies may cause only limited dampening of virus priming by the vaccine vector. Ad type C serotypes (including Ad5) can establish latent infections with intermittent reactivation in tonsils and adenoids, as modeled in human cell lines (41). However, we saw no GFP expression in lymphocytes from PBMCs or lymph nodes at any time point of biopsy or necropsy specimen collection for any macaque.
The broad Ad biodistribution regardless of immunization route and uniform targeting of particular cell types were mirrored by the induction of similar SIV-specific cellular immunity and Env-specific serum antibodies across all immunization groups. Slightly lower antibody titers developed in the SL group and slightly higher titers developed in the IR group. Whether this reflects the small number of macaques per group or a real difference will await further study. Notably, all groups developed gp120-specific mucosal IgA responses. Although the IVag group developed less SIV-specific IgA in BAL fluid, all immunization groups exhibited similar levels of gp120-specific sIgA in rectal secretions. The results suggest that replicating Ad vaccines may be administered by the easiest and safest route with the expectation that effective immunity will result.
With regard to safety, the Step trial raised concerns that immunization of Ad-immune individuals with Ad vectors might lead to activation of CD4+ T cells, targets of HIV infection, thereby enhancing HIV infection (4). We observed only transient activation of CD4+ CCR5+ cells in vitro in all immunization groups, although the vector persisted. Samples were not sufficient to examine activation of mucosal CD4+ T cells, more relevant to potential enhancement. Further studies designed to examine mucosal cells may clarify this question.
The SL route was found to be as effective as the i.n./IT route in eliciting SIV-specific immune responses. The efficacy of this route might be improved if the vaccine is formulated in a gel-like substance. Here it was administered in liquid form, which may have allowed quick dispersal of the Ad recombinants. Use of SL delivery in an improved formulation could preclude concerns about the IN route regarding potential trafficking of the vector to the olfactory bulb (24). Replicating wild-type oral Ad4 and Ad7 vaccines are fully licensed and have been administered to over 10 million people over a 25-year period (14). However, the oral route is not as immunogenic as the IN route, and the oral vaccine is more difficult to manufacture.
Our study demonstrates that there is no restriction for replicating Ad administration dependent on route. Mucosal cells are targeted, especially in the lung and rectum, and importantly for vaccine design and induction of comprehensive immunity, they persist in professional APCs. Continued development of these vectors is warranted. Future studies will draw on these results and seek to further optimize induction of systemic and mucosal immunity.
ACKNOWLEDGMENTS
We gratefully acknowledge Bioqual, Inc., veterinarians and animal caretakers for their expertise in animal care, sample collection, and administration of vaccines and challenge virus. We thank Charles Wira for helpful discussion.
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: SIVmac239 Gag peptides, complete set. This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
We have no conflicts of interest to report.
Footnotes
Published ahead of print 21 March 2012
REFERENCES
- 1. Reference deleted.
- 2. Bogers WM, et al. 2008. Systemic neutralizing antibodies induced by long interval mucosally primed systemically boosted immunization correlate with protection from mucosal SHIV challenge. Virology 382:217–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brocca-Cofano E, et al. 2011. Vaccine-elicited SIV and HIV envelope-specific IgA and IgG memory B cells in rhesus macaque peripheral blood correlate with functional antibody responses and reduced viremia. Vaccine 29:3310–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buchbinder SP, et al. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buge SL, et al. 1997. An adenovirus-simian immunodeficiency virus env vaccine elicits humoral, cellular, and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J. Virol. 71:8531–8541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen K, Cerutti A. 2010. Vaccination strategies to promote mucosal antibody responses. Immunity 33:479–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reference deleted.
- 8. Cuburu N, et al. 2009. Sublingual immunization with nonreplicating antigens induces antibody-forming cells and cytotoxic T cells in the female genital tract mucosa and protects against genital papillomavirus infection. J. Immunol. 183:7851–7859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cuburu N, et al. 2007. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine 25:8598–8610 [DOI] [PubMed] [Google Scholar]
- 10. Curran RM, et al. 2009. Vaginal delivery of the recombinant HIV-1 clade C trimeric gp140 envelope protein CN54gp140 within novel rheologically structured vehicles elicits specific immune responses. Vaccine 27:6791–6798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duerr A. 2010. Update on mucosal HIV vaccine vectors. Curr. Opin. HIV AIDS 5:397–403 [DOI] [PubMed] [Google Scholar]
- 12. Fahlen-Yrlid L, et al. 2009. CD11chigh dendritic cells are essential for activation of CD4+ T cells and generation of specific antibodies following mucosal immunization. J. Immunol. 183:5032–5041 [DOI] [PubMed] [Google Scholar]
- 13. Florese RH, et al. 2009. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared to multigenic vaccines. J. Immunol. 182:3718–3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gaydos CA, Gaydos JC. 1995. Adenovirus vaccines in the U.S. military. Mil. Med. 160:300–304 [PubMed] [Google Scholar]
- 15. Gomez-Roman VR, et al. 2006. An adenovirus-based HIV subtype-B prime/boost vaccine regimen elicits antibodies mediating broad antibody-dependent cellular cytotoxicity against non-subtype-B strains. J. Acquir. Immune Defic. Syndr. 43:270–277 [DOI] [PubMed] [Google Scholar]
- 16. Gomez-Roman VR, et al. 2005. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J. Immunol. 174:2185–2189 [DOI] [PubMed] [Google Scholar]
- 17. Graham BS, et al. 2010. Mucosal delivery of human papillomavirus pseudovirus-encapsidated plasmids improves the potency of DNA vaccination. Mucosal Immunol. 3:475–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hervouet C, et al. 2010. Sublingual immunization with an HIV subunit vaccine induces antibodies and cytotoxic T cells in the mouse female genital tract. Vaccine 28:5582–5590 [DOI] [PubMed] [Google Scholar]
- 19. Hidajat R, et al. 2010. Construction and immunogenicity of replication-competent adenovirus 5 host range mutant recombinants expressing HIV-1 gp160 of SF162 and TV-1 strains. Vaccine 28:3963–3971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hidajat R, et al. 2009. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J. Virol. 83:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalyanaraman VS, et al. 1990. Characterization of the secreted, native gp120 and gp160 of the human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses 6:371–380 [DOI] [PubMed] [Google Scholar]
- 22. Kaufman DR, Bivas-Benita M, Simmons NL, Miller D, Barouch DH. 2010. Route of adenovirus-based HIV-1 vaccine delivery impacts the phenotype and trafficking of vaccine-elicited CD8+ T lymphocytes. J. Virol. 84:5986–5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lakhashe SK, et al. 2011. Prime-boost vaccination with heterologous live vectors encoding SIV gag and multimeric HIV-1 gp160 protein: efficacy against repeated mucosal R5 clade C SHIV challenges. Vaccine 29:5611–5622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lemiale, et al. 2003. Enhanced mucosal immunoglobulin A response of intranasal adenoviral vector human immunodeficiency virus vaccine and localization in the central nervous system. J. Virol. 77:10078–10087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Q, et al. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148–1152 [DOI] [PubMed] [Google Scholar]
- 26. Lindsay RWB, et al. 2010. CD8+ T cell responses following replication-defective adenovirus serotype 5 immunization are dependent on CD11c+ dendritic cells but show redundancy in their requirement of TLR and nucleotide-binding oligomerization domain-like receptor signaling. J. Immunol. 185:1513–1521 [DOI] [PubMed] [Google Scholar]
- 27. Lore K, et al. 2007. Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. J. Immunol. 179:1721–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lubeck MD, et al. 1994. Immunogenicity of recombinant adenovirus-human immunodeficiency virus vaccines in chimpanzees following intranasal administration. AIDS Res. Hum. Retroviruses 10:1443–1449 [DOI] [PubMed] [Google Scholar]
- 29. Lubeck MD, et al. 1997. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat. Med. 3:651–658 [DOI] [PubMed] [Google Scholar]
- 30. Malkevitch NV, et al. 2006. Durable protection of rhesus macaques immunized with a replicating adenovirus-SIV multigene prime/protein boost vaccine regimen against a second SIVmac251 rectal challenge: role of SIV-specific CD8+ T cell responses. Virology 353:83–98 [DOI] [PubMed] [Google Scholar]
- 31. Mannering SI, et al. 2003. A sensitive method for detecting proliferation of rare autoantigen-specific human T cells. J. Immunol. Methods 283:173–183 [DOI] [PubMed] [Google Scholar]
- 32. Negri DRM, et al. 2010. Persistence of mucosal and systemic immune responses following sublingual immunization. Vaccine 28:4175–4180 [DOI] [PubMed] [Google Scholar]
- 33. Patterson LJ, et al. 2011. Rapid SIV Env-specific mucosal and serum antibody induction augments cellular immunity in protecting immunized, elite-controller macaques against high dose heterologous SIV challenge. Virology 411:87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patterson LJ, et al. 2003. Potent, persistent induction and modulation of cellular immune responses in rhesus macaques primed with Ad5hr-simian immunodeficiency virus (SIV) env/rev, gag, and/or nef vaccines and boosted with SIV gp120. J. Virol. 77:8607–8620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patterson LJ, Robert-Guroff M. 2008. Replicating adenovirus vector prime/protein boost strategies for HIV vaccine development. Expert Opin. Biol. Ther. 8:1347–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peng B, et al. 2005. Replicating rather than nonreplicating adenovirus-human immunodeficiency virus recombinant vaccines are better at eliciting potent cellular immunity and priming high titer antibodies. J. Virol. 79:10200–10209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qureshi H, et al. 2012. Low dose penile SIVmac251 exposure of rhesus macaques infected with adenovirus type 5 (Ad5) and then immunized with a replication-defective Ad5-based SIV gag/pol/nef vaccine recapitulates the results of the phase IIb Step trial of a similar HIV-1 vaccine. J. Virol. 86:2239–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang S-W, et al. 2004. An SHIV DNA/MVA rectal vaccination in macaques provides systemic and mucosal virus-specific responses and protection against AIDS. AIDS Res. Hum. Retroviruses 20:846–859 [DOI] [PubMed] [Google Scholar]
- 39. Wira CR, Fahey JV. 2008. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS 22:1909–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xiao P, et al. 2010. Multiple vaccine-elicited nonneutralizing anti-envelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J. Virol. 84:7161–7173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40a. Xiao P, et al. 2012. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIVmac251 challenge. J. Virol. 86:4644–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Y, Huang W, Ornelles DA, Gooding LR. 2010. Modeling adenovirus latency in human lymphocyte cell lines. J. Virol. 84:8799–8810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao J, et al. 2003. Improved protection of rhesus macaques against intrarectal simian immunodeficiency virus SIVmac251 challenge by a replication-competent Ad5hr-SIV env/rev and Ad5hr-SIV gag recombinant priming/gp120 boosting regimen. J. Virol. 77:8354–8365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zolla-Zazner S, et al. 1998. Induction of neutralizing antibodies to T cell line-adapted and primary human immunodeficiency virus type 1 isolates with a prime/boost vaccine regimen in chimpanzees. J. Virol. 72:1052–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]