Abstract
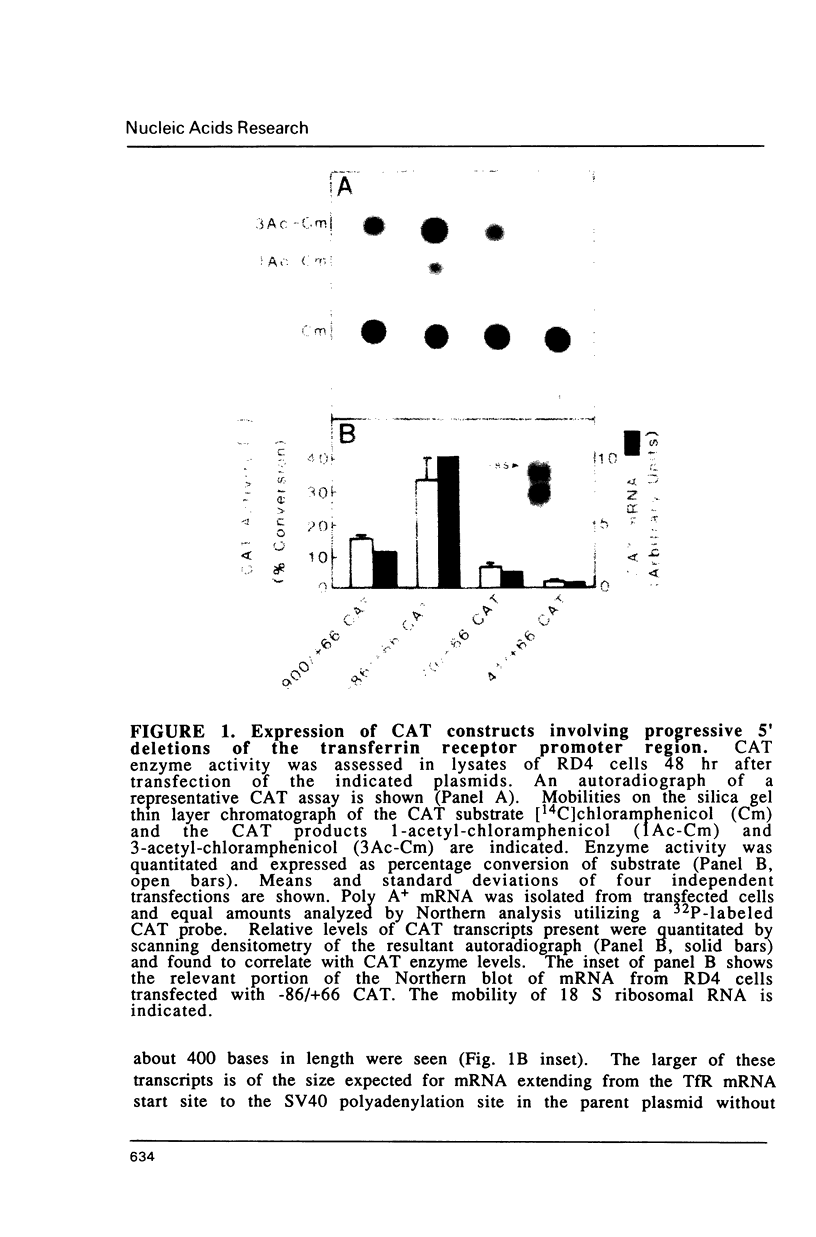
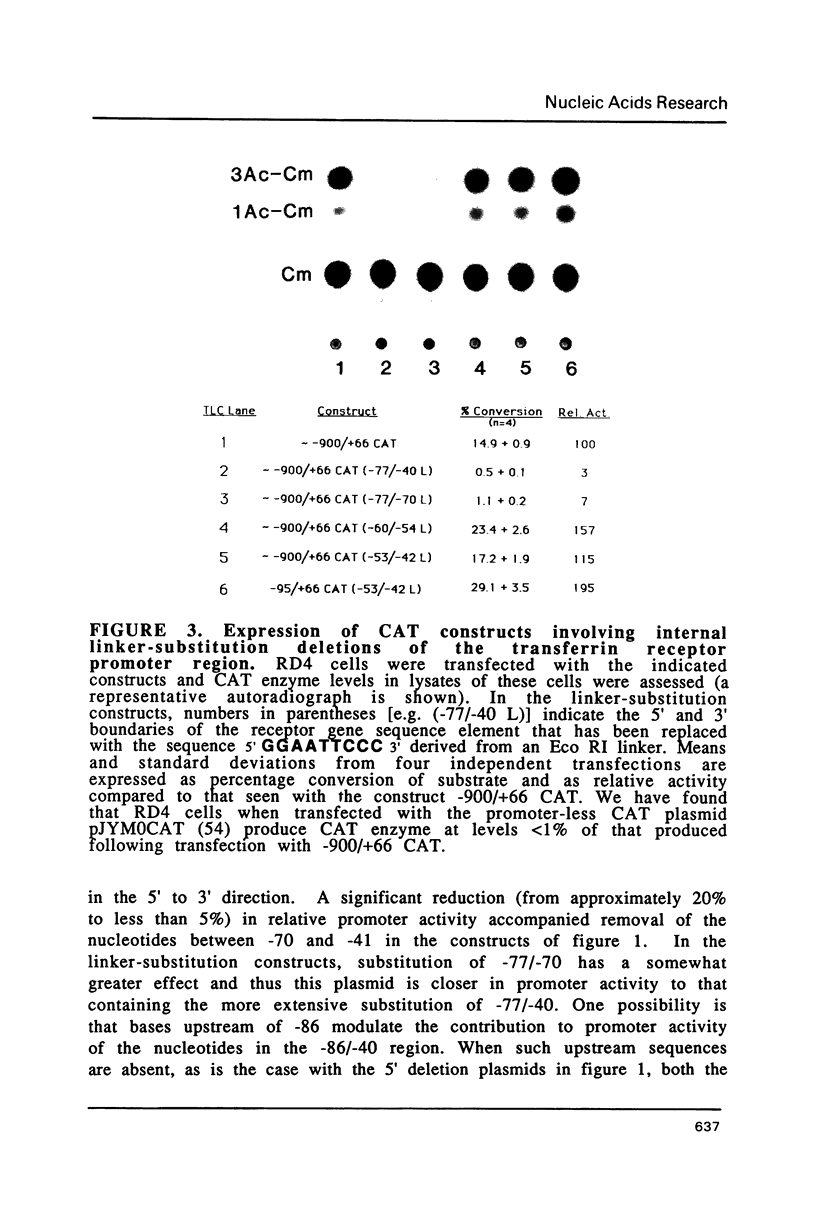
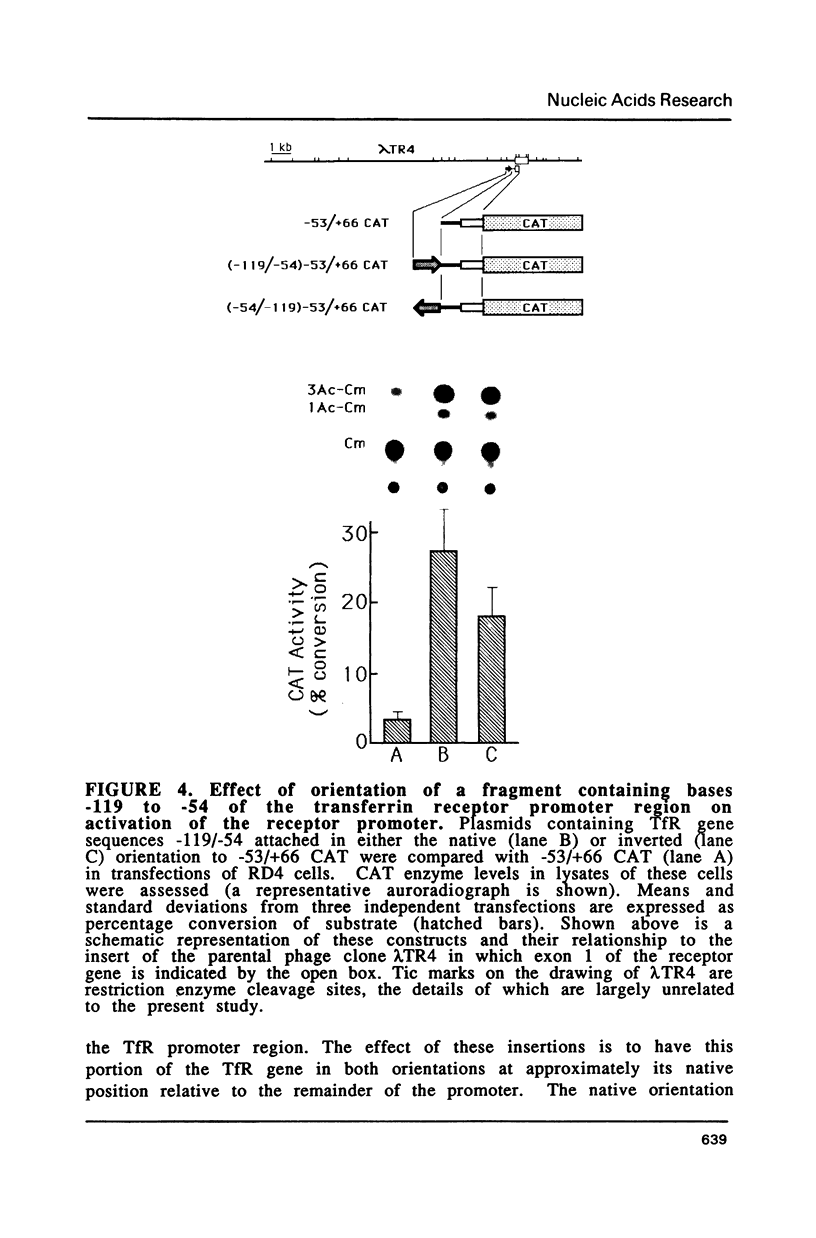
Fragments of human genomic DNA corresponding to the promoter region of the gene for the transferrin receptor have been cloned upstream of the bacterial gene for chloramphenicol acetyltransferase and these constructs used to assess promoter activity following transfection into a human rhabdomyosarcoma cell line. Progressive 5' deletions as well as internal linker-substitution constructs support a critical role in gene expression of a sequence element approximately 70 bp upstream of the mRNA start site. In this region, the receptor gene was found to contain 11bp that are identical to a segment of the enhancers of polyoma virus and adenovirus. A fragment encompassing this element was shown to increase gene expression when the fragment was placed in either orientation upstream of the remainder of the transferrin receptor promoter but the same fragment did not activate an enhancer-less SV40 promoter. Removal from within the receptor promoter of three potential binding sites for the transcription factor Sp1 did not decrease the promoter's activity.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Barnes D., Sato G. Serum-free cell culture: a unifying approach. Cell. 1980 Dec;22(3):649–655. doi: 10.1016/0092-8674(80)90540-1. [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Bretscher M. S. Transferrin receptor and its recycling in HeLa cells. EMBO J. 1982;1(3):351–355. doi: 10.1002/j.1460-2075.1982.tb01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Chitambar C. R., Massey E. J., Seligman P. A. Regulation of transferrin receptor expression on human leukemic cells during proliferation and induction of differentiation. Effects of gallium and dimethylsulfoxide. J Clin Invest. 1983 Oct;72(4):1314–1325. doi: 10.1172/JCI111087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delia D., Greaves M. F., Newman R. A., Sutherland D. R., Minowada J., Kung P., Goldstein G. Modulation of T leukaemic cell phenotype with phorbol ester. Int J Cancer. 1982 Jan 15;29(1):23–31. doi: 10.1002/ijc.2910290106. [DOI] [PubMed] [Google Scholar]
- Galbraith R. M., Galbraith G. M. Expression of transferrin receptors on mitogen-stimulated human peripheral blood lymphocytes: relation to cellular activation and related metabolic events. Immunology. 1981 Dec;44(4):703–710. [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S., Burstein H., Maniatis T. The human beta-interferon gene enhancer is under negative control. Cell. 1986 May 23;45(4):601–610. doi: 10.1016/0092-8674(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford J., Wolkoff A. W., Ashwell G., Klausner R. D. Monensin inhibits intracellular dissociation of asialoglycoproteins from their receptor. J Cell Biol. 1983 Jun;96(6):1824–1828. doi: 10.1083/jcb.96.6.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A enhancer contains two functionally distinct domains: one is specific for E1A and the other modulates all early units in cis. Cell. 1986 Apr 25;45(2):229–236. doi: 10.1016/0092-8674(86)90387-9. [DOI] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A transcriptional control region contains a duplicated enhancer element. Cell. 1983 Jul;33(3):695–703. doi: 10.1016/0092-8674(83)90012-0. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Hu H. Y., Gardner J., Aisen P. Inducibility of transferrin receptors on friend erythroleukemic cells. Science. 1977 Aug 5;197(4303):559–561. doi: 10.1126/science.267327. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Ashwell G., van Renswoude J., Harford J. B., Bridges K. R. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., van Renswoude J., Kempf C., Rao K., Bateman J. L., Robbins A. R. Failure to release iron from transferrin in a Chinese hamster ovary cell mutant pleiotropically defective in endocytosis. J Cell Biol. 1984 Mar;98(3):1098–1101. doi: 10.1083/jcb.98.3.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryszke M. H., Piette J., Yaniv M. Induction of a factor that binds to the polyoma virus A enhancer on differentiation of embryonal carcinoma cells. Nature. 1987 Jul 16;328(6127):254–256. doi: 10.1038/328254a0. [DOI] [PubMed] [Google Scholar]
- Kühn L. C., McClelland A., Ruddle F. H. Gene transfer, expression, and molecular cloning of the human transferrin receptor gene. Cell. 1984 May;37(1):95–103. doi: 10.1016/0092-8674(84)90304-0. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Modulation of cell surface iron transferrin receptors by cellular density and state of activation. J Supramol Struct. 1979;11(4):579–586. doi: 10.1002/jss.400110415. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Mattia E., Rao K., Shapiro D. S., Sussman H. H., Klausner R. D. Biosynthetic regulation of the human transferrin receptor by desferrioxamine in K562 cells. J Biol Chem. 1984 Mar 10;259(5):2689–2692. [PubMed] [Google Scholar]
- McAllister R. M., Melnyk J., Finkelstein J. Z., Adams E. C., Jr, Gardner M. B. Cultivation in vitro of cells derived from a human rhabdomyosarcoma. Cancer. 1969 Sep;24(3):520–526. doi: 10.1002/1097-0142(196909)24:3<520::aid-cncr2820240313>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Melin F., Pinon H., Reiss C., Kress C., Montreau N., Blangy D. Common features of polyomavirus mutants selected on PCC4 embryonal carcinoma cells. EMBO J. 1985 Jul;4(7):1799–1803. doi: 10.1002/j.1460-2075.1985.tb03853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskimins W. K., McClelland A., Roberts M. P., Ruddle F. H. Cell proliferation and expression of the transferrin receptor gene: promoter sequence homologies and protein interactions. J Cell Biol. 1986 Nov;103(5):1781–1788. doi: 10.1083/jcb.103.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L. M., Yenokida G., James S. P. The role of the transferrin receptor in human B lymphocyte activation. J Immunol. 1984 Nov;133(5):2437–2441. [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S., Minowada J. Human cell-surface glycoprotein with unusual properties. Nature. 1980 Aug 28;286(5776):888–891. doi: 10.1038/286888a0. [DOI] [PubMed] [Google Scholar]
- Owen D., Kühn L. C. Noncoding 3' sequences of the transferrin receptor gene are required for mRNA regulation by iron. EMBO J. 1987 May;6(5):1287–1293. doi: 10.1002/j.1460-2075.1987.tb02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B. M. Coated vesicles from human placenta carry ferritin, transferrin, and immunoglobulin G. Proc Natl Acad Sci U S A. 1982 Jan;79(2):451–455. doi: 10.1073/pnas.79.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicci P. G., Tabilio A., Thomopoulos P., Titeux M., Vainchenker W., Rochant H., Testa U. Hemin regulates the expression of transferrin receptors in human hematopoietic cell lines. FEBS Lett. 1982 Aug 23;145(2):350–354. doi: 10.1016/0014-5793(82)80198-1. [DOI] [PubMed] [Google Scholar]
- Piette J., Yaniv M. Two different factors bind to the alpha-domain of the polyoma virus enhancer, one of which also interacts with the SV40 and c-fos enhancers. EMBO J. 1987 May;6(5):1331–1337. doi: 10.1002/j.1460-2075.1987.tb02372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K. K., Shapiro D., Mattia E., Bridges K., Klausner R. Effects of alterations in cellular iron on biosynthesis of the transferrin receptor in K562 cells. Mol Cell Biol. 1985 Apr;5(4):595–600. doi: 10.1128/mcb.5.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K., Harford J. B., Rouault T., McClelland A., Ruddle F. H., Klausner R. D. Transcriptional regulation by iron of the gene for the transferrin receptor. Mol Cell Biol. 1986 Jan;6(1):236–240. doi: 10.1128/mcb.6.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K., van Renswoude J., Kempf C., Klausner R. D. Separation of Fe+3 from transferrin in endocytosis. Role of the acidic endosome. FEBS Lett. 1983 Aug 22;160(1-2):213–216. doi: 10.1016/0014-5793(83)80969-7. [DOI] [PubMed] [Google Scholar]
- Ruley H. E., Fried M. Sequence repeats in a polyoma virus DNA region important for gene expression. J Virol. 1983 Jul;47(1):233–237. doi: 10.1128/jvi.47.1.233-237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., Kurkinen M., Greaves M. Isolation of cDNA clones for the human transferrin receptor. EMBO J. 1983;2(12):2259–2263. doi: 10.1002/j.1460-2075.1983.tb01732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Gruss P. Specific interaction between enhancer-containing molecules and cellular components. Cell. 1984 Feb;36(2):403–411. doi: 10.1016/0092-8674(84)90233-2. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Durand D. B., Bressler P., Holbrook N. J., Norris C. A., Kamoun M., Kant J. A., Crabtree G. R. Promoter region of interleukin-2 gene undergoes chromatin structure changes and confers inducibility on chloramphenicol acetyltransferase gene during activation of T cells. Mol Cell Biol. 1986 Sep;6(9):3042–3049. doi: 10.1128/mcb.6.9.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Newman R. A., Domingo D. L., Sauvage C. Transferrin receptors: structure and function. Biochem Pharmacol. 1984 Mar 15;33(6):925–932. doi: 10.1016/0006-2952(84)90447-7. [DOI] [PubMed] [Google Scholar]
- Wu B. J., Kingston R. E., Morimoto R. I. Human HSP70 promoter contains at least two distinct regulatory domains. Proc Natl Acad Sci U S A. 1986 Feb;83(3):629–633. doi: 10.1073/pnas.83.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke M., Grundström T., Matthes H., Wintzerith M., Schatz C., Wildeman A., Chambon P. Multiple sequence motifs are involved in SV40 enhancer function. EMBO J. 1986 Feb;5(2):387–397. doi: 10.1002/j.1460-2075.1986.tb04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]