Abstract
A versatile transformation system for thraustochytrids, a promising producer for polyunsaturated fatty acids and fatty acid-derived fuels, was established. G418, hygromycin B, blasticidin, and zeocin inhibited the growth of thraustochytrids, indicating that multiple selectable marker genes could be used in the transformation system. A neomycin resistance gene (neor), driven with an ubiquitin or an EF-1α promoter-terminator from Thraustochytrium aureum ATCC 34304, was introduced into representatives of two thraustochytrid genera, Aurantiochytrium and Thraustochytrium. The neor marker was integrated into the chromosomal DNA by random recombination and then functionally translated into neor mRNA. Additionally, we confirmed that another two genera, Parietichytrium and Schizochytrium, could be transformed by the same method. By this method, the enhanced green fluorescent protein was functionally expressed in thraustochytrids. Meanwhile, T. aureum ATCC 34304 could be transformed by two 18S ribosomal DNA-targeting vectors, designed to cause single- or double-crossover homologous recombination. Finally, the fatty acid Δ5 desaturase gene was disrupted by double-crossover homologous recombination in T. aureum ATCC 34304, resulting in an increase of dihomo-γ-linolenic acid (C20:3n-6) and eicosatetraenoic acid (C20:4n-3), substrates for Δ5 desaturase, and a decrease of arachidonic acid (C20:4n-6) and eicosapentaenoic acid (C20:5n-3), products for the enzyme. These results clearly indicate that a versatile transformation system which could be applicable to both multiple transgene expression and gene targeting was established for thraustochytrids.
INTRODUCTION
Omega-3 polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid (EPA; C20:5n-3) and docosahexaenoic acid (DHA; C22:6n-3) are beneficial both nutritionally and pharmacologically (4, 16, 18). These PUFAs are mainly produced in oily fish such as salmon and sardine and currently supplied as nutritional supplements and medicines (23). However, the supply of fish oils is unstable due to declines in fish stocks, and thus, alternatives are required. One promising substitute for fish oils is thraustochytrids (class Labyrinchulomycetes, Stramenopiles) (2), unicellular eukaryotic marine protists, including those of the genera Aurantiochytrium (formerly known as the genus Schizochytrium), Parietichytrium, Schizochytrium sensu stricto, and Thraustochytrium (26, 27). Thraustochytrids accumulate large amounts of DHA and n-6 docosapentaenoic acid (C22:5n-6) mainly in their lipid droplets; however, they produce little EPA or arachidonic acid (C20:4n-6) (5, 11). Thus, molecular breeding using gene manipulation is necessary for the selective production of PUFAs in thraustochytrids. Such approaches have been conducted in fungi (22), plants (1, 20, 25), and animals (7, 10, 21); however, thraustochytrids are thought to be superior to those candidates on the aspects of the production and accumulation of PUFAs, possibly because of the higher productivity of PUFAs by both PUFA synthase and standard pathways (12) and the higher accumulation of PUFAs in well-developed lipid droplets (9). Furthermore, thraustochytrids are expected to be a source of fatty acid (FA)/squalene-derived fuels (8, 24), which will necessitate an increase in FA/squalene production by gene manipulation. In this context, genes coding fatty acid synthase (FAS) and squalene synthase could be good targets for the molecular breeding of thraustochytrids. Nevertheless, basic information and tools for genetic systems are still lacking for thraustochytrids. It is worth noting that the scope of all reports describing the molecular tools for thraustochytrids is limited to the genus Aurantiochytrium (formerly assigned to Schizochytrium) (6, 12, 17). Thus, the development of a genetic system for multiple transgene expression and targeted gene knockout in thraustochytrids is urgently required for mass and/or tailor-made production of beneficial PUFAs as well as FA/squalene-based fuels.
In the present study, we established a transformation system for thraustochytrids representative of four genera, Aurantiochytrium, Thraustochytrium, Parietichytrium, and Schizochytrium sensu stricto. The neomycin resistance gene (neor) driven with an ubiquitin or EF-1α promoter-terminator system was randomly integrated into the thraustochytrid genomes when electroporation or particle bombardment was used. Alternatively, the gene was specifically incorporated into 18S ribosomal DNA (rDNA) by single- and double-crossover homologous recombination. This technique could be applied to the targeted disruption of the Δ5 desaturase gene in Thraustochytrium aureum ATCC 34304 (TauΔ5des). Furthermore, the enhanced green fluorescence protein (EGFP) was functionally expressed in thraustochytrids. This report, the first to describe a versatile transformation system for thraustochytrids applicable to both multiple transgene expression and gene targeting, could facilitate the molecular breeding of thraustochytrids for the production of beneficial PUFAs and FA/squalene-based fuels.
MATERIALS AND METHODS
Materials.
The restriction enzymes were purchased from Nippon Gene (Tokyo, Japan), and T4 DNA ligase was purchased from Promega (Madison, WI). Synthetic oligonucleotides were obtained from Hokkaido System Science (Hokkaido, Japan) and Genenet (Fukuoka, Japan). The antibiotics neomycin (G418), hygromycin B, and blasticidin were purchased from Nacalai Tesque (Kyoto, Japan), and zeocin was purchased from Invitrogen (CA). Sealife (artificial seawater mixture) was obtained from MarineTech (Tokyo, Japan). All other reagents were of the highest purity available.
Strains and culture.
The phylogenetic analysis of thraustochytrids was performed on the basis of 18S ribosomal DNA sequences, as described previously (26, 27), and four selected strains: Aurantiochytrium limacinum mh0186 (representative of the genus Aurantiochytrium) (19), Thraustochytrium aureum ATCC 34304 (Thraustochytrium), Parietichytrium sp. strain TA04Bb (Parietichytrium), and Schizochytrium sp. strain SEK 579 (Schizochytrium sensu stricto). T. aureum ATCC 34304 was purchased from the American Type Culture Collection (ATCC). A. limacinum mh0186 and Parietichytrium sp. TA04Bb were previously isolated from the Abusuki River in Tanegashima Island, Kagoshima, Japan. Schizochytrium sp. SEK 579 was isolated from brackish water at the mouth of the Shuku River in Hyogo, Japan. T. aureum ATCC 34304 was repurified from a stock culture by repeated zoospore isolation, as follows. Cells grown in 1 ml of PD liquid medium (0.48% [wt/vol] potato dextrose and 1.75% [wt/vol] Sealife, pH 6.0) were spread on a d-GPY agar plate (0.2% [wt/vol] glucose, 0.1% [wt/vol] polypeptone, 0.25% [wt/vol] yeast extract, 1.5% [wt/vol] agar, and 1.75% Sealife, 15 by 150 mm) and incubated at 25°C for 5 days. Ten milliliters of d-GPY liquid medium was then gently poured onto the plate and incubated at 25°C until zoospores were released. An aliquot of liquid medium containing zoospores was carefully withdrawn and diluted by d-GPY liquid medium to ∼5 × 101 zoospores/ml. Two hundred microliters of diluted medium (containing ∼101 zoospores) was then spread on a d-GPY agar plate and incubated at 25°C until colonies were formed. A single colony derived from one zoospore was then picked and incubated in 1 ml of PD liquid medium at 25°C for 5 days. These isolation procedures were repeated five times.
Screening of antibiotics.
Precultured strains of thraustochytrids were inoculated in 5 ml of PD liquid medium containing various antibiotics at 25°C with shaking at 150 rpm, and turbidity was measured with an Ultrospec 3000 spectrophotometer at 600 nm after 4 days. The antibiotics used and their concentrations were as follows: G418 (2 mg/ml), zeocin (1 mg/ml), puromycin (100 μg/ml), blasticidin (100 μg/ml), hygromycin B (2 mg/ml), chloramphenicol (30 μg/ml), kanamycin (50 μg/ml), penicillin (500 μg/ml), streptomycin (500 μg/ml), and tetracycline (100 μg/ml). Subsequently, the MICs of antibiotics were determined by spotting 10 μl of culture of each strain on PD agar plates (0.78 [wt/vol] potato dextrose agar [Nissui], 1.21% [wt/vol] agar, and 1.75% [wt/vol] Sealife) containing various antibiotics and incubated at 25°C for 7 days.
Isolation of EF-1α and ubiquitin promoter and terminator regions.
T. aureum ATCC 34304 was grown at 25°C in GY liquid medium (3% [wt/vol] glucose, 1% [wt/vol] yeast extract, and 1.75% [wt/vol] Sealife) with shaking at 150 rpm. Cells in a logarithmic growth phase were harvested by centrifugation (3,500 × g, 4°C, 10 min), and total RNA was extracted using Sepasol RNA I Super solution (Nacalai Tesque). Poly(A)+ RNA was purified using an Oligotex-dT Super mRNA purification kit (TaKaRa Bio, Shiga, Japan). A cDNA library was constructed with a SMART RACE cDNA amplification kit (Clontech, CA), and 3′ and 5′ rapid amplification of cDNA ends (RACE) PCRs were carried out according to the manufacturer's instructions.
For the EF-1α gene, a 980-bp 3′ RACE product was obtained using a degenerate primer targeting the conserved region of EF-1α, primer EF-F1 (5′-THG AYG CNC CNG GNC AYM G-3′). The sequence was highly homologous to EF-1α, and thus, the primer EF-1r (5′-GTG AAG GCC AGA AGG GCG TG-3′) was designed and a 496-bp 5′ RACE PCR product was obtained. Consequently, we identified 1,396 bp of the EF-1α cDNA of T. aureum ATCC 34304, which included a 1,023-bp open reading frame (ORF) encoding 341 amino acid residues. The flanking 5′ and 3′ sequences of the gene, considered to be functional EF-1α promoter and terminator regions, respectively, were then isolated using an LA PCR in vitro cloning kit (TaKaRa Bio) according to the manufacturer's instructions. The PCR primers used were as follows: primer r3 (5′-CCT CCT TCT CGA ACT TCT CGA TCG TG-3′) for isolation of the EF-1α promoter and primers EF-t-F1 (5′-CAT GGT CAA GAT GTA TCC CCT CCA A-3′) and EF-t-F2 (5′-TCA CCA AGG GCG ACA AAT AAA TTC T-3′) for isolation of the EF-1α terminator. As a result, 615-bp and 1,414-bp EF-1α promoter and terminator regions, respectively, were identified.
For the ubiquitin gene, a 278-bp 3′ RACE product was obtained using a degenerate primer targeting the conserved region of ubiquitin, primer 2F (5′-ATG CAR ATH TTY GTK AAR ACY YTS-3′). The sequence was highly homologous to ubiquitin, and thus, primers 1R (5′-CAG GAC TAG GTG GAG CGT GGA-3′) and 2R (5′-ACT CCT TCT GGA TGT TGT AGT CGC TG-3′) were designed and a 260-bp 5′ RACE product was obtained using a 5′ RACE system for rapid amplification of cDNA ends, version 2.0 (Invitrogen). Subsequently, in the same manner described above, 801-bp and 584-bp ubiquitin promoter and terminator regions, respectively, were identified. The PCR primers used were as follows: primers REVERS-U PR-1 (5′-CAC GTT CTC GAT GGT GTC GCT-3′) and REVERS-U PR-2 (5′-GAT CTG CAT GTT GGC TAG TGT TGC T-3′) for isolation of the ubiquitin promoter and primers ubqterminalf1 (5′-CTA TAC TCG AAT CAT GCT GCC CTG-3′) and ter2F (5′-AAC TAA GCT ATC TGT AGT ATG TGC-3′) for isolation of the ubiquitin terminator.
Construction of neor expression cassettes.
neor was synthesized with optimized codon usage to reflect the codon bias of T. aureum ATCC 34304 (9). The neor expression cassette, driven with an EF-1α promoter-terminator system, was generated by fusion PCR and designated EF-Neor. Similarly, the 18S ribosomal DNA fragment of T. aureum ATCC 34304 was fused with the neor expression cassette, driven with an ubiquitin promoter-terminator (Ubi-Neor) system, by fusion PCR and designated 18S-Ubi-Neor. EF-Neor and 18S-Ubi-Neor were subcloned into the Escherichia coli vector pGEM-T Easy (Promega) and the NdeI/KpnI site of pUC18 (TaKaRa Bio) to generate the plasmids pEF-Neor and p18S-Ubi-Neor, respectively. Schematic structures of EF-Neor and 18S-Ubi-Neor along with the primers used for fusion PCR are shown in Fig. S1A and B in the supplemental material, respectively. Linear DNA fragments of EF-Neor and Ubi-Neor (Fig. 1A) were then amplified by PCR using pEF-Neor and p18S-Ubi-Neor as the template and used for transformation of thraustochytrids.
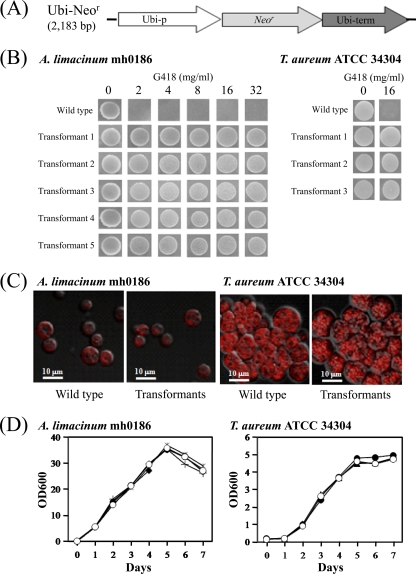
Fig 1.
Transformants of A. limacinum mh0186 and T. aureum ATCC 34304. (A) Schematic diagram of Ubi-Neor. Ubi-p, ubiquitin promoter from T. aureum ATCC 34304; Ubi-term, ubiquitin terminator from T. aureum ATCC 34304; Neor, neomycin resistance gene. (B) G418-resistant phenotypes of transformants generated by Ubi-Neor. Transformants were passaged 5 times in GY liquid medium without G418 and inoculated on PD agar plates containing G418 at the concentrations indicated. Incubation was carried out at 25°C for 7 days. The wild type was used as a control. (C) Fluorescence images of wild-type strains and transformants generated by Ubi-Neor showing lipid droplets. Lipid droplets were stained with Nile red as described in Materials and Methods. (D) Growth curves of wild-type strains and transformants generated by Ubi-Neor. Precultured cells were inoculated in 20 ml of GY liquid medium without G418 and incubated at 25°C with shaking at 150 rpm, and growth (optical density [OD] at 600 nm) was measured at the indicated times. Open circle, wild-type strain; closed circle, transfectant 1; closed triangle, transfectant 2; closed diamond, transfectant 3; asterisk, transfectant 4; plus mark, transfectant 5.
Transformation of thraustochytrids.
DNA cassettes were introduced into thraustochytrids by electroporation and particle bombardment. For electroporation, cells in a logarithmic growth phase were harvested by centrifugation (3,500 × g, 4°C, 10 min) and washed with 1.75% (wt/vol) Sealife. Cells (5 × 106) and 5 μg of DNA in 80 μl of Nucleofector solution L (Amaxa Biosystems, MD) were transferred to a 0.1-cm-gap cuvette and electroporated (pulsed conditions, 50 μF, 50 Ω, 7.5 kV/cm, 2 times) by using a Gene Pulser II apparatus (Bio-Rad, CA). Immediately, 1 ml of fresh GY medium was added to the solution and the mixture was incubated at 25°C for 20 h. The culture was then spread on a PD agar plate (15 by 150 mm) containing G418 and incubated at 25°C until colonies of transformants were formed. For particle bombardment, cells in a logarithmic growth phase were spread on a PD agar plate (15 by 60 mm) without G418 and bombarded in a Biolistic PDS-1000/He system (Bio-Rad) with DNA-coated gold microcarriers (diameter, 0.6 μm) according to the manufacturer's instructions (bombardment conditions, 900, 1,100, or 1,350 lb/in2; 6-cm target distance; 26-in. Hg vacuum). The plate was incubated at 25°C for 20 h, and cells were collected to be suspended in PD medium and respread on a PD agar plate (15 by 150 mm) containing G418. The G418 concentration of the PD agar plate was 0.5 mg/ml for A. limacinum mh0186 and 2 mg/ml for T. aureum ATCC 34304, Parietichytrium sp. TA04Bb, and Schizochytrium sp. SEK 579.
Nile red staining and fluorescence microscopy.
Wild-type and transformed cells in a logarithmic growth phase were collected and washed with phosphate-buffered saline (PBS) and resuspended in PBS containing 1 μg/ml of Nile red (WaKo). After incubation at room temperature for 5 min, cells were washed two times with PBS and observed using a confocal laser scanning microscope (Digital Eclipse C1; Nikon, Tokyo, Japan).
Genomic PCR analysis.
Transformants were cultured in GY medium containing G418 at a concentration of 0.5 mg/ml for A. limacinum mh0186 and 1 mg/ml for T. aureum ATCC 34034. After several passages, genomic DNA was prepared and PCR was performed to amplify neor using the primers FU2FA (5′-GAC CTA AGC AAC ACT AGC CAA CAT GAT TGA ACA GGA CGG CCT TCA-3′) and FU2RA (5′-TAT AGC ACA TAC TAC AGA TAG CTC AAA AGA ACT CGT CCA GGA GGC G-3′).
Southern blot analysis.
Genomic DNA (2 μg) prepared from wild-type or transformed cells was digested with appropriate restriction enzymes and subjected to 0.7% agarose gel electrophoresis. The DNA was then transferred to nylon membranes (Hybond N+; GE Healthcare, Buckingham, United Kingdom). A digoxigenin (DIG)-labeled neor DNA probe was prepared by using a PCR DIG probe synthesis kit (Roche, IN). Hybridization and detection were performed according to the manufacturer's instructions.
RT-PCR analysis.
Total RNA was prepared from wild-type and transformed cells with a Sepasol RNA I Super, RNeasy minikit (Qiagen, CA) and DNase I (TaKaRa Bio), followed by reverse transcription (RT) with PrimeScript reverse transcriptase (TaKaRa Bio). PCR was then performed for neor amplification as described above.
Expression of EGFP in thraustochytrids.
The egfp expression cassette driven with an ubiquitin promoter-terminator system was constructed by fusion PCR and subcloned into the pGEM-T Easy vector (Promega), and the plasmid generated was designated pUbi-EGFP. A schematic diagram of Ubi-EGFP along with the primers used for fusion PCR is shown in Fig. S1C in the supplemental material. PCR was performed with primers Ubi-pro-F1 (5′-TCG GTA CCC GTT AGA ACG CGT AAT ACG AC-3′; the KpnI site is underlined) and Ubi-term R2 (5′-TCG GTA CCA CCG CGT AAT ACG ACT CAC TAT AGG GAG ACT GCA GTT-3′) using pUbi-EGFP as the template. The amplified PCR product was digested with KpnI and ligated into the same site of pEF-Neor. The plasmid harboring neor and egfp expression cassettes was designated pEF-Neor/Ubi-EGFP. Subsequently, a linear DNA fragment of EF-Neor/Ubi-EGFP, which contains neor and egfp expression cassettes (see Fig. 4A), was amplified by PCR with the primers 2F (5′-GGT TTC CGT AGT GAA CCT GCA ATT CAA AAA AAG CCG TTA CTC ACA T-3′) and pUC18-R (5′-AAC AGC TAT GAC CAT GAT TAC GAA TTC GAG CTC GG-3′) and used as the neor and egfp expression construct. Similarly, a DNA fragment of EF-Neor was amplified by PCR with primers 2F and terminator 5R (5′-CAC TGC AGC GAA AGA CGG GCC GTA AGG ACG-3′) using pEF-Neor as the template and employed as the neor expression control construct. EF-Neor/Ubi-EGFP and EF-Neor were then separately introduced into A. limacinum mh0186 and T. aureum ATCC 34304 by electroporation and particle bombardment, respectively. To verify that EF-Neor/Ubi-EGFP was integrated into the chromosomal DNA, genomic PCR was conducted with the primer 2F and terminator 5R for the detection of EF-Neor and 2F and pUC18-R for the detection of EF-Neor/Ubi-EGFP. Transformants were then observed under the confocal laser scanning microscope (Digital Eclipse C1).
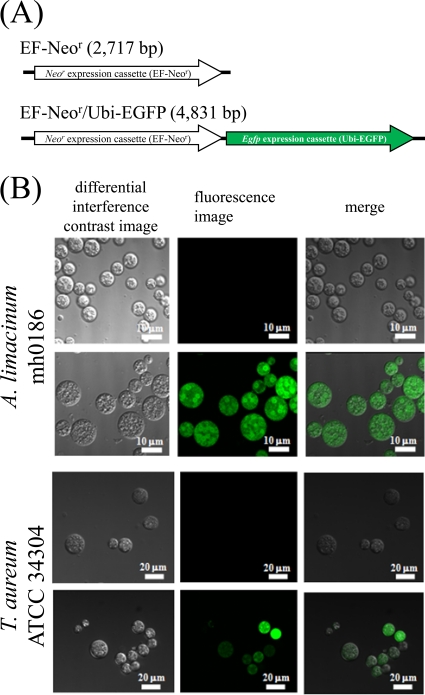
Fig 4.
Expression of EGFP gene in thraustochytrids. (A) Schematic diagram of the neor expression control construct (negative control; top) and neor and egfp expression construct (bottom). (B) EGFP-derived fluorescent images of transfectants of the neor expression control construct (negative control; top rows) and neor and egfp expression construct (bottom rows) of A. limacinum mh0186 and T. aureum ATCC 34304. The neor expression control construct and neor and egfp expression construct were separately transfected into A. limacinum and T. aureum ATCC 34304 and observed with a confocal laser scanning microscope. Details are given in Materials and Methods.
Construction of 18S rDNA-targeting vectors.
18S rDNA was amplified by PCR with the primers Tau18S F (5′-TAT ATC AGT TAT AGT TTC TTT GAT AGT G-3′) and Tau18S R (5′-GGA TCG TTC AAT CGG TAG GTG CGA C-3′) using genomic DNA of T. aureum ATCC 34304 as the template. As a result, we found the sequence variations in 18S ribosomal DNA by direct sequencing of PCR-amplified 18S ribosomal DNA of T. aureum ATCC 34304 (1,591 bp). Thus, we purified the strain by isolating zoospores as described before and subjected 6 clones to sequencing analysis. Some substitutions were still observed between nucleotide positions (nt) 577 and 594 (Fig. S3 in the supplemental material). The amplified PCR product was subcloned into pGEM-T Easy and designated pTau18S. PCR was then performed using pTau18S as the template with two sets of primers: primers svec NheI (5′-ATC CGC GCT AGC TTC CAC ACA ACA TAC GAG CCG GAA GC-3′; the NheI site is underlined) and sdvec HindIII (5′-CGC TGC AAG CTT ATC CTC CCC CGG TCT TTT GGG CTG G-3′; the HindIII site is underlined) and primers dvec NheI (5′-CGA CGC GCT AGC CAC CGC GCG CCA AGG TCG GCC CTA-3′) and sdvec HindIII. The amplified PCR products were designated TSC and TDC, respectively. The neor expression cassette was amplified by PCR with the primers sdins NheI (5′-ATC CGC GCT AGC GCC GCA GCG CCT GGT GCA CCC GCC GGG-3′) and sdins HindIII (5′-CGC TGC AAG CTT ACC GCG TAA TAC GAC TCA CTA TAG GGA GCDA TGC-3′) using p18S-Ubi-Neor as the template. Both DNA fragments were digested with NheI and HindIII and ligated into TSC and TDC, which were digested with the same enzymes, to generate the plasmids pTSC1Neor and pTDCNeor, respectively. Subsequently, site-directed mutagenesis was carried out with primers pTSC SmaI F (5′-ACT CAA CCC GGG AAA ACT TAC CAG GT-3′; the SmaI site is underlined) and pTSC SmaI R (5′-TTT CCC GGG TTG AGT CAA ATT AAG CC-3′) by using a PrimeSTAR mutagenesis kit (TaKaRa Bio). As a result, a SmaI site was introduced into the 18S rDNA sequence of pTSC1Neor and the resultant plasmid was designated pTSCNeor. Schematic structures of pTSCNeor and pTDCNeor are shown in Fig. S5A and B in the supplemental material, respectively. PTSCNeor and pTDCNeor should achieve single-crossover homologous recombination by 18S R (18S rDNA at nt 602 to 1,591) and double-crossover homologous recombination between 18S F (nt 1 to 566) and 18S R (nt 602 to 1,591), respectively (see Fig. S6A and B in the supplemental material). It is worth noting that the variable region of 18S rDNA (nt 577 to 594) was excluded in the two vectors.
PCR was then performed with the primers Tau18S F and Tau18S R using pTDCNeor as the template (see Fig. S5B in the supplemental material), and the amplified linear DNA fragment was designated TDCNeor (see Fig. S5D in the supplemental material). pTSCNeor was digested with SmaI, and the resultant linear DNA fragment was designated TSCNeor (see Fig. S5C in the supplemental material). Thereafter, pTSCNeor (circular), TSCNeor (linear), pTDCNeor (circular), and TDCNeor (linear) (see Fig. S5A to D in the supplemental material) were separately introduced into T. aureum ATCC 34304. Transformants were checked by genomic PCR with four sets of primers: primers Ta18S F1 (5′-TTA AAA AGC TCG TAG TTG AA-3′) and Svec det R (5′-AGT CAG TGAGCG AGG AAG CGG AAG AGC-3′) and primers Sneo F (5′-TCG GGA GCC AGC CGG AAA CAG GTT CAA AAG AAC TCG TCC AGG AGG CGG TAG A-3′) and Ta18S R (5′-AAA TGA ATC AGC CAC AAC AGA ATC C-3′) for pTSCNeor and TSCNeor transfectants and primers Ta18S F2 (5′-GGC TTA TAC TCT GAA ACT GCG AAC G-3′) and Sneo R (5′-GCT GCG CTG CTT TGT AAA CGC GAC CAT GAT TGA ACA GGA CGG CCT TCA CGC T-3′) and primers Sneo F and Ta18S R for pTDCNeor and TDCNeor transfectants. These sets of primers gave rise to PCR products only when pTSCNeor/TSCNeor and pTDCNeor/TSCNeor were integrated by homologous recombination (see Fig. S6A and B in the supplemental material). On the other hand, genomic PCR was performed with primers Sneo F and Sneo R to examine whether the transfected DNA was integrated into chromosomal DNA.
Disruption of Δ5 desaturase gene in T. aureum ATCC 34304.
Molecular cloning of the Δ5 desaturase gene from T. aureum ATCC 34304 (TauΔ5des) was performed by the method described previously (8). A TauΔ5des-targeting construct harboring neor and one harboring the hygromycin resistance gene (hygr) marker were generated by fusion PCR and were designated TauΔ5desKoNeor and TauΔ5desKoHygr, respectively. Schematic structures of TauΔ5desKoNeor and TauΔ5desKoHygr along with the primers used for fusion PCR are shown in Fig. S1D and E in the supplemental material. TauΔ5desKoNeor was introduced into T. aureum, and transformants were checked by genomic PCR with three sets of primers: primers FU2FA and FU2FR for detection of neor, primers FU2FA and com1R (5′-GCG AAG CGA GTG GCC TAA GCG-3′) for detection of homologous recombination, and primers D5ORFcomF (5′-GAC GCG GCG GCG AAG GTC AGG-3′) and D5ORFcomR (5′-CTT GCT GTG CTG AAC GCG CCA C-3′) for detection of the TauΔ5des ORF. Annealing sites of these primers are shown in Fig. 6A. The TauΔ5des-disrupted transformants were found to retain at least one wild-type allele (see Fig. S7A in the supplemental material), and therefore, TauΔ5desKoHygr was reintroduced into the transformants, followed by incubation on PD agar plates containing 2 mg/ml of G418 and hygromycin B. The transformants were rechecked by genomic PCR with three sets of primers: primers hygro F (5′-ATG AAA AAG CCT GAA CTC ACC GCG ACG TCT G-3′) and hygro R (5′-CTA TTC CTT TGC CCT CGG ACG AGT GCT GG-3′) for detection of hygr, primers hygro F and com1R for detection of homologous recombination, and primers D5ORFcomF and D5ORFcomR for detection of the TauΔ5des ORF. Reverse transcription-PCR for detection of TauΔ5des mRNA was performed with primers D5ORFcomF and D5ORFcomR. Gas-liquid chromatography (GC) analysis using fatty acid methyl esters (FAMEs) was carried out as described previously (8).
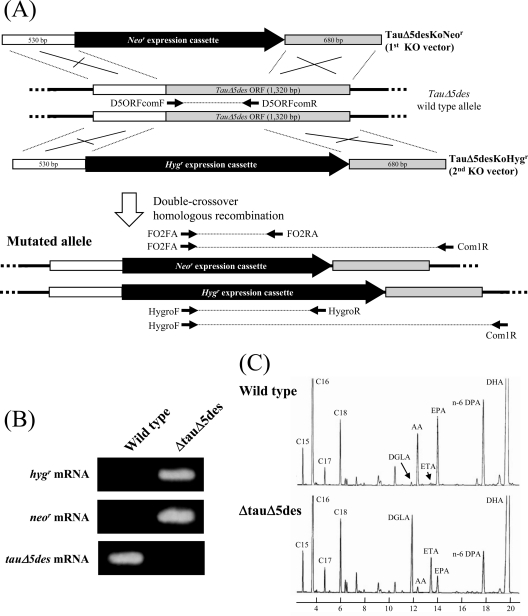
Fig 6.
Gene disruption of Δ5 desaturase in T. aureum ATCC 34304. (A) Scheme for disruption of the Δ5 desaturase gene in T. aureum ATCC 34304 (TauΔ5des) by TauΔ5desKoNeor (targeting construct for 1st allele) and TauΔ5desKoHygr (targeting construct for 2nd allele). Annealing sites for PCR primers are shown. (B) Reverse transcription-PCR detecting the TauΔ5des mRNA in the wild type and TauΔ5des-disrupted mutant (ΔtauΔ5des) of T. aureum. (C) GC analysis of FAMEs from the wild type and TauΔ5des-disrupted mutant (ΔtauΔ5des) of T. aureum. Details are given in Materials and Methods.
RESULTS
Screening of antibiotics.
To select marker genes suitable for thraustochytrids, various antibiotics were subjected to growth inhibition tests with Aurantiochytrium limacinum mh0186 and Thraustochytrium aureum ATCC 34304 using PD liquid medium as described in Materials and Methods. The growth of both strains was completely inhibited by G418 (2 mg/ml), hygromycin B (2 mg/ml), and blasticidin (100 μg/ml) in PD liquid medium. Zeocin (1 mg/ml) was effective against A. limacinum but not T. aureum, and puromycin (100 μg/ml) and tetracycline (100 μg/ml) did not affect either strain. Subsequently, MICs of antibiotics were examined using PD agar plates (see Fig. S2A and B in the supplemental material). The MICs of G418, hygromycin B, and blasticidin were 0.5, 2.0, and 1.2 mg/ml, respectively, for A. limacinum and 1.0, 2.0, and 0.4 mg/ml, respectively, for T. aureum. Zeocin was effective against A. limacinum but not T. aureum on the PD agar plates, the MIC being 0.5 mg/ml. The same experiment was performed using the other genera of thraustochytrids, Parietichytrium (strain TA04Bb) and Schizochytrium sensu stricto (strain SEK 579) (see Fig. S2C and D in the supplemental material). G418 and hygromycin B were effective against both strains, and the MICs were determined to be 0.5 and 2.0 mg/ml, respectively. Blasticidin was effective against Parietichytrium sp. TA04Bb at a MIC of 0.8 mg/ml, but zeocin was not effective against either strain. These results indicate that multiple selectable marker genes are available in the transformation system, as shown in Table 1. It was confirmed that neomycin and hygromycin B were effective, and thus, the neomycin resistance gene (neor) was selected as a marker. The G418 concentration used was 2.0 mg/ml for T. aureum ATCC 34304, Parietichytrium sp. TA01Bb, and Schizochytrium sp. SEK 579 and 0.5 mg/ml for A. limacinum mh0186.
Table 1.
Selectable marker genes could be available in the thraustochytrid transformation system
| Strain | Selectable marker genesa |
|---|---|
| A. limacinum mh0186 | neor, hygr, blar, bler |
| T. aureum ATCC 34304 | neor, hygr, blar |
| Parietichytrium sp. TA04Bb | neor, hygr, blar |
| Schizochytrium sp. 204-06 m | neor, hygr |
neor, neomycin resistance gene; hygr, hygromycin B resistance gene; blar, blasticidin resistance gene; bler, zeocin resistance gene.
Transformation of thraustochytrids by Ubi-Neor.
A linear DNA fragment of Ubi-Neor (Fig. 1A) was introduced into A. limacinum mh0186 and T. aureum ATCC 34304 by electroporation and particle bombardment. The efficiency of transformation was found to depend on the method of gene transfer, i.e., electroporation, but not particle bombardment, which was suitable for A. limacinum, while for T. aureum, particle bombardment was superior to electroporation under the conditions used (Table 2). The transformants obtained were very stable and grew on PD agar plates containing G418 at a concentration of up to 32 mg/ml for A. limacinum and 16 mg/ml for T. aureum, after being maintained in GY liquid medium without G418 for five passages (∼1 month) (Fig. 1B). No prominent morphological differences, in cell shape and lipid droplets, for example, were observed between the transformants and wild-type strains (Fig. 1C). Furthermore, the growth of transformants was almost the same as that of wild-type cells (Fig. 1D). Subsequently, genomic PCR amplifying neor was carried out to examine whether the introduced DNA was integrated into chromosomal DNA. As shown in Fig. 2A, 0.8-kbp PCR products corresponding to the size of neor were amplified in 5 and 3 different transformants of A. limacinum and T. aureum, respectively. Southern blotting showed that a single hybridized band varying in molecular size was detected in each transformant when DIG-labeled neor was used as the probe (Fig. 2B), indicating that one copy of neor was integrated into the chromosomal DNA at a random position. Furthermore, reverse transcription-PCR revealed that transcripts of neor were detected in these transformants but not the wild-type strains (Fig. 2C). These results clearly indicate that neor was incorporated into the chromosomal DNA of these thraustochytrids by random integration, being translated to neor mRNA in vivo.
Table 2.
Transformation frequencies of thraustochytridsa
| Strain | Transformation method | No. of colonies per 1 μg vector DNA |
|---|---|---|
| A. limacinum mh0186 | Microprojectile bombardment | Extremely rare |
| Electroporation | 1.6 × 102 | |
| T. aureum ATCC 34304 | Microprojectile bombardment | 1.9 × 102 |
| Electroporation | Extremely rare | |
| Parietichytrium sp. TA04Bb | Microprojectile bombardment | 5.0 × 101 |
| Electroporation | 0 | |
| Schizochytrium sp. 204-06 m | Microprojectile bombardment | 4.6 × 101 |
| Electroporation | 0 |
Ubi-Neor was transfected into cells by both electroporation and particle bombardment, followed by incubation on PD agar plates containing 0.5 mg/ml (A. limacinum mh0186) or 2 mg/ml (T. aureum ATCC 34304, Parietichytrium sp. TA94Bb, and Schizochytrium sp. 204-06 m) of G418 at 25°C until G418-resistant colonies were formed. The number of transformants was then determined by counting the colonies on the plates.
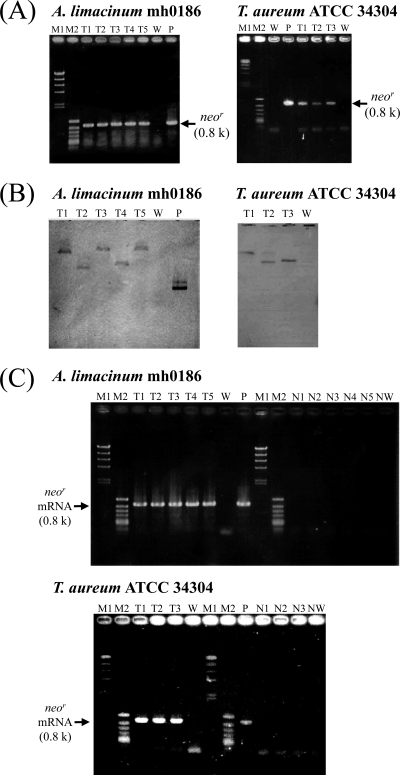
Fig 2.
Molecular characterization of transformants of A. limacinum mh0186 and T. aureum ATCC 34304. (A) Genomic PCR detecting neor in transformants generated by Ubi-Neor. The PCR product corresponding to neor is 0.8 kb. Lanes: M1, bacteriophage λ HindIII digest (marker); M2, phage ϕX174 HincII digest (marker); T, transformants (each number corresponds to the transformant's number); W, wild type; P, positive control (Ubi-Neor was used as the PCR template). (B) Southern blot detecting neor in transformants generated by Ubi-Neor with the neor-specific probe. Genomic DNA (2 μg) of the wild type and transformants was digested with PstI (A. limacinum mh0186) and NotI (T. aureum ATCC 34304) and subjected to Southern blotting. Lanes: T, transformants (each number corresponds to the transformant's number); W, wild type; P, positive control (Ubi-Neor). (C) Reverse transcription-PCR detecting the neor mRNA in transformants generated by Ubi-Neor. Lanes: M1, bacteriophage λ HindIII digest (marker); M2, phage ϕX174 HincII digest (marker); T, transformants (each number corresponds to the transformant's number); W, negative control (wild type); P, positive control (Ubi-Neor was used as a PCR template); N, negative-control reactions without reverse transcriptase (each number corresponds to the transformant's number and W is wild type). k, kilobases. Details are provided in Materials and Methods.
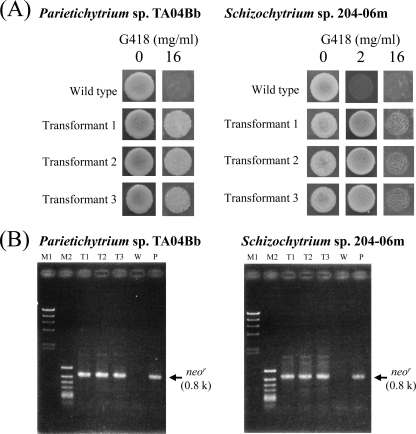
To confirm the usability of the method, strains belonging to other genera, Parietichytrium and Schizochytrium sensu stricto, were used for the transformation. Transformants were obtained from these two strains by particle bombardment, but not electroporation, as observed for T. aureum ATCC 34304 (Table 2). Transformants of both strains exhibited the G418-resistant phenotypes on PD agar plates containing G418 (Fig. 3A), and chromosomal integration of neor was confirmed by a genomic PCR analysis (Fig. 3B).
Fig 3.
Molecular characterization of transformants of Parietichytrium sp. TA04Bb and Schizochytrium sp. SEK 579. (A) G418-resistant phenotypes of transformants generated by transformation with Ubi-Neor. Transformants were inoculated on PD agar plates containing G418 at the concentrations indicated, with wild-type strains used as a control. Incubation was carried out at 25°C for 7 days. (B) Genomic PCR detecting neor in transformants generated by transformation with Ubi-Neor. The PCR product corresponding to neor is 0.8 kb. Lanes: M1, bacteriophage λ HindIII digest (marker); M2, phage ϕX174 HincII digest (marker); T, transformants (each number corresponds to the transformant's number); W, wild-type strain; P, positive control (Ubi-Neor was used as the PCR template). Details are given in Materials and Methods.
Expression of EGFP gene in thraustochytrids.
We expressed the EGFP gene in thraustochytrids using the EF-Neor-conjugated egfp expression cassette driven with an ubiquitin promoter-terminator (EF-Neor/Ubi-EGFP) (Fig. 4A). The neor and egfp expression construct (EF-Neor/Ubi-EGFP) and the neor expression control construct (EF-Neor) (Fig. 4A) were separately introduced into A. limacinum mh0186 and T. aureum ATCC 34304. Transformants were subjected to genomic PCR, and chromosomal integration of EF-Neor/Ubi-EGFP was identified (see Fig. S4 in the supplemental material). These transformants were then observed under a fluorescence microscope. As shown in Fig. 4B, transformants harboring the neor and egfp expression construct but not the neor expression control construct showed EGFP-derived fluorescence. These results clearly indicated that the transformation system established in this study is applicable to the heterozygous expression of genes.
Transformation of thraustochytrids by 18S rDNA-targeting Ubi-Neor.
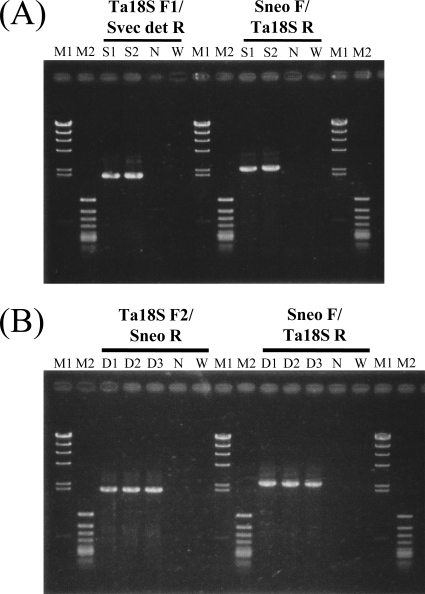
Four 18S ribosomal DNA-targeting vectors, pTSCNeor (circular), TSCNeor (linear), pTDCNeor (circular), and TDCNeor (linear) (see Fig. S5A to D in the supplemental material), were separately introduced into T. aureum ATCC 34304 by particle bombardment. Genomic PCR of the respective transformants was then performed with appropriate primers: primers Ta18S F1/Svec det R and Sneo F/Ta18S R for the pTSCNeor and TSCNeor transformants and primers Ta18S F2 Sneo R and Sneo F/Ta18S R for the pTDCNeor and TDCNeor transformants. These primer sets should generate expected PCR products only when pTSCNeor and TSCNeor (see Fig. S6A in the supplemental material) or pTDCNeor and TDCNeor (see Fig. S6B in the supplemental material) were integrated in 18S ribosomal DNA by homologous recombination. On the other hand, integration of these vectors would be detected by genomic PCR amplification when SNeo F and SNeo R were used as the primers regardless of the type of recombination, i.e., homologous or random integration. We examined 20, 20, 15, and 15 transformants with the pTSCNeor, TSCNeor, pTDCNeor, and TDCNeor vectors, respectively, by genomic PCR. Two of the 20 TSCNeor transformants were confirmed to be single-crossover homologous recombinants, while 3 of the 15 TDCNeor transformants were double-crossover homologous recombinants (Fig. 5A and B). However, neor was likely to be inserted at a random position in the other transformants. The homologous integration frequencies of transformants with pTSCNeor, TSCNeor, pTDCNeor, and TDCNeor are summarized in Table 3. No transformants were obtained by homologous recombination when circular DNA vectors were used, suggesting that linear DNA, not circular DNA, promotes homologous recombination in thraustochytrids.
Fig 5.
Examination of mode of homologous integration in transformants of T. aureum ATCC 34304 by PCR. (A) Genomic PCR for detection of single-crossover homologous recombination in TSCNeor transfectants. Primer sets used and sizes of amplicons are as follows: primers Ta18S F1 and Svec det R (2.0 kbp) and primers Sneo F and Ta18S R (2.4 kbp). Lanes: M1, bacteriophage λ HindIII digest (marker); M2, phage ϕX174 HincII digest (marker); S, TSCNeor transfectants (each number corresponds to the transformant's number); N, negative control (pTSCNeor was used as the PCR template); W, negative control (wild type). (B) Genomic PCR for detection of a double-crossover homologous recombination in TDCNeor transfectants. Primer sets used and sizes of amplicons are as follows: primers Ta18S F2 and Sneo R (2.0 kbp) and primers Sneo F and Ta18S R (2.4 kbp). Lanes: M1, bacteriophage λ HindIII digest (marker); M2, phage ϕX174 HincII digest (marker); D, TDCNeor transfectants (each number corresponds to the transformant's number); N, negative control (pTDCNeor was used as the PCR template); W, negative control (wild type). Details are described in Materials and Methods.
Table 3.
Homologous recombination frequencies of pTSCNeor, TSCNeor, pTDCNeor, and TDCNeor transfectantsa
| Introduced DNA | No. of transformants: |
HR frequency (%) | ||
|---|---|---|---|---|
| Subjected to analyses | With single-crossover HR | With double-crossover HR | ||
| pTSCNeor (circular) | 20 | 0 | 0 | |
| TSCNeor (linear) | 20 | 2 | 10 | |
| pTDCNeor (circular) | 15 | 0 | 0 | |
| TDCNeor (linear) | 15 | 3 | 20 | |
Vectors for pTSCNeor, TSCNeor, pTDCNeor, and TDCNeor were separately transfected into T. aureum ATCC 34304 cells by particle bombardment, followed by incubation on PD agar plates containing 2 mg/ml of G418 at 25°C until colonies of transformants formed. Transformants were then cultured in GY liquid medium containing 1 mg/ml of G418 at 25°C for 5 days and genomic DNA was prepared. Homologous recombination (HR) frequencies were determined by genomic PCR with primers, as follows: Sneo F Sneo R for detection of vector integration, Ta18S F1 Sneo R and Sneo F/Ta18S R for detection of single-crossover homologous recombination by pTSCNeor and TSCNeor, and Ta18S F2 Sneo R and Sneo F/Ta18S R for detection of double-crossover homologous recombination by pTDCNeor and TDCNeor. Annealing sites of primers are shown in Fig. S6A and B in the supplemental material.
Disruption of Δ5 desaturase gene in T. aureum ATCC 34304.
To provide an example of targeted gene disruption by double-crossover homologous recombination, we disrupted the Δ5 desaturase gene (TauΔ5des) in T. aureum ATCC 34304. The linear targeting construct TauΔ5desKoNeor, composed of a neor marker inserted into the TauΔ5des locus (Fig. 6A), was introduced into T. aureum ATCC 34304. The transformants were subjected to genomic PCR analysis. As a result, 8 of 14 transformants showed that TauΔ5des was disrupted but at least one wild-type allele still remained (see Fig. S7A in the supplemental material). Thus, another construct with a different gene marker (hygr) (TauΔ5desKoHygr; Fig. 6A) was introduced into the transformants, resulting in the complete disruption of TauΔ5des (see Fig. S7B in the supplemental material). This result suggests that T. aureum is diploid under the conditions that we employed. Furthermore, disruption of TauΔ5des was also confirmed at the transcriptional level in TauΔ5des disruption mutants, designated ΔTauΔ5des, by reverse transcription-PCR (Fig. 6B).
Fatty acid profiles of the wild type and TauΔ5des disruption mutants were determined by GC using fatty acid methyl esters (FAMEs). As shown in Fig. 6C, disruption of TauΔ5des led to the marked accumulation of dihomo-γ-linolenic acid (DGLA; C20:3n-6) and eicosatetraenoic acid (ETA; C20:4n-3), which are substrates of TauΔ5des. In contrast, arachidonic acid (AA; C20:4n-6) and EPA (C20:5n-3), which are products for TauΔ5des, decreased in ΔTauΔ5des. However, the amount of DHA (C22:6n-6) was almost the same as that in the wild type (120.0 ± 7.23 and 129.1 ± 9.86 mg per g of dry cell weight in the wild type and ΔTauΔ5des, respectively), suggesting that DHA is mainly produced in T. aureum via the PUFA synthase pathway.
DISCUSSION
The aim here was to establish a versatile transformation system for thraustochytrids. Extensive screening of antibiotics revealed multiple selectable markers to be available in the transformation system (Table 1). This result indicated that multiple gene expression and gene targeting could be performed in thraustochytrids and, furthermore, indicated that the targeting could be performed even if thraustochytrid strains are polyploidy, as observed for T. aureum ATCC 34304.
If one could integrate the genes into the chromosomal DNA by site-specific homologous recombination, the ribosomal DNA, which shares high sequence identity among a wide range of organisms and possesses multiple copies, would be suitable as a site for the recombination of transgenes. The multiple copies of ribosomal DNA would provide more chances for genes to integrate and prevent the disruption of vital gene loci. Ribosomal DNA has frequently been used for recombination sites of transformation vectors for yeasts, fungi, and plants (3, 13, 14) to achieve high and stable expression of target genes. However, we found that DNA fragments of selectable markers (neor expression cassettes designated Ubi-Neor) were efficiently integrated into the chromosomal DNA of four species of thraustochytrids at random positions. It is worth noting that the efficiency of transfection through random integration is superior to that through homologous recombination on 18S rDNA. Importantly, the G418-resistant phenotype of transformants was retained after 5 passages (∼1 month) in medium without G418 (Fig. 1B), indicating that the marker is stably incorporated into the chromosomal DNA.
In this study, the EGFP gene with a selectable marker (EF-Neor/Ubi-EGFP) (Fig. 4A) was efficiently integrated into chromosomal DNA of A. limacinum mh0186 and T. aureum ATCC 34304 when administered as a linear DNA fragment, with the strains expressing EGFP-derived fluorescence (Fig. 4B). The expression of EGFP was stable for at least 3 passages.
To conduct gene disruption, the marker should be introduced into the target gene by homologous recombination. With this process, single- or double-crossover homologous recombination could be utilized. In this study, we transformed T. aureum ATCC 34304 using two different 18S ribosomal DNA-targeting vectors harboring the 18S ribosomal DNA sequence as a recombination site and neor as a marker gene (see Fig. S5A to D in the supplemental material), aiming to achieve single- or double-crossover homologous recombination (see Fig. S6A and B in the supplemental material). We obtained transformants through single- and double-crossover homologous recombination with 10 and 20% frequencies, respectively (Table 3). These results indicated that gene targeting could be performed for thraustochytrids by either single- or double-crossover homologous recombination. In this study, we actually succeeded in disrupting the Δ5 desaturase gene in T. aureum ATCC 34304 by double-crossover homologous recombination.
In conclusion, we established a versatile transformation system for thraustochytrids. This system has been successfully applied to heterozygous gene expression in Aurantiochytrium (8, 15). However, we stress that this is the first report to demonstrate the transformation of thraustochytrids, except for the genus Aurantiochytrium (formerly identified as Schizochytrium). Additionally, we showed that this system would be applicable to both multiple transgene expression and gene targeting. This study should facilitate the molecular breeding of thraustochytrids in order to generate superior mutants for production of PUFAs and FA/squalene-based fuels.
Supplementary Material
Footnotes
Published ahead of print 17 February 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Abbadi A, et al. 2004. Biosynthesis of very-long-chain polyunsaturated fatty acids in transgenic oilseeds: constraints on their accumulation. Plant Cell 16:2734–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adel SM, et al. 2005. The new higher level of eukaryotes with emphasis on the taxonomy of protists. J. Eukaryot. Microbiol. 52:399–451 [DOI] [PubMed] [Google Scholar]
- 3. Amador E, Martin JF, Castro JM. 2000. A Brevibacterium lactofermentum 16S rRNA gene used as target site for homologous recombination. FEMS Microbiol. Lett. 185:199–204 [DOI] [PubMed] [Google Scholar]
- 4. Berge JP, Barnathan G. 2005. Fatty acids from lipids of marine organisms: molecular biodiversity, roles as biomarkers, biologically active compounds, and economical aspects. Adv. Biochem. Eng. Biotechnol. 96:49–125 [DOI] [PubMed] [Google Scholar]
- 5. Bowles RD, Hunt AE, Bremer GB, Duchars MG, Eaton RA. 1999. Long-chain n-3 polyunsaturated fatty acid production by members of the marine protistan group the thraustochytrids: screening of isolates and optimization of docosahexaenoic acid production. J. Biotechnol. 70:193–202 [Google Scholar]
- 6. Cheng R-B, et al. 2011. Establishment of a transgene expression system for the marine microalga Schizochytrium by 18S rDNA-targeted homologous recombination. World J. Microbiol. Biotechnol. 27:737–741 [Google Scholar]
- 7. Kang JX, Wang J, Wu L, Kang ZB. 2004. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature 427:504. [DOI] [PubMed] [Google Scholar]
- 8. Kaya K, et al. 2011. Thraustochytrid Aurantiochytrium sp. 18W-13a accumulates high amounts of squalene. Biosci. Biotechnol. Biochem. 75:2246–2248 [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi T, et al. 2011. Increase of eicosapentaenoic acid in thraustochytrids through thraustochytrid ubiquitin promoter-driven expression of a fatty acid {delta}5 desaturase gene. Appl. Environ. Microbiol. 77:3870–3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lai L, et al. 2006. Generation of cloned transgenic pigs rich in omega-3 fatty acids. Nat. Biotechnol. 24:435–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lewis TE, Nichols PD, Mcmeekin TA. 1999. The biotechnological potential of thraustochytrids. Mar. Biotechnol. 1:580–587 [DOI] [PubMed] [Google Scholar]
- 12. Lippmeier JC, et al. 2009. Characterization of both polyunsaturated fatty acid biosynthetic pathways in Schizochytrium sp. Lipids 44:621–630 [DOI] [PubMed] [Google Scholar]
- 13. Lopes TS, et al. 1989. High-copy-number integration into the ribosomal DNA of Saccharomyces cerevisiae: a new vector for high-level expression. Gene 79:199–206 [DOI] [PubMed] [Google Scholar]
- 14. Mackenzie DA, Wongwathanarat P, Carter AT, Archer DB. 2000. Isolation and use of a homologous histone H4 promoter and a ribosomal DNA region in a transformation vector for the oil-producing fungus Mortierella alpina. Appl. Environ. Microbiol. 66:4655–4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsuda T, et al. 2011. Molecular cloning of a Pinguiochrysis pyriformis oleate-specific microsomal Δ12-fatty acid desaturase and functional analysis in yeasts and thraustochytrids. J. Biochem. 150:375–383 [DOI] [PubMed] [Google Scholar]
- 16. Meireles LA, Guedes AC, Malcata FX. 2003. Lipid class composition of the microalga Pavlova lutheri: eicosapentaenoic and docosahexaenoic acid. J. Agric. Food. Chem. 51:2237–2241 [DOI] [PubMed] [Google Scholar]
- 17. Metz JG, et al. 2009. Biochemical characterization of polyunsaturated fatty acid synthesis in Schizochytrium: release of the products as free fatty acids. Plant Physiol. Biochem. 47:472–478 [DOI] [PubMed] [Google Scholar]
- 18. Muskiet FA, Kemperman RF. 2006. Folate and long-chain polyunsaturated fatty acids in psychiatric disease. J. Nutr. Biochem. 17:717–727 [DOI] [PubMed] [Google Scholar]
- 19. Nagano N, Taoka Y, Honda D, Hayashi M. 2009. Optimization of culture conditions for growth and docosahexaenoic acid production by a marine thraustochytrids, Aurantiochytrium limacinum mh0186. J. Oleo Sci. 58:623–628 [DOI] [PubMed] [Google Scholar]
- 20. Robert SS, et al. 2005. Metabolic engineering of Arabidopsis to produce nutritionally important DHA in seed oil. Funct. Plant Biol. 32:473–479 [DOI] [PubMed] [Google Scholar]
- 21. Saeki K, et al. 2004. Functional expression of a delta12 fatty acid desaturase gene from spinach in transgenic pigs. Proc. Natl. Acad. Sci. U. S. A. 101:6361–6366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sakuradani E, Ando A, Ogawa J, Shimizu S. 2009. Improved production of various polyunsaturated fatty acids through filamentous fungus Mortierella alpina breeding. Appl. Microbiol. Biotechnol. 84:1–10 [DOI] [PubMed] [Google Scholar]
- 23. Saravanan P, Davidson NC, Schmidt EB, Calder PC. 2010. Cardiovascular effects of marine omega-3 fatty acids. Lancet 375:540–550 [DOI] [PubMed] [Google Scholar]
- 24. Tracy NI, Crunkleton DW, Price GL. 2011. Catalytic cracking of squalene to gasoline-range molecules. Biomass Bioenergy 35:1060–1065 [Google Scholar]
- 25. Wu GH, et al. 2005. Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nat. Biotechnol. 23:1013–1017 [DOI] [PubMed] [Google Scholar]
- 26. Yokoyama R, Honda D. 2007. Taxonomic rearrangement of the genus Schizochytrium sensu lato based on morphology, chemotaxonomic characteristics, and 18S rRNA gene phylogeny (Chraustochytriaceae Labyrinthulomycetes): emendation for Schizochytrium and erection of Aurantiochytrium and Oblongichytrium gen. nov. Mycoscience 48:199–211 [Google Scholar]
- 27. Yokoyama R, Salleh B, Honda D. 2007. Taxonomic rearrangement of the genus Ulkenia sensu lato based on morphology, chemotaxonomical characteristics, and 18S rRNA gene phylogeny (Thraustochytriaceae Labyrinthulomycetes): emendation for Ulkenia and erection of Botryochytrium Parietichytrium and Sicyoidochytrium gen. nov. Mycoscience 48:329–341 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.