Abstract
The effect of a number of N-aroyl-3,5-bis(benzylidene)-4-piperidones 2 and related quaternary ammonium compounds 3 on the rates of respiration in rat liver mitochondria were determined. All of the compounds stimulated respiration and the greatest effect was displayed by the compounds in series 3 which caused swelling of mitochondria.
1. Introduction
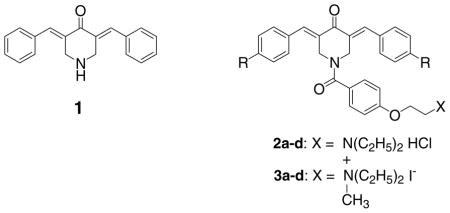
3,5-bis(Benzylidene)-4-piperidone (1) displays potent cytotoxicity to a number of transformed and neoplastic cell lines (Dimmock et al. 2001; Das et al. 2007). In addition, it has excellent antimycobacterial properties (Das et al. 2008). Thus 1 was used as the lead molecule in the simultaneous development of further candidate cytotoxins and antimycobacterials, including the formation of the corresponding N-aroyl derivatives 2a–d and related quaternary ammonium salts (3a–d) (Das et al. 2007). Most of the compounds in series 2 and 3 also possess excellent cytotoxic and antimycobacterial properties (Das et al. 2007; Das et al. 2008).
2. Investigations, results and discussion
The aim of the present study was to investigate how these compounds exert their bioactivities. A possible target organelle is the mitochondrion for a number of reasons including the following considerations. First, respiration in rat liver mitochondria was stimulated and then inhibited as the concentrations of an acyclic Mannich base of a conjugated arylidene ketone increased (Hamon et al. 1982). The compounds in series 1–3 are cyclic Mannich bases (3-aminoketones) of arylidene ketones and hence may affect mitochondrial respiration in a similar fashion. Second, some compounds which are structurally related to 1 and 2 stimulate respiration in mitochondria at a low concentration (10 μM) (Das et al. 2008). Third, some antineoplastic agents alter the rates of respiration in mitochondria (Marín-Hernández et al. 2003; Lemeshko and Kugler 2007).
The data in the Table indicate that the compounds 1–3 stimulate respiration in rat liver mitochondria. At the concentrations employed in this study, no inhibition of respiration was noted although on occasions reduction of stimulation was observed as the concentrations of the compounds increased. This observation may have been due to the coexistence of the induction of both inhibitory and stimulatory effects on respiration in mitochondria. An attempt was made to ascertain whether the extent of respiration was controlled by the electronic, hydrophobic and steric properties of the atom or group in the arylidene aryl rings in series 2 and 3. Thus linear and semilogarithmic plots were constructed between the Hammett σ, Hansch π and molar refractivity constants of the R group and the percentage increase in respiration when 50 μM of the compounds were employed. A trend to a positive correlation was noted only in series 2 with the σ values when linear (p = 0.081) and semilogarithmic (p = 0.100) plots were made. In the remaining cases, no correlations were observed (p > 0.1). Thus the insertion of strongly electron- attracting substituents in the arylidene aryl rings of compounds related to 2a–d such as the 3,5-dinitro group (Σσ = 1.42) (Hansch and Leo 1979) would be predicted to increase respiration. Conversely the placement of one or more electron-donating groups such as the 4-methylamino substituent (σ= −0.84) (Hansch and Leo 1979) would likely reduce respiration compared to the biodata generated for 2a–d.
Table.
Stimulation of respiration in rat liver mitochondria by 1, 2a–d and 3a–d
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compd. | R | Percentage increase in respirationa
|
|||||||
| 10 μM | Δb | 20 μM | Δb | 50 μM | Δb | 100 μM | Δb | ||
| 1 | – | 20.2 ± 1.22 | – | 22.8 ± 0.61 | – | 43.3 ± 4.51 | – | 48.3 ± 4.80 | – |
| 2a | H | 27.7 ± 2.80 | 28.4 ± 2.17 | 61.2 ± 5.14 | 87.2 ± 11.6 | ||||
| 3a | H | 32.4 ± 5.68 | 1.2c | 53.6 ± 3.86 | 1.9 | 134 ± 2.49 | 2.2 | 168 ± 10.5 | 1.9 |
| 2b | Cl | 25.4 ± 3.81 | 29.7 ± 6.97 | 52.5 ± 9.35 | 62.4 ± 11.3 | ||||
| 3b | Cl | 136 ± 3.67 | 5.4 | 119 ± 3.45 | 4.0 | 100 ± 2.78 | 1.9 | 80.0 ± 4.13 | 1.3 |
| 2c | CH3 | 23.6 ± 2.60 | 36.0 ± 6.71 | 41.2 ± 7.60 | 67.8 ± 8.58 | ||||
| 3c | CH3 | 60.0 ± 3.03 | 2.5 | 83.7 ± 2.20 | 2.3 | 254 ± 10.6 | 6.2 | 83.9 ± 1.79 | 1.2 |
| 2d | NO2 | 32.3 ± 2.11 | 63.5 ± 2.76 | 88.9 ± 8.22 | 155 ± 4.46 | ||||
| 3d | NO2 | 66.8 ± 2.91 | 2.1 | 54.7 ± 3.50 | 0.9 | 94.9 ± 2.52 | 1.1c | 60.3 ± 3.56 | 0.4 |
The rates of oxygen consumption by mitochondria (1 mg protein/mL) respiring on succinate were calculated for the period 1 min prior to, and 1 min after, addition of the compound
The Δ figures are the quotients of the percentage increases in respiratory stimulation of the compound in series 3 with the analog in series 2 which has the same aryl substituent
The differences between the percentage increases in respiration are not statistically significantly different
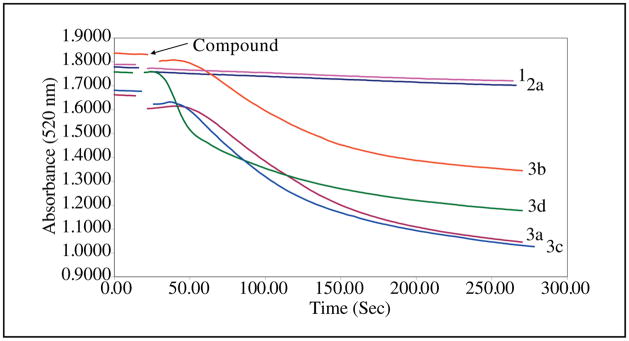
Stimulation of respiration in mitochondria can be caused by a number of biochemical mechanisms including the induction of swelling of these organelles. In order to explore this possibility, 1, 2a and 3a–d were evaluated for this property. The results which are portrayed in the Figure reveal that swelling in mitochondria was caused by the quaternary ammonium compounds in series 3 while this effect was absent in 1 and 2a. Mitochondrial swelling has been observed previously, such as with the anticancer quaternary ammonium compound erucylphosphohomocholine (Lemeshko and Kugler 2007).
An important consideration in deciding to develop compounds as candidate drugs is their mammalian toxicity. A previous study revealed that when a dose of 300 mg/kg of 1, 2a–d and 3a–d was administered to mice and the animals observed at the end of 4 h, there were no mortalities caused by the compounds in series 1 and 2 but all of the mice receiving 3a, c, d were dead. Reducing the dose to 100 mg/kg led to the deaths of most of the mice receiving 3a, d (Das et al. 2008). It is conceivable that these differences in tolerability in mice may be due, at least in part, to variations in the effects on mitochondria. Thus, in general, stimulation of respiration is greater in series 3 than 2 as the Δ values in the Table indicate. This observation may be due to the fact that quaternary ammonium salts being completely ionized can interact with anionic binding sites in the mitochondria. On the other hand, amines such as 2a–d exist in solution as a mixture of protonated and unprotonated molecules (Albert 1985) and hence the extent of their interacting with anionic sites will be lower than is capable with 3a–d. Furthermore, the compounds in series 3 cause swelling of mitochondria. These two observations are important, since the toxicity of quaternary ammonium compounds is often attributed to neurological deficit (Pandeya and Dimmock 1997) and hence future toxicity studies of these compounds should take into consideration their effect on mitochondria.
In conclusion, the mode of action of the promising bioactive molecules 1–3 includes stimulation of respiration in mitochondria and in the case of the quaternary ammonium compounds swelling of these organelles takes place.
3. Experimental
The preparation of 1, 2a–d and 3a–d has been described previously (Dimmock et al. 2001; Das et al. 2007). The Hammett σ, Hansch π and molar refractivity constants were taken from the literature (Hansch and Leo 1979) and the linear and semilogarithmic plots between these values and the percentage increases in mitochondrial respiration were made using a commercial software package (SPSS 2005).
A literature method was used to isolate the mitochondria (Kowaltowski et al. 1996) and the increase in mitochondrial oxygen consumptions was determined polarographically by a previously reported procedure (Estabrook 1967). A reference compound carbonyl cyanide 3-chlorophenylhydrazone caused an increase in respiration of 606 ± 5.34% when 10 μM was utilized. Mitochondrial swelling was determined spectrophotometrically at 520 nm as described previously (Kowaltowski et al. 1996). For obtaining the data in the Table and the Fig., the mitochondria at 1 mg protein/mL were incubated at 30 °C in an aqueous buffer pH 7.2 containing sucrose (125 mM), HEPES (10 mM), potassium phosphate (5 mM), magnesium chloride (1 mM) and succinate (5 mM).
Fig.
Evaluation of 50 μM of 1, 2a and 3a–d for causing swelling of rat liver mitochondria
References
- Albert A. Selective toxicity. 7. Chapman and Hall; London: 1985. pp. 642–643. [Google Scholar]
- Das U, Alcorn J, Shrivastav A, Sharma RK, De Clercq E, Balzarini J, Dimmock JR. Design, synthesis and cytotoxic properties of novel 1-[4-(2-alkylaminoethoxy)phenylcarbonyl]-3,5-bis(arylidene)-4-piperidones and related compounds. Eur J Med Chem. 2007;42:71–80. doi: 10.1016/j.ejmech.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Das U, Das S, Bandy B, Stables JP, Dimmock JR. N-Aroyl-3,5-bis(benzylidene)-4-piperidones: a novel class of antimycobacterial agents. Bioorg Med Chem. 2008;16:3602–3607. doi: 10.1016/j.bmc.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmock JR, Padmanilayam MP, Puthucode RN, Nazarali AJ, Motaganahalli NL, Zello GA, Quail JW, Oloo EO, Kraatz H-B, Prisciak JS, Allen TM, Santos CL, Balzarini J, De Clercq E, Manavathu EK. A conformational and structure-activity relationship study of cytotoxic 3,5-bis(arylidene)-4-piperidones and related N-acryloyl analogues. J Med Chem. 2001;44:586–593. doi: 10.1021/jm0002580. [DOI] [PubMed] [Google Scholar]
- Estabrook RW. Mitochondrial respiratory control and the polarographic measurement of ADP :O ratios. Methods Enzymol X. 1967:41–47. [Google Scholar]
- Hamon NW, Kirkpatrick DL, Chow EWK, Dimmock JR. Effect of 4-dimethylaminomethyl-1-(3-hydroxyphenyl)-1-nonen-3-one hydrochloride and related compounds on respiration in rat liver mitochondria. J Pharm Sci. 1982;71:25–29. doi: 10.1002/jps.2600710106. [DOI] [PubMed] [Google Scholar]
- Hansch C, Leo AJ. Substituent constants for correlation analysis in chemistry and biology. John Wiley and Sons; New York: 1979. p. 49. [Google Scholar]
- Kowaltowski AJ, Castilho RF, Grijalba MT, Bechara EJ, Vercesi AE. Effect of inorganic phosphate concentration on the nature of inner mitochondrial membrane alterations mediated by Ca2+ ions: A proposed model for phosphate-stimulated lipid peroxidation. J Biol Chem. 1996;271:2929–2934. doi: 10.1074/jbc.271.6.2929. [DOI] [PubMed] [Google Scholar]
- Lemeshko VV, Kugler W. Synergistic inhibition of mitochondrial respiration by anticancer agent erucylphosphohomocholine and cyclosporin A. J Biol Chem. 2007;282:37303–37307. doi: 10.1074/jbc.C700134200. [DOI] [PubMed] [Google Scholar]
- Marín-Hernández A, Gracia-Mora I, Ruiz-Ramírez L, Moreno-Sánchez R. Toxic effects of copper-based antineoplastic drugs (Casiopeinas®) on mitochondrial functions. Biochem Pharmacol. 2003;65:1979–1989. doi: 10.1016/s0006-2952(03)00212-0. [DOI] [PubMed] [Google Scholar]
- Pandeya SN, Dimmock JR. An introduction to drug design. New Age International (P) Limited, Publishers; New Delhi: 1997. p. 99. [Google Scholar]
- Statistical Package for Social Sciences. SPSS for Windows, Standard Version, release 14.0. SPSS Inc; 2005. [Google Scholar]