Abstract
Many people in Japan often detect an unpleasant odor generated from laundry that is hung to dry indoors or when using their already-dried laundry. Such an odor is often described as a “wet-and-dirty-dustcloth-like malodor” or an “acidic or sweaty odor.” In this study, we isolated the major microorganisms associated with such a malodor, the major component of which has been identified as 4-methyl-3-hexenoic acid (4M3H). The isolates were identified as Moraxella osloensis by morphological observation and biochemical and phylogenetic tree analyses. M. osloensis has the potential to generate 4M3H in laundry. The bacterium is known to cause opportunistic infections but has never been known to generate a malodor in clothes. We found that M. osloensis exists at a high frequency in various living environments, particularly in laundry in Japan. The bacterium showed a high tolerance to desiccation and UV light irradiation, providing one of the possible reasons why they survive in laundry during and even after drying.
INTRODUCTION
An unpleasant odor generated from laundry hung to dry has been a concern for a long time, particularly in Japan, where most people wash laundry at low temperatures and increasingly hang it indoors to dry (23). Such an unpleasant odor is often and generally described as a “wet-and-dirty-dustcloth-like malodor” or an “acidic or sweaty odor,” and many people in Japan often detect the malodor of laundry that is hung indoors to dry or even laundry that is already dry. In a previous survey of 200 persons in Japan, 84% of the respondents reported that they had hung their laundry indoors and 63% of them had encountered the problem of an unpleasant odor when hanging the clothes indoors to dry (23). In our survey in 2008, 86% of the respondents (580 women whose ages ranged from 20 to 60 and who lived in the Tokyo metropolitan district) reported detecting a “wet-and-dirty-dustcloth-like malodor” of laundry. Recently, Takeuchi et al. (35) have identified the agent that is frequently detected in clothes with a strong malodor as 4-methyl-3-hexenoic acid (4M3H), which accounts for the sweaty and wet-and-dirty-dustcloth-like malodor. Although 4M3H is present in trace amounts (up to 5 μg/50 g of cloth) in clothes, it contributes most highly to the overall strength of such a malodor (35). Similar branched unsaturated fatty acids have been reported. 3-Methyl-2-hexenoic acid (3M2H) is present in human underarm sweat and confers the characteristic axillary odor (24). 5-Methyl-4-hexenoic acid (5M4H) has been reported as the substance responsible for Eugenia uniflora aroma (8), and 5-methyl-2-hexenoic acid (5M2H) is a component of black tea aroma (20). 4-Methyl hexenoic acid was detected in hops during storage (37). 4M3H was reported as a compound bioconverted from linalool by species closely related to Pseudomonas pseudomallei (21), but it has never been found as a causative agent of malodor of clothes.
On the other hand, it has been known that microorganisms survive in laundry during washing and drying (15, 22, 25, 39, 41), and some of the malodors generated from laundry are caused by the surviving microorganisms (22). Munk et al. (22) used Staphylococcus epidermidis, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa as the bacterial models in clothes and examined their survival and transfer in cloths during washing and their association with the odors generated after washing. Moreover, regarding the human environment, the relationships between microorganisms and malodors have been reported also for human axillary odor (14) and foot odor (2). In the present study, to elucidate the involvement of microorganisms in the generation of a particular agent causing malodor, namely, 4M3H, in laundry, we isolated the major microorganisms associated with the generation of 4M3H, identified the isolates as Moraxella osloensis, and analyzed their characteristics.
MATERIALS AND METHODS
Bacterial strains.
The strains used were M. osloensis ATCC 19976T, M. osloensis NCIMB10693, E. coli NBRC3972, S. aureus NBRC13276, and the bacteria isolated from clothes in the present study.
Sample collection and preparation.
Samples such as bath towels, hand towels, T-shirts, and other clothes with malodor were collected from 15 homes in Japan from 2008 to 2009. The total numbers of bath towels, hand towels, T-shirts, and others with malodor were 16, 7, 3, and 4, respectively. Another 59 samples from items that were washed and kept in drawers, including those both with or without malodor, were randomly collected from 26 homes in Japan from 2009 to 2011. The items were towels, T-shirts, underwear, socks, bottoms, bath mats, pillow cases, and shirts (n = 21, 6, 6, 10, 5, 6, 3, and 2, respectively). All of these samples were stored at room temperature until analysis within one or 2 weeks.
Bacterial isolation from samples.
One piece (3 by 3 cm) cut from each sample was placed in a 20 ml of a “Diluent with Lecithin & Polysorbate 80” solution (Wako Pure Chemical Industries, Ltd., Tokyo, Japan) in a filtered stomacher bag (190 by 280 mm; GSI Creos, Tokyo, Japan) and mixed using a stomacher (IUL, Barcelona, Spain) for 1 min at 1,500 rpm. An aliquot (100 μl) of the solution was spread on soybean casein digest agar with lecithin and polysorbate 80 (SCDA-LP; Wako Pure Chemical Industries, Ltd.) plates. The plates were incubated at 30°C for 2 days, and then bacterial colonies were isolated.
Odor sensory test.
The bacteria cultivated on SCDA (Wako Pure Chemical Industries, Ltd.) plates at 30°C for 2 days were suspended in sterile deionized water (SDW). An aliquot (100 μl) of the bacterial suspension (106 CFU/ml) was inoculated onto small pieces (2 by 2 cm) of sterilized used-towel samples and incubated at 30 or 35°C for 1 day. The odor sensory test of the samples was performed by the method of Takeuchi et al. (35) as follows. Three panelists who had been trained in the test for at least 3 years directly sniffed the samples and ranked the overall odor intensities of the samples.
Quantitative assay of 4M3H.
4M3H was quantitated by the method of Takeuchi et al. (35) as follows. The volatile compounds present in each sample were isolated by extraction using dichloromethane, and the extract was evaporated to a volume of 1 ml. After the addition of 50 ml of dichloromethane, this diluted extract was separated into neutral, basic, and acidic fractions by treatment with 1 M NaOH aqueous solution (50 ml), followed by acidification with 2 M HCl aqueous solution (50 ml). The acidic fraction was then extracted twice by adding another 50 ml of dichloromethane, dried using magnesium sulfate, and again concentrated to 1 ml. Gas chromatography-mass spectrometry (GC/MS) was performed using a gas chromatograph (6890N; Agilent, Palo Alto, CA) equipped with a mass spectrometer (HP5973; Agilent). GC preparative fraction collection (PFC) was performed using a gas chromatograph (6890N) equipped with a flame ionization detector (FID) and a preparative fraction collector (Gerstel GmbH, Mülheim an der Ruhr, Germany). The capillary column used was DB-1 (30 m by 0.53 mm [inner diameter], 1-μm film thickness; J&W Scientific, Inc., Folsom, CA). Helium was used as the carrier gas at a flow rate of 5.5 ml/min. The acidic extract (2 μl) was injected in the splitless mode at 40°C. The temperature program was as follows: 40°C for 1 min, increased to 60°C at a rate of 6°C/min, and further increased to 300°C at a rate of 4°C/min. The split ratio for the FID and PFC devices was 1:99. The effluent with a retention time between a retention index (RI) of 1000 and RI 1500 was trapped using 200 mg of Tenax TA packed into the glass tube (6-mm inner diameter, 117-mm length). This fractionation was repeated 30 times. The thermal desorption system (TDS)-GC/MS analysis of the concentrated fraction was performed using a gas chromatograph (6890N) equipped with a mass spectrometer (HP5973) and a TDS (Gerstel GmbH). The capillary column was DB-FFAP (30 m by 0.25 mm [inner diameter], 0.25-μm film thickness; J&W Scientific). Helium was used as the carrier gas at a flow rate of 0.9 ml/min. Thermodesorption of trapped volatile compounds was carried out at 250°C (purge flow, 50 ml/min; purge duration time, 3 min). The temperature program for the GC oven was as follows: increased to 60°C at a rate of 6°C/min and to 240°C at a rate of 2°C/min. Mass spectra were obtained in the electron-impact mode (70 eV). The recovery yield of 4M3H obtained by the extraction and quantification procedures was ca. 93%.
Analysis of biochemical characteristics of M. osloensis.
The biochemical characteristics of M. osloensis were analyzed using API-ZYM (Sysmex; bioMérieux Co., Ltd., Tokyo, Japan).
rRNA sequence.
We analyzed ∼500 bp to identify each isolate and almost the full length (1,500 bp) of the 16S rRNA sequence to determine the phylogenetic positions of the representative strains. The total genomic DNA was extracted using PrepMan Ultra sample preparation reagent (×100; Applied Biosystems). The obtained genomic DNA was PCR amplified in a thermal cycler (Takara Bio, Inc.) using a MicroSeq 500 16S rDNA bacterial identification PCR kit (Applied Biosystems) to obtain ∼500 bp and PrimeSTAR HS DNA Polymerase (Takara Bio, Inc.) and the primers 9F (5′-GAGTTTGATCCTGGCTCAG-3′; E. coli positions 9 to 27) and 1510R (5′-GGCTACCTTGTTACGA-3′; E. coli positions 1510 to 1495) to obtain ∼1,500 bp. The PCR products were purified with a High-Pure PCR product purification kit (Roche, Basel, Switzerland). The cycle sequencing reaction of ∼500 bp of the 16S rRNA fragments was performed using a MicroSeq 500 16S rDNA bacterial sequencing kit (Applied Biosystems). That of ∼1,500 bp of the 16S rRNA fragments was performed by using the primers 9F, 785F (5′-GGATTAGATACCCTGGTAGTC-3′; E. coli positions 785 to 805), 802R (5′-TACCAGGGTATCTAATCC-3′; E. coli positions 802 to 785), and 1510R, and a BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems). The reaction products were purified using a Dye Ex 2.0 Spin kit (Qiagen, CA) and examined using an ABI Prism 3130 genetic analyzer (Applied Biosystems). The sequence data were compiled using DNASIS-Pro software (Hitachi, Tokyo, Japan). The percent sequence similarities of the fragments were searched using the Aporon DB-FU 2.0. (Technosuruga Lab., Co., Shizuoka, Japan) database.
Phylogenetic tree analysis.
The 16S rRNA gene sequence (∼1,500 bp) data of the strains were aligned using ClustalX version 2.0 (17). The alignment was carried out to construct a phylogenetic tree by the neighbor-joining method, and confidence in the branching order was tested by bootstrap analysis (1,000 trials) using algorithms in ClustalX. The phylogenetic tree was viewed using NJplot (26).
Detection of M. osloensis in samples.
From the collected cloth samples both with and without an unpleasant odor, M. osloensis was detected by the isolation method described above.
Detection of M. osloensis on hard surfaces in living environments.
The sampling points were the various places or household items, such as the entrance hall, kitchen sink, dishwashing sponges, kitchen drain, the floor around the washstand, washbasins, bathroom drains, body wash towels, bathroom walls, toilet floors, and filters in washing machines, in 21 houses located in various prefectures in Japan. These houses were included in the houses where we collected the washed and dried laundry. The total number of sampling points was 230 from 10 to 11 places and items in the houses. M. osloensis was detected by both the isolation and specific PCR methods. To isolate bacteria from the surfaces in living abodes, SCD-LP stamp-medium plates (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) were stamped on the sampling points and incubated at 30°C for 24 h. Colonies were isolated from SCDA (Wako Pure Chemical Industries, Ltd.) plates, and the isolates were identified by sequencing of the 16S rRNA gene (∼500 bp).
When the number of the colonies was large, M. osloensis was detected on the basis of specific PCR. All of the colonies on each stamp-medium plate were collected, and the total genomic DNA of the colony mixture was extracted using PrepMan Ultra sample preparation reagent (×100; Applied Biosystems) and subjected to PCR using the primers primer 1 forward (5′-GGTGAAAGGGGGCGCAAGC-3′) and primer 2 reverse (5′-GTGCTTATTCTGCAGGTAACGTCTAATC-3′).
On the basis of 16S rRNA gene sequencing of M. osloensis, we developed the above-mentioned primers used in the present study and confirmed that they were specific for M. osloensis prior to the present study (data not shown). Each PCR solution consisted of 1× ExTaq buffer (Takara Bio, Inc.), 0.2 mM deoxynucleoside triphosphate, 0.4 μM concentrations of each primer, 1 μl of DNA, and 1 U of ExTaq HS DNA polymerase (Takara Bio, Inc.). Sterilized water was added to the PCR solution up to 20 μl. Amplification was carried out in a PCR thermal cycler (Takara Bio, Inc.) as follows. The samples were incubated at 98°C for 2 min and then subjected to 30 cycles of 98°C for 10 s and 68°C for 1 min. PCR amplification was confirmed by electrophoresis on 2% agarose gels.
Desiccation tolerance assay.
Bacteria were incubated in SCD broth (Wako Pure Chemical Industries, Ltd.) in glass tubes (Iwaki, 18 mm [diameter] by 150 mm; Asahi Techno Glass Co., Ltd., Tokyo, Japan) at 35°C for 24 h to obtain cells in the stationary phase. To analyze the survival of bacterial cells in clothes under dry and wet environmental conditions, 50 μl of the bacterial broth diluted with SCD broth (Wako Pure Chemical Industries, Ltd.) was inoculated onto sterilized cotton stockinette test cloths (1 by 1 cm) to obtain a bacterial initial concentration of 104 CFU/cm2, and the samples were incubated at 35°C for 24 h to obtain a bacterial cell concentration in the range of 107 to 108 CFU/cm2. The cloths were air dried for 1 h, placed in sterilized 24-well polystyrene microtiter plates (Iwaki 1820-024; Asahi Techno Glass Co., Ltd.), and incubated at 30°C for 35 days under dry (11.3% relative humidity [RH%], equilibrium RH% was obtained using saturated LiCl solution) and wet (∼100 RH%) conditions controlled using sterile saturated LiCl solution and sterile deionized water (SDW), respectively, in sealed cases. The cloths were rehydrated by adding 1,000 μl of saline, and appropriate dilutions were made in saline before plating on SCDA plates. The number of viable cells was counted following 1 day of cultivation at 35°C.
To analyze tolerance to desiccation, that is, the survival of bacterial cells after drying, the broth was centrifuged at 8,000 × g for 10 min, and the obtained bacterial cell precipitates were rinsed and resuspended in 0.85% (wt/vol) saline. The suspension was vacuum filtered through 12-mm-diameter membrane filters (Millipore, 0.2 μm; initial concentration, 106 to 107 CFU/cm2), and the bacterial cells on the filters were air dried for 1 h and then further dried for another 23 h using silica gel in sealed cases at 30°C. The bacterial cells were resuspended by adding 1,000 μl of saline, and aliquots (100 μl) of appropriate dilutions with saline were plated onto SCDA plates. The number of viable cells was counted after 1 day of cultivation at 35°C to determine the survival. The data presented are means ± the standard deviations obtained from three independent experiments.
UV light irradiation tolerance assay.
Immediately after the suspended bacterial cells (100 μl) were plated onto SCDA (Wako Pure Chemical Industries, Ltd.) plates, the plates were irradiated with UV light using a UV lamp GL15 (Hitachi) attached to the biological safety cabinet SCV1305EC (Hitachi) for 60, 80, 100, and 120 s at room temperature. The number of viable cells was counted after 1 day of cultivation without UV light irradiation at 35°C. The data obtained were from three independent experiments.
Surfactant tolerance assay.
Sodium dodecyl sulfate (SDS; Wako Pure Chemical Industries, Ltd.) and alkylbenzene sulfonate (ABS; Neoperex; Kao Corp., Tokyo, Japan) were dissolved in 0.85% (wt/vol) saline to obtain final concentrations of 25, 50, and 100 ppm. Portions (1 ml) of cultured cells (106 to 107 CFU/ml) were placed in 9 ml of each solution in glass tubes (Iwaki, 18 mm [diameter] by 150 mm) for 10 min at room temperature, and 0.85% (wt/vol) saline was used as the control instead of the test solutions. Aliquots (1 ml) of these cells were suspended in 9 ml of diluent with Lecithin & Polysorbate 80 (Wako Pure Chemical Industries, Ltd.), and aliquots (100 μl) of the suspension or diluted solution were plated onto SCDA-LP plates. The plates were incubated for 24 h at 35°C, and the number of viable cells was determined. The data presented are means ± the standard deviations obtained from three independent experiments.
Nucleotide sequence accession numbers.
The sequences of the 16S rRNA genes of M. osloensis strains have been deposited in GenBank/EMBL/DDJB under the accession numbers AB643586 to AB643593, AB643595, and AB643596 for the isolates from laundry, AB643597 for NCIMB10693, and AB643598 for ATCC 19976T.
RESULTS
Isolation of major microorganisms responsible for malodor generation.
We isolated the bacteria from the plates in which the extracts from 30 samples (16 bath towels, 7 hand towels, 3 T-shirts, and 4 others) with malodor from 15 homes were incubated. The major isolates were divided into groups on the basis of the colony and biochemical characteristics. There were four major groups detected at frequencies ≧30% with a detection limit below 2 × 102 CFU/cm2 (Table 1). Group A bacteria, whose detection frequency was the highest (87%), generated a high intensity of malodor and a high level of 4M3H (>50 μg/2 by 2 cm) as determined by the odor sensory test and quantitative assay, respectively. Bacterial isolates in groups B and D did not generate malodor and generated 4M3H at levels below the detection limit (3.4 μg/2 by 2 cm), and those in group C generated a weak odor and a low level of 4M3H (<10 μg/2 by 2 cm). The representative bacteria in each major group were identified on the basis of the sequence similarity of the 16S rRNA gene (∼500 bp) to the most closely related type strains and are presented in Table 2. The representative bacteria in other groups whose detection frequency was <30% included Acinetobacter spp. (A. radioresistens, A. junii, and Acinetobacter sp.), Bacillus cereus, and Escherichia hermannii.
Table 1.
Detection frequencies of bacterial groups
| Group | % detection frequencya |
||||
|---|---|---|---|---|---|
| Bath towel | Hand towel | T-shirts | Others | All samplesb | |
| A | 75 | 100 | 100 | 100 | 87 |
| B | 68 | 71 | 33 | 75 | 67 |
| C | 75 | 57 | 33 | 75 | 67 |
| D | 25 | 42 | 33 | 25 | 30 |
The total number of samples of each item was used as the denominator in the calculation of the detection frequency (%).
Samples were collected from 15 homes in Japan. The total numbers of samples of bath towels, hand towels, T-shirts, and others were16, 7, 3, and 4, respectively.
Table 2.
Representative strains isolated from laundry with malodor
| Group | Representative strain |
|||
|---|---|---|---|---|
| Genus | Closest relative | Accession no.a | % sequence similarityb | |
| A | Moraxella | Moraxella osloensis ATCC 19976T | AB643598 | 99.2 |
| B | Micrococcus | Micrococcus luteus JCM1464T | AJ536198/Tecsrg0047 | 99.6 |
| C | Pseudomonas | Pseudomonas oleovorans IAM1508T | D84018 | 99.2 |
| Pseudomonas | Pseudomonas aeruginosa ATCC 10145T | AF094713 | 100 | |
| D | Roseomonas | Roseomonas mucosa MDA5527T | AF538712 | 100 |
Accession numbers based on the 16S rRNA partial gene sequence.
Sequence similarity (16S rRNA, ∼500 bp) was searched using Aporon DB-FU 2.0. (Technosuruga Lab. Co., Shizuoka, Japan).
Characteristics of isolates producing 4M3H.
The representative strains in group A showed milky white colonies on SCD plates and good growth at 30 to 37°C. The strains were 0.5- to 1.0-μm by 1.0- to 1.5-μm short rod-shaped bicellular Gram-negative bacteria (data not shown). The biochemical traits of strains in group A were almost identical to those of the reference strains of M. osloensis (see Table S1 in the supplemental material). The sequence similarities of the 16S rRNA gene (∼1,500 bp) between the 10 representative strains in group A, which were isolated from different samples, and the type strain of M. osloensis (ATCC 19976T) were 99.2 to 99.7%. Based on 16S rRNA phylogenetic tree analysis, we classified the representative strains in group A into the M. osloensis group (see Fig. S1 in the supplemental material).
4M3H-producing ability of M. osloensis.
M. osloensis ATCC 19976T (isolate from cerebrospinal fluid), M. osloensis NBRC10693 (isolate from urine), and 10 M. osloensis isolates from cloth samples in the present study (KMC41, KMC43, KMC44, KMC45, KMC46, KMC47, KMC48, KMC49, KMC411, and KMC412) showed a high malodor intensity, as determined by the odor sensory test.
Detection of M. osloensis in cloth samples and living environments.
A total of 59 samples, which were already washed and dried and collected with or without an unpleasant odor from 26 homes in Japan, were analyzed. M. osloensis was detected at a high frequency (≥50%) in towels, T-shirts, socks, pillowcases, and shirts. More than 50% of these items had a large population (>104 CFU/cm2) of M. osloensis (Table 3). The detection limit of the PCR detection method used here was 102 CFU/cm2, and M. osloensis was detected at frequencies of 14 to 57% on the hard surfaces in living environments (Table 4).
Table 3.
Detection of Moraxella osloensis in cloth samplesa
| Item | No. of samples | % detection frequencyb | Bacterial count (CFU/cm2)c | % detection frequency at >104 CFU/cm2d |
|---|---|---|---|---|
| Towels | 21 | 86 | 1.0 × 101 to 1.3 × 107 | 52 |
| T-shirts | 6 | 50 | 1.9 × 105 to 1.6 × 106 | 50 |
| Underwear | 6 | 33 | 1.8 × 102 to 2.4 × 104 | 16 |
| Socks | 10 | 50 | 2.1 × 103 to 2.8 × 105 | 50 |
| Bottoms | 5 | 0 | NDe | ND |
| Bath mat | 6 | 17 | 2.7 × 105 | 17 |
| Pillowcase | 3 | 67 | 1.2 × 103 to 2.9 × 104 | 67 |
| Shirts | 2 | 50 | 1.0 × 101 to 4.4 × 101 | 0 |
A total of 59 samples were collected from 26 homes in Japan.
The number of samples of each item was used as the denominator in the calculation of the detection frequency (%).
The bacterial count ranges for each item are indicated.
The detection frequency (%) in samples at which M. osloensis was detected at >104 CFU/cm2.
ND, not detected.
Table 4.
Detection of Moraxella osloensis on hard surfaces in houses
| Sampling point | % detection frequencya |
|---|---|
| Entrance hall | 14 |
| Kitchen sink | 57 |
| Dishwashing sponge | 33 |
| Kitchen drain | 33 |
| Floor around washstand | 29 |
| Washbasin | 24 |
| Bathroom drain | 48 |
| Body washing towel | 19 |
| Bathroom wall | 33 |
| Toilet floor | 24 |
| Filter in washing machine | 33 |
The total number of all samples was used as the denominator in calculating the detection frequency (%). The number of samples was 11 each in the entrance hall, kitchen sink, dishwashing sponge, kitchen drain, floor around the washstand, washbasin, bathroom drain, body washing towel, bathroom wall, and toilet floor. That in the filter in the washing machine was 10.
Tolerance of M. osloensis to environmental stresses.
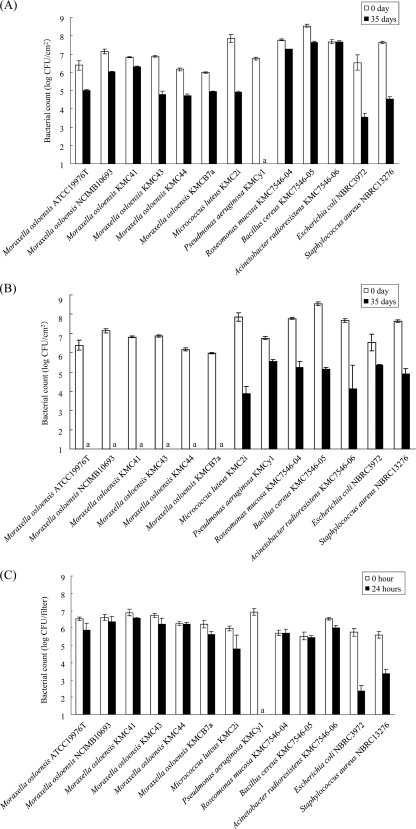
Figure 1 shows the survival levels of two subdivided (ATCC 19976T and NCIMB10693) and four isolated (KMC41, KMC43, KMC44 [from laundries], and KMCB7a [from living environments]) strains of M. osloensis, five other representative bacterial isolates from laundries in the present study, and control bacteria (E. coli NBRC3972 and S. aureus NBRC13276) in clothes under dry (Fig. 1A) and wet (Fig. 1B) environmental conditions and during desiccation (Fig. 1C). All of the M. osloensis strains tested and all of the isolates except for P. aeruginosa KMCy1 showed high survival levels, with their counts decreasing by <3 log10 CFU/cm2 under dry environmental conditions and during desiccation. P. aeruginosa KMCy1, E. coli NBRC3972, and S. aureus NBRC13276 showed low survival levels under dry condition and during desiccation.
Fig 1.
Survival levels of Moraxella osloensis ATCC 19976T, M. osloensis NCIMB10693, M. osloensis KMC41, M. osloensis KMC43, M. osloensis KMC44, M. osloensis KMCB7a, Micrococcus luteus KMC2i, Pseudomonas aeruginosa KMCy1, Roseomonas mucosa KMC7546-04, Bacillus cereus KMC7546-05, Acinetobacter radioresistens KMC7546-06, Escherichia coli NBRC3972, and Staphylococcus aureus NBRC13276 under dry (A) and wet (B) environmental conditions and during desiccation (C). The bacterial cells in test clothes were incubated at 30°C for 35 days under dry conditions (A), which were controlled using saturated LiCl solution, and wet conditions (B), which were controlled using SDW. The 1-h air-dried bacterial cells on the filters were incubated with silica gel at 30°C for 24 h (C). The error bars indicate the standard deviations of three independent samples. a, below the detection limit.
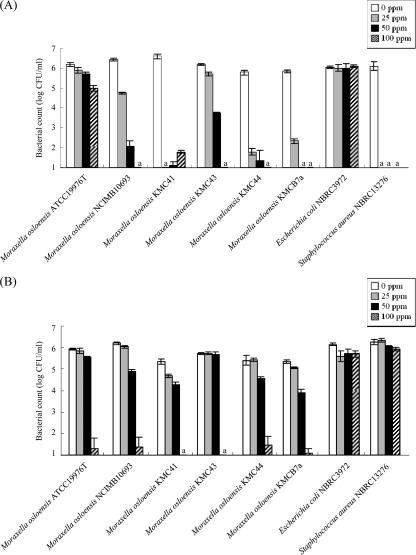
After UV light irradiation, all of the M. osloensis strains, M. luteus KMC2i, and A. radioresistens KMC7546-06 showed a higher survival level than P. aeruginosa KMCy1, E. coli NBRC3972, and S. aureus NBRC13276 (Table 5). On the other hand, the M. osloensis strains did not show a high tolerance level against surfactants such as ABS and SDS (Fig. 2).
Table 5.
Effect of UV light irradiation on cell survival
| Strain | Time (s)a |
|---|---|
| Moraxella osloensis ATCC 19976T | 80 |
| Moraxella osloensis NCIMB10693 | 120 |
| Moraxella osloensis KMC41 | 100 |
| Moraxella osloensis KMC43 | 120 |
| Moraxella osloensis KMC44 | >120 |
| Moraxella osloensis KMCB7a | 80 |
| Micrococcus luteus KMC2i | 120 |
| Pseudomonas aeruginosa KMCy1 | 20 |
| Acinetobacter radioresistens KMC7546-06 | 100 |
| Escherichia coli NBRC3972 | 60 |
| Staphylococcus aureus NBRC13276 | 40 |
The time required to decrease the bacterial count by 4 orders of magnitude.
Fig 2.
Tolerance of M. osloensis ATCC 19976T, M. osloensis NCIMB10693, M. osloensis KMC41, M. osloensis KMC43, M. osloensis KMC44, M. osloensis KMCB7a, E. coli NBRC3972, and S. aureus NBRC13276 to ABS (A) and SDS (B). Bacterial cells were suspended in ABS (A) and SDS (B) solution at the concentrations indicated and incubated for 10 min. The error bars indicate the standard deviations of three independent samples. a, below the detection limit.
DISCUSSION
The major component responsible for unpleasant odors generated from laundry, 4M3H, has never been reported as a component of major dirt in laundry such as grime, sebum, sweat, or soil. Nevertheless, it has been reported that a large number of microorganisms survive in laundry during washing and drying (15, 22, 25, 39, 41), and bacteria in clothes produce malodors (22). Assuming that 4M3H is a bacterial metabolite, we isolated the bacteria that had high detection frequencies and counts of more than 2 × 102 CFU/cm2 in the laundry samples with malodors even after washing and drying. As a preliminary test, we compared the results obtained by the cultivation method to those obtained by a noncultivation method, i.e., PCR-denaturing gradient gel electrophoresis (DGGE) using universal primers of 16S rRNA. The numbers of bacteria showing major bands in DGGE were limited in washed and dried laundry samples, and all of the major bacteria analyzed by DGGE were also isolated by the cultivation method (data not shown). These findings suggest that nonculturable bacteria could not be the major bacteria in the samples in the present study or the bacteria primarily involved in the generation of 4M3H.
The major causative bacteria were screened and determined among the isolates by the assays of malodor generation in used towels sterilized after washing and drying. The bacteria causing malodor or producing 4M3H were then classified and identified as M. osloensis by morphological observation and phylogenetic tree and biochemical characteristic analyses. M. osloensis was reported as a cause of bacterial opportunistic infections and was detected from patients with meningitis (28), lymphadenitis (1), endophthalmitis (4), bacteremia (12), and hematological malignancy (31). It is also associated with nematode parasites of slugs (36). The species has been isolated or detected in living organisms and foods, such as insects (43), the kidney tissue of Chinook salmon (10), beef (34), and milk and cheese (6) and in natural environments worldwide such as a soda lake in Russia (11), glacial melt water on Mount Everest (18), Antarctic ice cores (29), an arsenic-contaminated river in Chile (9), household air in the United Kingdom (44), restaurants in China (5), the surfaces of a men's restroom at a university in the United States (19), and compost in Portugal (38). To the best of our knowledge, however, there has been no report on M. osloensis detection at a high frequency in laundry or clothes, nor has an association of M. osloensis with specific malodor occurrence been demonstrated. Not only 10 strains of M. osloensis isolated from laundry with malodors, but also M. osloensis ATCC 19976T originating from cerebrospinal fluid, a type strain of this species, and M. osloensis NBRC10693 originating from urine caused a high-intensity malodor, as determined by the odor sensory test, suggesting that the species M. osloensis has the potential to generate 4M3H rather than the particular M. osloensis strains isolated from laundry.
To examine the agent responsible for malodor generation of M. osloensis, an odor sensory test using sterilized used and new towels was performed. M. osloensis KMC41 produced 4M3H only in used towels and not in new towels (data not shown). This finding suggests that the coexistence of M. osloensis and dirt such as grime, sebum, sweat, or soil in laundry, which remain even after washing, is an essential factor for the malodor generation; however, no bacteria that convert such components of dirt to 4M3H have been reported. Biodegradations by Moraxella sp. were reported for p-nitrophenol (32), naphthalene-2,6- and naphthalene-1,6-disulfonic acid (42), and 4-chloroaniline (45) but not for 4M3H. Mizutani et al. (21) reported that 4M3H is converted from linalool by species closely related to Pseudomonas pseudomallei, which is a linalool-utilizing bacterium. However, it appears unlikely that linalool is a precursor in laundry; the actual precursors could be a component of dirt, and M. osloensis may use a different metabolic pathway to produce 4M3H in laundry. In a study of human axillary odor, short (C2 to C5)- and medium (C6 to C11)-chain-length volatile fatty acids, which are among the primary causal molecules of axillary malodor, were shown to be produced from lipid substrates readily available for the bacteria residing on the axillary skin (14). Likewise, another possibility is that the conversion to 4M3H occurs after the formation of precursors of 4M3H by natural conversion or other microbial metabolisms of primary components in dirt. To clarify the pathway, our next goal is to identify the essential components of dirt or the precursors of the conversion to 4M3H in laundry.
We found that M. osloensis exists in large populations (102 to 107 CFU/cm2) in the laundry or clothes, even after washing and drying, which were collected from various regions and families in Japan, and was detected frequently. Moreover, the species also exists on various hard surfaces in houses in Japan. As the second step, we considered the ecological implications of M. osloensis in laundry. All of the M. osloensis strains tested showed higher tolerances under dry conditions in cloths, which was a test condition used as a model of drying and keeping clothes, and higher tolerances to desiccation stress (Fig. 1) than did E. coli NBRC3972 and S. aureus NBRC13276, which are the strains recommended for use in preservative efficacy tests or antibacterial tests by the International Organization for Standardization, U.S. Pharmacopoeia, Personal Care Product Council, European Pharmacopoeia, European Standard, Japanese Pharmacopoeia, and Japanese Industrial Standard. All of the M. osloensis strains tested here also showed a higher tolerance to UV light irradiation than did E. coli NBRC3972 and S. aureus NBRC13276. Interestingly, however, they did not have high tolerance against surfactants. These results suggest that M. osloensis strains, regardless of their origin, can survive and generate 4M3H during the drying process because of their high tolerance to desiccation and UV light irradiation. The tolerance of M. osloensis strains to desiccation has not been reported to the best of our knowledge, whereas the irradiation of UV-light-tolerant Moraxella strains (16) and isolation of M. osloensis strains tolerant to environmental stresses such as gamma radiation (40), penicillin (13), and streptomycin (13) have been reported. Lipids and fatty acids in the cell membrane have an important role in the desiccation tolerance of various microorganisms (3, 27, 30). The particular fatty acid composition in the cell membrane of the species M. osloensis (33) might be related to desiccation tolerance. UV-light-resistant Acinetobacter has a high catalase activity, which reduces oxidative stress following UV light irradiation (7). M. osloensis KMC41 showed a higher catalase activity of their culture broth than E. coli NBRC 3972 (data not shown). Further examination is necessary to determine whether catalase activity of M. osloensis strains is high even in laundry and whether the catalase activity is the major factor for M. osloensis to survive.
In the present study, M. osloensis, regardless of its origin, showed a higher tolerance to desiccation and UV light irradiation than the subdivided bacteria, which are commonly used in preservative efficacy tests or antibacterial tests. However, interestingly, the bacteria isolated at high frequencies and large populations also had such tolerance, suggesting that tolerance to desiccation and UV light irradiation limits the bacterial flora in laundry during the washing and drying cycles in daily laundering. Moreover, both the environmental stress tolerance during drying and the metabolic pathway for 4M3H production are essential characteristics for a microorganism to generate a malodor in laundry, and M. osloensis has these characteristics. Because M. osloensis has been isolated elsewhere in the world as described above, it is possible that the same malodor problems caused by the bacterium are encountered in some countries where people usually hang their laundry indoors to dry or in other countries where machine washing and drying tend to be carried out at lower temperatures and under mild conditions, which are favorable for the survival of the bacterium.
Supplementary Material
ACKNOWLEDGMENT
We thank Miyuki Ojima of Kao Corp. for the survey of consumers' attitudes regarding malodors generated from laundry.
Footnotes
Published ahead of print 24 February 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Angelakis E, Roux V, Raoult D, Rolain J-M. 2009. Real-time PCR strategy and detection of bacterial agents of lymphadenitis. Eur. J. Clin. Microbiol. Infect. Dis. 28:1363–1368 [DOI] [PubMed] [Google Scholar]
- 2. Ara K, et al. 2006. Foot odor due to microbial metabolism and its control. Can. J. Microbiol. 52:357–364 [DOI] [PubMed] [Google Scholar]
- 3. Beney L, Gervais P. 2001. Influence of the fluidity of the membrane on the response of microorganisms to environmental stresses. Appl. Microbiol. Biotechnol. 57:34–42 [DOI] [PubMed] [Google Scholar]
- 4. Berrocal AM, Scott IU, Miller D, Flynn HW., Jr 2002. Endophthalmitis caused by Moraxella osloensis. Graefe's Arch. Clin. Exp. Ophthalmol. 240:329–330 [DOI] [PubMed] [Google Scholar]
- 5. Chan PL, Yu PHF, Cheng YW, Chan CY, Wong PK. 2009. Comprehensive characterization of indoor airborne bacterial profile. J. Environ. Sci. 21:1148–1152 [DOI] [PubMed] [Google Scholar]
- 6. Delbès C, Ali-Mandjee L, Montel M-C. 2007. Monitoring bacterial communities in raw milk and cheese by culture-dependent and -independent 16S rRNA gene-based analyses. Appl. Environ. Microbiol. 73:1882–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Capua C, Bortolotti A, Farías ME, Cortez N. 2011. UV-resistant Acinetobacter sp. isolates from Andean wetlands display high catalase activity. FEMS Microbiol. Lett. 317:181–189 [DOI] [PubMed] [Google Scholar]
- 8. El Alfy TS, Ibrahim TA, Sleem AA. 2003. Phytochemical and biological study of the essential oils and petroleum ether extract of the leaves and flowers of Eugenia uniflora L. grown in Egypt (part 1). Bull. Fac. Pharm. 41:83–92 [Google Scholar]
- 9. Escalante G, et al. 2009. Arsenic resistant bacteria isolated from arsenic contaminated river in the Atacama Desert (Chile). Bull. Environ. Contam. Toxicol. 83:657–661 [DOI] [PubMed] [Google Scholar]
- 10. Evans ML, Neff BD. 2009. Major histocompatibility complex heterozygote advantage and widespread bacterial infections in populations of Chinook salmon (Oncorhynchus tshawytscha). Mol. Ecol. 18:4716–4729 [DOI] [PubMed] [Google Scholar]
- 11. Foti MJ, et al. 2008. Bacterial diversity and activity along a salinity gradient in soda lakes of the Kulunda Steppe (Altai, Russia). Extremophiles 12:133–145 [DOI] [PubMed] [Google Scholar]
- 12. Han XY, Tarrand JJ. 2004. Moraxella osloensis blood and catheter infections during anticancer chemotherapy. Am. J. Clin. Pathol. 121:581–587 [DOI] [PubMed] [Google Scholar]
- 13. Hansen W, Butzler JP, Fuglesang JE, Henriksen SD. 1974. Isolation of penicillin and streptomycin resistant strains of Moraxella osloensis. Acta Pathol. Microbiol. Scand. Sect. 82:318–322 [DOI] [PubMed] [Google Scholar]
- 14. James AG, Casey J, Hyliands D, Mycock G. 2004. Fatty acid metabolism by cutaneous bacteria and its role in axillary malodor. World J. Microbiol. Biotechnol. 20:787–793 [Google Scholar]
- 15. Jaska JM, Fredell DL. 1980. Impact of detergent systems on bacterial survival on laundered fabrics. Appl. Environ. Microbiol. 39:743–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keller LC, Thompson TL, Maxcy RB. 1982. UV light-induced survival response in a highly radiation-resistant isolate of the Moraxella-Acinetobacter group. Appl. Environ. Microbiol. 43:424–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Larkin MA, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, et al. 2009. Culturable bacteria in glacial meltwater at 6,350 m on the East Rongbuk Glacier, Mount Everest. Extremophiles 13:89–99 [DOI] [PubMed] [Google Scholar]
- 19. Meschke S, et al. 2009. The effect of surface charge, negative and bipolar ionization on the deposition of airborne bacteria. J. Appl. Microbiol. 106:1133–1139 [DOI] [PubMed] [Google Scholar]
- 20. Mick W, Götz E-M, Schreier P. 1984. Volatile acids of black tea aroma. Lebensm.-Wiss. U-Technol. 17:104–106 [Google Scholar]
- 21. Mizutani S, Hyashi T, Ueda H, Tatsumi C. 1971. Microbiological conversion of terpens. IX. Conversion of linalool. Nippon Nougeikagakukaishi 8:368–373 (In Japanese.) [Google Scholar]
- 22. Munk S, Johansen C, Stahnke LH, Adler-Nissen J. 2001. Microbial survival and odor in laundry. J. Surfact. Deterg. 4:385–394 [Google Scholar]
- 23. Nagoh Y, Tobe S, Watanabe T, Mukaiyama T. 2005. Analysis of odorants produced from indoor drying laundries and effects of enzyme for preventing malodor generation. Tenside Surf. Det. 42:7–12 [Google Scholar]
- 24. Natsch A, Derrer S, Flachsmann F, Schmid J. 2006. A broad diversity of volatile carboxylic acids, released by a bacterial aminoacylase from axilla secretions, as candidate molecules for the determination of human-body odor type. Chem. Biodivers. 3:1–20 [DOI] [PubMed] [Google Scholar]
- 25. O'Toole J, Sinclair M, Leder K. 2009. Transfer rates of enteric microorganisms in recycled water during machine clothes washing. Appl. Environ. Microbiol. 75:1256–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perrière G, Gouy M. 1996. WWW-query: an on-line retrieval system for biological sequence banks. Biochimie 78:364–369 [DOI] [PubMed] [Google Scholar]
- 27. Potts M. 1994. Desiccation tolerance of prokaryotes. Microbiol. Rev. 58:755–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roh KH, et al. 2010. Three cases of Moraxella osloensis meningitis: a difficult experience in species identification and determination of clinical significance. J. Korean Med. Sci. 25:501–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Segawa T, Ushida K, Narita H, Kanda H, Kohshima S. 2010. Bacterial communities in two Antarctic ice cores analyzed by 16S rRNA gene sequencing analysis. Polar Sci. 4:215–227 [Google Scholar]
- 30. Singh SC, Sinha RP, Häder D-P. 2002. Role of lipids and fatty acids in stress tolerance in cyanobacteria. Acta Protozool. 41:297–308 [Google Scholar]
- 31. Skovbjerg S, et al. 2009. Optimization of the detection of microbes in blood from immunocompromised patients with haematological malignancies. Clin. Microbiol. Infect. 15:680–683 [DOI] [PubMed] [Google Scholar]
- 32. Spain JC, Gibson DT. 1991. Pathway for biodegradation of p-nitrophenol in a Moraxella sp. Appl. Environ. Microbiol. 57:812–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sugimoto C, Miyagawa E, Nakazawa M, Mitani K, Ishizawa Y. 1983. Cellular fatty acid composition comparisons of Haemophilus equigenitalis and Moraxella species. Int. J. Syst. Bacteriol. 33:181–187 [Google Scholar]
- 34. Takahashi H, et al. 2008. Use of single-strand conformation polymorphism of amplified 16S rDNA for grouping of bacteria isolated from foods. J. Food Prot. 71:839–844 [DOI] [PubMed] [Google Scholar]
- 35. Takeuchi K, Hasegawa Y, Ishida H, Kashiwagi M. 2012. Identification of novel malodor compounds in laundry. Flavour Fragr. J. 27:89–94 [Google Scholar]
- 36. Tan L, Grewal PS. 2001. Pathogenicity of Moraxella osloensis, a bacterium associated with the nematode Phasmarhabditis hermaphrodita, to the slug Deroceras reticulatum. Appl. Environ. Microbiol. 67:5010–5016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tressl R, Friese L, Fendesack F, Köppler H. 1978. Studies of the volatile composition of hops during storage. J. Agric. Food Chem. 26:1426–1430 [Google Scholar]
- 38. Vaz-Moreira I, Silva ME, Manaia CM, Nunes OC. 2008. Diversity of bacterial isolates from commercial and homemade composts. Microb. Ecol. 55:714–722 [DOI] [PubMed] [Google Scholar]
- 39. Walter WG, Schillinger JE. 1975. Bacterial survival in laundered fabrics. Appl. Microbiol. 29:368–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Welch AB, Maxcy RB. 1975. Characterization of radiation-resistant vegetative bacteria in beef. Appl. Microbiol. 30:242–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wiksell JC, Pickett MS, Hartman PA. 1973. Survival of microorganisms in laundered polyester-cotton sheeting. Appl. Microbiol. 25:431–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wittich RM, Rast HG, Knackmuss H-J. 1988. Degradation of naphthalene-2,6- and naphthalene-1,6-disulfonic acid by Moraxella sp. Appl. Environ. Microbiol. 54:1842–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yamoah E, et al. 2008. Microbial population and diversity on the exoskeletons of four insect species associated with gorse. Aust. J. Entomology. 47:370–379 [Google Scholar]
- 44. Yuan I, et al. 2007. Molecular identification of environmental bacteria in indoor air in the domestic home: description of a new species of Exiguobacterium. Int. J. Environ. Health Res. 17:75–82 [DOI] [PubMed] [Google Scholar]
- 45. Zeyer J, Wasserfallen A, Timmis KN. 1985. Microbial mineralization of ring-substituted anilines through an ortho-cleavage pathway. Appl. Environ. Microbiol. 50:447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.