Abstract
Campylobacter jejuni commensally colonizes the cecum of birds. The RacR (reduced ability to colonize) response regulator was previously shown to be important in avian colonization. To explore the means by which RacR and its cognate sensor kinase RacS may modulate C. jejuni physiology and colonization, ΔracR and ΔracS mutations were constructed in the invasive, virulent strain 81-176, and extensive phenotypic analyses were undertaken. Both the ΔracR and ΔracS mutants exhibited a ∼100-fold defect in chick colonization despite no (ΔracS) or minimal (ΔracR) growth defects at 42°C, the avian body temperature. Each mutant was defective for colony formation at 44°C and in the presence of 0.8% NaCl, both of which are stresses associated with the heat shock response. Promoter-reporter and real-time quantitative PCR (RT-qPCR) analyses revealed that RacR activates racRS and represses dnaJ. Although disregulation of several other heat shock genes was not observed at 38°C, the ΔracR and ΔracS mutants exhibited diminished upregulation of these genes upon a rapid temperature upshift. Furthermore, the ΔracR and ΔracS mutants displayed increased length heterogeneity during exponential growth, with a high proportion of filamented bacteria. Filamented bacteria had reduced swimming speed and were defective for invasion of Caco-2 epithelial cells. Soft-agar studies also revealed that the loss of racR or racS resulted in whole-population motility defects in viscous medium. These findings reveal new roles for RacRS in C. jejuni physiology, each of which is likely important during colonization of the avian host.
INTRODUCTION
Campylobacter jejuni is a Gram-negative, highly motile, microaerophilic, capnophilic, and modestly thermophilic bacterium. C. jejuni is the leading cause of bacterial diarrheal disease in the developed world, accounting for more infections than Escherichia coli O157:H7, Salmonella spp., and Shigella spp. combined (3, 4, 10). Although the ingestion of contaminated drinking water and milk is a concern, it is believed that 50 to 70% of C. jejuni infections result from the consumption of poultry and related products (3). Acute campylobacteriosis presents as stomach cramps, fever, and severe, often bloody, diarrhea (3, 15). Although gastroenteritis associated with C. jejuni is generally self-limiting, bacteremia and other complications can occur in immunocompromised individuals. Infections can also lead to more serious medical sequelae, including Guillain-Barré syndrome, a demyelinating polyneuropathy causing bilateral paralysis (2). Furthermore, the emergence of antibiotic-resistant C. jejuni isolates has accelerated in recent years (14). Despite its prevalence as a human pathogen, many aspects of C. jejuni physiology are poorly understood.
Although a pathogen in humans, C. jejuni is able to colonize the gastrointestinal tract of birds in an asymptomatic manner. The optimal growth temperature of C. jejuni in the laboratory is 37°C to 42°C, correlating with the body temperatures of humans (37°C) and birds (42°C). Once colonization occurs, the bacteria can quickly spread through a chicken flock (56). The primary site of colonization is the deep crypts of the cecum (6), and in poultry, C. jejuni can propagate to 1010 CFU per gram of intestinal tissue (77). Chemotaxis and motility are required to reach this privileged niche (28, 47). Relative to other enteric Gram-negative pathogens, C. jejuni has a significantly high swimming speed, which increases further with elevated viscosity (22, 55), and it is likely that this adaptation allows the organisms to traverse the viscous mucus layer that covers the intestinal epithelium. Although the number of transcriptional regulatory genes in C. jejuni is limited, owing partly to its small genome size (48), a relatively high proportion of C. jejuni regulatory elements is devoted to the synthesis of its bipolar flagella, motility, and chemotaxis (32, 41). Other factors, such as its short corkscrew morphology, are also predicted to play an important role in motility of C. jejuni (76).
The ability of a bacterium to counter environmental insults is dependent on its genetic repertoire. As bacteria sense environmental changes that challenge their intracellular homeostasis, they must carefully modulate the expression of specific genes and pathways to alleviate stress. The regulation of this can be performed by bacterial two-component systems (59). These systems typically consist of a membrane-anchored sensor kinase (SK) and a cognate response regulator (RR). The canonical SK commonly has both kinase and phosphatase activities on its cognate RR, which translates into a dynamic and continuous response to extracellular stimuli (71). The phosphate on the histidine of the SK is transferred onto an aspartate residue on the RR, which in many cases is a transcription factor. The phosphorylated RR acquires a high affinity for DNA and binds at specific regulatory sequences to activate or repress transcription (58, 59).
The C. jejuni genome is predicted to encode 11 RRs, 6 SKs, and 1 hybrid sensor response regulator protein (48). To date, mutants of genes encoding the RRs, CbrR, CheY, FlgR, RacR, and DccR as well as the sensor kinase CprS have all been shown to have defects in colonization or the ability to cause disease in an animal model (12, 50, 64, 70, 73). Of these, the ΔcheY and ΔflgR mutants were nonmotile, whereas the ΔcprS mutant was modestly hypermotile. RacRS was one of the first two-component systems identified in C. jejuni. RacR was previously shown to be important for avian colonization and growth at 42°C, the body temperature of chickens (12). Proteomic analyses indicated that RacR influences the expression of up to 11 proteins in both temperature-dependent and -independent manners (12). Genome analysis has revealed that the RacRS two-component system is conserved among multiple Campylobacter spp. (24), and racR was detected in 98% of C. jejuni clinical isolates (18). The racRS operon was disregulated under several in vitro conditions similar to the environments encountered by C. jejuni during infection (E. C. Gaynor, unpublished observations). Notably, some of these conditions were independent of the switch between 37°C and 42°C, suggesting that the system may play multiple roles in C. jejuni physiology, colonization, and pathogenesis.
In the present study, we provide evidence that RacR and RacS enable C. jejuni to overcome stresses associated with the heat shock response. We also reveal the importance of this two-component system in maintaining bacterial motility and cell length population homogeneity.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Studies were performed primarily using C. jejuni parent strain 81-176 but also 81116 and 11168, where indicated. C. jejuni-derived strains are listed in Table 1. C. jejuni bacteria were cultured in Mueller-Hinton (MH) broth (Oxoid, Hampshire, England) or on agar plates. C. jejuni growth medium was supplemented with 10 μg ml−1 vancomycin and 5 μg ml−1 trimethoprim (MH-TV). When appropriate, bacterial growth media were supplemented with kanamycin and/or chloramphenicol (Sigma, Oakville, Ontario, Canada) at 50 or 20 μg ml−1, respectively. C. jejuni bacteria were routinely cultured in a 12% CO2–6% O2 atmosphere, using either a Sanyo tri-gas incubator (plates) or an Oxoid CampyGen system (broth cultures). Unless otherwise noted, the standard C. jejuni culture temperature was 38°C. DNA manipulations were performed in an E. coli DH5α background strain, which were routinely cultured at 37°C in Luria-Bertani broth (Sigma) with 100 μg ml−1 ampicillin, 50 μg ml−1 kanamycin, or 30 μg ml−1 chloramphenicol, when appropriate.
Table 1.
C. jejuni strains used in this study
| Strain | Descriptiona | Reference |
|---|---|---|
| 81-176 | 81-176 wild type | 30 |
| DRH461 | 81-176 ΔastA Strr | 29 |
| 11168 | 11168 wild type | 25 |
| 81116 | 81116 wild type | 49 |
| 81-176 ΔracR mutant | 81-176 racR::aphA-3 Knr | This study |
| 11168 ΔracR mutant | 11168 racR::aphA-3 Knr | This study |
| 81116 ΔracR mutant | 81116 racR::aphA-3 Knr | This study |
| 81-176 ΔracS mutant | 81-176 racS::aphA-3 Knr | This study |
| 81-176 ΔracRC mutant | 81-176 racR::aphA-3PracR-racR Knr Cmr | This study |
| 81-176 ΔdnaJ mutant | 81-176 dnaJ::cat Cmr | This study |
| 81-176 ΔracR ΔdnaJ mutant | 81-176 racR::aphA-3dnaJ::cat Knr Cmr | This study |
| 81-176(PdnaJ-lux pRY112) | Wild-type 81-176 with the luxCDABE operon under the transcriptional regulation of the dnaJ promoter in plasmid pRY112; Cmr | This study |
| 81-176(PracR-lux pRY112) | Wild-type 81-176 with the luxCDABE operon under the transcriptional regulation of the racR promoter in plasmid pRY112; Cmr | This study |
| 81-176 ΔracR mutant (PdnaJ-lux pRY112) | 81-176 racR::aphA-3 with the luxCDABE operon under the transcriptional regulation of the dnaJ promoter in plasmid pRY112; Cmr Knr | This study |
| 81-176 ΔracR mutant (PracR-lux pRY112) | 81-176 racR::aphA-3 with the luxCDABE operon under the transcriptional regulation of the racR promoter in plasmid pRY112; Cmr Knr | This study |
| 81-176 ΔracS mutant (PdnaJ-lux pRY112) | 81-176 racS::aphA-3 with the luxCDABE operon under the transcriptional regulation of the dnaJ promoter in plasmid pRY112; Cmr Knr | This study |
| 81-176 ΔracS mutant (PracR-lux pRY112) | 81-176 racS::aphA-3 with the luxCDABE operon under the transcriptional regulation of the racR promoter in plasmid pRY112; Cmr Knr | This study |
| 81-176(PatpF′-gfp pRY112) | Wild-type 81-176 with the GFP ORF under the transcriptional regulation of the atpF′ promoter in plasmid pRY112; Cmr | This study |
| 81-176 ΔracR mutant (PatpF′-gfp pRY112) | 81-176 racR::aphA-3 with the GFP ORF under the transcriptional regulation of the atpF′ promoter in plasmid pRY112; Cmr Knr | This study |
Abbreviations: Cmr, chloramphenicol resistant; Knr, kanamycin resistant; Strr, streptomycin resistant.
Recombinant DNA techniques.
Recombinant DNA manipulation was performed as described previously by Sambrook and Russell (52). DNA-modifying enzymes were purchased from New England BioLabs (Mississauga, Ontario, Canada) and Invitrogen (Burlington, Ontario, Canada). Plasmids were isolated from bacteria by utilizing the Qiagen Qiaprep Spin miniprep kit (Qiagen, Mississauga, Ontario, Canada).
Construction of the ΔracR, ΔracS, ΔdnaJ, ΔracR ΔdnaJ, and ΔracRC strains.
The ΔracR mutant was constructed by the PCR amplification of racR from 81-176 genomic DNA using primers racR-1 and racR-2 and by the cloning of the PCR product into the commercial vector pGEM-T (Promega) (see Table S1 in the supplemental material for primers used in this study). Inverse PCR was then performed by using primers racR-3 and racR-4. The resulting amplicon and plasmid pUC18K-2, carrying a nonpolar kanamycin resistance (aphA-3) cassette (26), were each digested with BamHI and EcoRI restriction enzymes and ligated to form plasmid pGEM-racR-KO. The plasmid was introduced into C. jejuni 81-176, 11168, or 81116 cells by natural transformation. Kanamycin-resistant colonies were isolated, and ΔracR mutants were confirmed via PCR and sequencing analysis.
The ΔracS mutant was constructed in a manner similar to that for ΔracR, except that the initial primers were racS-1 and racS-2 and inverse PCR was performed by using primers racS-3 and racS-4.
The ΔdnaJ mutant was constructed in a manner similar to that for ΔracR, except that the initial primers were dnaJ-1 and dnaJ-2 and inverse PCR was performed by using primers dnaJ-3 and dnaJ-4. The resulting amplicon and plasmid pRY109, carrying a chloramphenicol resistance (cat) cassette (72), were digested with EcoRI and ligated to form plasmid pGEM-dnaJ-KO. The cat cassette was confirmed to be in the same orientation as the dnaJ open reading frame (ORF). The plasmid was introduced into C. jejuni wild-type (wt) strain 81-176 or the 81-176 ΔracR mutant by natural transformation to create the ΔdnaJ and ΔracR ΔdnaJ strains, respectively.
To generate a complemented ΔracR strain (ΔracRC), the racR ORF and 323 bp of upstream DNA were PCR amplified from 81-176 genomic DNA using primers 5′-racR-SpeI and 3′-racR-EcoRI. The amplicon was digested with SpeI and EcoRI and ligated into plasmid pRRC (34), which was digested with MfeI and XbaI to generate compatible DNA overhangs. The E. coli-derived plasmid was introduced by natural transformation into the ΔracR mutant. Chloramphenicol- and kanamycin-resistant colonies were isolated, and the presence of the complementing DNA at an rRNA spacer region as well as the retention of the racR deletion at the native locus were confirmed by PCR.
Physiological studies of the ΔracR, ΔracS, ΔdnaJ, ΔdnaJ ΔracR, and ΔracRC strains.
Growth curves were performed with MH-TV broth from C. jejuni cultures grown overnight and diluted to an initial optical density at 600 nm (OD600) of 0.05, and the OD600 was measured at various time points specific to the experiment. To investigate C. jejuni colony formation at various temperatures, broth cultures were normalized to an OD600 of 0.1, and 10-fold serial dilutions were spot plated onto solid medium and incubated at 38°C, 42°C, 44°C, 46°C, 38°C with 0.8% NaCl, and 42°C with 0.8% NaCl for 48 h, at which time CFU were enumerated.
Chick colonization assays.
The colonization assays were performed essentially as described previously (28). Briefly, day-of-hatch chicks (White Leghorn; Charles River Laboratories) were orally inoculated with ∼104 CFU from a broth culture grown overnight and diluted in phosphate-buffered saline (PBS). After 6 days, birds were sacrificed, and the ceca were removed. Ceca were weighed, homogenized, serially diluted, and plated onto MH agar containing 30 μg ml−1 cefoperazone and 10 μg ml−1 trimethoprim. C. jejuni colonies were enumerated, and the CFU g−1 cecal matter were recorded.
Construction and utilization of a luciferase reporter vector.
To construct a luciferase promoter reporter system in C. jejuni, the luxCDABE operon of Photorhabdus luminescens was PCR amplified from vector pCS26 (9) using primers 5′-SmaI-luxC and 3′-PstI-luxE. The amplicon was digested with SmaI and PstI and subsequently ligated into similarly digested plasmid pRY112 (72). The resulting vector, pRY112-luxCDABE, can be used to swap the transcriptional regulatory sequence upstream of the luxCDABE cassette encoding luciferase biosynthetic enzymes. The racR and dnaJ regulatory sequences were PCR amplified from 81-176 genomic DNA and subcloned upstream of the lux operon to generate PracR-luxCDABE pRY112 and PdnaJ-luxCDABE pRY112 (see Table S2 in the supplemental material). Through triparental mating with E. coli cells, performed as previously described (44), the chloramphenicol resistance plasmids were introduced into C. jejuni strain DRH461, a streptomycin-resistant 81-176 derivative (29). Once luminescent colonies of strain DRH461 were isolated, the plasmids were purified and transformed into 81-176 or the ΔracR and ΔracS mutant derivatives.
Growth curves were performed at 38°C as described above, and measurements of light production were carried out at 0, 3, 6, 9, and 12 h postinoculation, during which 100 μl of culture was removed and optical density and luminescence measurements were performed by using a Varioskan Flash luminometer (Thermo Scientific).
To investigate if RacR directly binds the racR-dnaJ intergenic region, a dnaJ promoter-luciferase fusion was assessed in E. coli cells also expressing recombinant RacR. To construct the expression plasmid, the racR ORF was amplified by using primers 5′-racR-EcoRI and 3′-racR-PstI and ligated into pBAD24 (27) to form pBAD24-racR. Using primers 5′-pBAD-XhoI and 3′-pBAD-ApaI, araC or racR-araC was PCR amplified from pBAD24 or pBAD24-racR, respectively. The amplicons were digested with XhoI and ApaI and ligated into PdnaJ-luxCDABE pRY112 to form PdnaJ-luxCDABE/araC and PdnaJ-luxCDABE/araC/PBAD-racR pRY112. The generated vectors were transformed into E. coli strain BW27783 (37). Luciferase expression experiments were performed by growing an E. coli culture to the early log phase in LB medium supplemented with 30 μg ml−1 chloramphenicol and inducing racR expression by the addition of 0.02% arabinose. Luciferase-dependent light activity was measured 6 h after induction as described above.
Real-time reverse transcription-qPCR (RT-qPCR).
C. jejuni cells were grown to early log phase (OD600 of ∼0.15) at 38°C under microaerophilic conditions and shifted to 44°C for 15 min. A control culture was grown in parallel and remained at 38°C. RNA isolation and cDNA synthesis were carried out as previously described (64). Quantitative PCRs (qPCRs) were performed for dnaJ, dnaK, and groEL by using designed primers (see Table S1 in the supplemental material). The expression of rpoA was used as an internal control, as this gene was previously shown to be invariant during temperature upshifts (57). Reactions were set up with IQ SYBR green Supermix (Bio-Rad) and performed with a CFX96 real-time PCR detection system (Bio-Rad). Expression differences were calculated by using the ΔΔCT method.
Bright-field microscopy.
For bright-field microscopy of log-phase bacteria, C. jejuni cells were harvested from MH-TV broth cultures (OD600 of 0.25 to 0.35) 6 h after inoculation from a culture grown overnight, and samples were immediately spotted onto glass slides overlaid with 1% agarose pads. For the tracking of swimming bacteria, C. jejuni cells were harvested at the same density, diluted 1/10 in prewarmed MH-TV broth, and spotted onto glass microscope slides. For the tracking of swimming speed, 50 individual bacteria were selected from each strain and, in the case of ΔracR cells, represented the observed length variation among the mutant populations. Images were captured with a Nikon Eclipse TE2000-U microscope equipped with 40× and 100× objectives and a Hamamatsu Orca camera system. Micrograph analyses were performed by using NIS-Elements AR imaging software and ImageJ (NIH).
Soft-agar motility assays.
C. jejuni broth cultures were propagated for 12 to 16 h in MH-TV broth. Cultures were diluted to an OD600 of 0.02, and 2.5 μl of the suspension was stab inoculated into plates containing MH-TV medium and 0.4%, 0.5%, or 0.6% agar. When the coinoculation of wt and ΔracR strains was performed, the bacterial culture of each strain was normalized to an OD600 of 0.02, the cultures were mixed at a 1:1 ratio, and 2.5 μl of the culture was stab inoculated into the motility plate. Plates were incubated at 38°C for 28 h, at which time motility was quantified by measuring the diameter of the migration front. When microscopic examinations of cells in the motility plate were performed, a sterile toothpick was used to harvest bacteria from specific locations in the semisolid motility halo, and light microscopy was carried out as described above. To quantify the CFU ratios of wt and ΔracR mutant cells in coinoculated motility plates, bacteria were harvested from the semisolid agar with a sterile toothpick, serially diluted 10-fold, and plated onto MH agar and MH agar with kanamycin.
Construction of GFP-expressing strains, tissue culture infections, and immunofluorescent analysis.
The green fluorescent protein (GFP) ORF was amplified from plasmid pFPV25 (17) by using primers 5′-SmaI-gfp and 3′-XhoI-gfp and subcloned into plasmid pRY112. The transcriptional regulatory sequence of the atpF′ ORF (CJJ81176_0137) was amplified with primers 5′-NotI-atpF′ and 3′-SmaI-atpF′ from strain 81-176 and subcloned upstream of the GFP ORF to generate plasmid PatpF′-gfp pRY112 (see Table S2 in the supplemental material). The plasmid was introduced into C. jejuni 81-176 cells as described above.
Tissue culture infections were performed with Caco-2 intestinal epithelial cells at a C. jejuni multiplicity of infection (MOI) of ∼25 (2.5 × 106 bacteria per well), essentially as described previously (26). Briefly, 105 Caco-2 cells were seeded onto glass coverslips in 24-well plates and grown overnight. Log-phase cultures grown overnight of C. jejuni harboring PatpF′-gfp were washed twice with minimal essential medium (MEM) and resuspended at an OD600 of 0.0005. One milliliter of bacterial suspension was added per well, and Caco-2 and C. jejuni cells were incubated for 3 h. Samples were washed twice in MEM, and cell-associated bacteria were fixed in a 4% formalin solution in PBS.
Samples were permeabilized for 15 min in PBS with 0.2% Triton X-100 when appropriate. Immunostaining was performed by treating the fixed samples with rabbit anti-Campylobacter antibodies (1:200; U.S. Biological) and Alexa 568 (red)-conjugated goat anti-rabbit secondary antibody (1:500; Molecular Probes). Samples were mounted onto glass slides with ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen). Fluorescent images were captured with an Olympus FluoView FV1000 confocal laser scanning microscope (Olympus) equipped with appropriate fluorescent filters. The experiment was performed three times for each strain, during which five individual fields containing 25 to 50 bacteria per image were acquired.
Statistical analysis.
Data obtained from microscopic tracking and halo diameters in motility assays and luciferase expression experiments were analyzed by using the Student two-tailed t test. Statistical analysis of the data from the chick infection experiment was performed by using the two-sided Mann-Whitney U test. P values are indicated when appropriate.
RESULTS
C. jejuni strain 81-176 ΔracR and ΔracS mutants are defective in chick colonization but display only modest (ΔracR) to no (ΔracS) growth defects at 42°C.
The ΔracR mutant of strain 81116 was reported previously to have a lower in vitro growth rate at 42°C and to be significantly defective in chick colonization (12). We wanted to assess the role of racR and racS in in vitro growth as well as chick colonization in the highly invasive C. jejuni strain 81-176. Neither the ΔracR nor ΔracS mutant was defective for growth at 38°C (Fig. 1A). At the avian body temperature of 42°C, the ΔracR mutant reached a modestly lower final optical density in broth culture than did the wild type (wt), although the growth rate in early stages was unaffected (Fig. 1B). The ΔracS mutant exhibited no defects in either the growth rate or the final optical density at 42°C.
Fig 1.
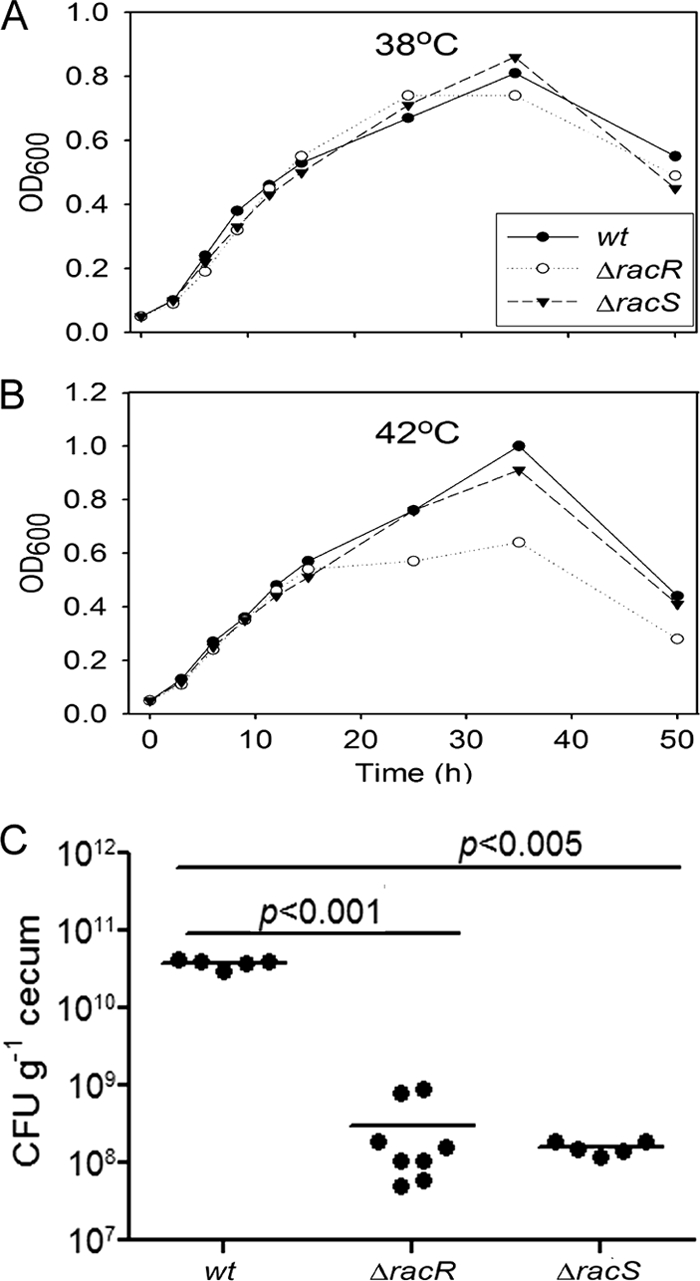
In vitro growth curves of wt and mutant strains at 38°C and 42°C and colonization of day-of-hatch White Leghorn chicks. The growth patterns of 81-176 wt, ΔracR, and ΔracS strains at 38°C (A) and 42°C (B) are shown. Values shown are representative of three independent experiments. OD600, optical density at 600 nm. (C) Chicks were orally inoculated with 104 CFU of C. jejuni resuspended in PBS. At 6 days postinfection, the birds were sacrificed. The bacterial load in the cecum was quantified and is represented as CFU g−1 cecum. Each dot represents the bacterial load in one chicken, and the horizontal lines represent the means.
To establish a requirement for RacRS in avian colonization for strain 81-176, we tested the ΔracR and ΔracS mutants in a 1-day-old chick colonization model. A total of 104 CFU were orally administered to day-of-hatch White Leghorn chicks, and cecal colonization was enumerated at 6 days postinfection. Both the ΔracR and ΔracS mutants were recovered at approximately 100-fold fewer CFU g−1 of cecal tissue than the wt strain 81-176 (Fig. 1C). The ΔracR colonization data are consistent with findings reported previously by Bras et al. demonstrating the importance of racR for chicken colonization (12). These data also identify a role for racS in colonization despite having no apparent growth defect in vitro at 42°C and suggest that RacRS likely influences physiological properties other than the ability to grow at 42°C that are also important for colonization.
RacR activates racRS and represses dnaJ.
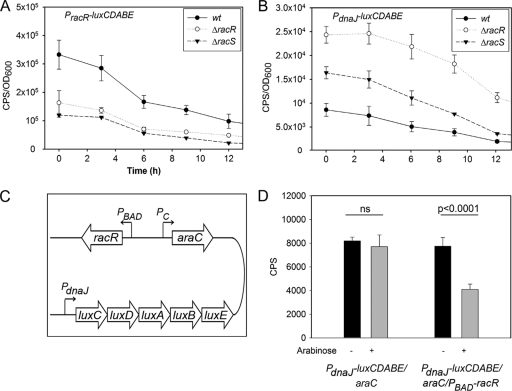
To identify potential factors contributing to the diminished in vivo fitness of the ΔracR and ΔracS mutants, we first investigated whether the expression of racRS and/or dnaJ was disregulated in the mutant strains. We tested racRS because transcription factors often regulate their own expression, and dnaJ, encoding a heat shock chaperone previously shown to be important for chick colonization (39), because it is divergently transcribed from racRS, with 157 bp separating the two operons. For studies of C. jejuni, we utilized a luciferase promoter-reporter fusion which enables the measurement of gene expression in real time at different points of the bacterial growth curve (1, 35). Promoter-luciferase fusions (PracR-luxCDABE and PdnaJ-luxCDABE) were constructed in the C. jejuni replicating vector pRY112 and introduced into the C. jejuni wt, ΔracR, and ΔracS strains as described in Materials and Methods. The PracR-luxCDABE reporter had a lower level of expression in both mutants at 38°C than in the wt parent strain (Fig. 2A). Conversely, the PdnaJ-luxCDABE fusion was expressed at a level approximately 3.3-fold higher at 38°C in the ΔracR mutant than in the wt, with the ΔracS mutant exhibiting an intermediate level of expression (Fig. 2B). Similar trends were observed at 42°C, with analysis of dnaJ expression by RT-qPCR also showing a ∼2-fold increase in levels in the ΔracR strain compared to the wt (data not shown). Several other heat shock gene operons (groESL, grpE-dnaK, and clpB) and hspR (encoding a repressor of those operons) were assessed by promoter-luciferase fusions and RT-qPCR analyses, but no expression differences in the ΔracR strain compared to the wt at 38°C were observed (data not shown).
Fig 2.
Measurements of light production of promoter-luciferase fusions in C. jejuni and E. coli. The racR and dnaJ transcriptional regulatory sequences were subcloned in front of the luxCDABE operon in pRY112, and the vector was transformed into wt C. jejuni and the ΔracR and ΔracS mutants. Measurements were made for the first 12 h of bacterial growth. Light counts per second (CPS) were normalized to the OD600 at the time of measurement. (A and B) Data for PracR-luxCDABE (A) and PdnaJ-luxCDABE (B) in wt C. jejuni and the ΔracR and ΔracS mutants are shown. Data are averages of data from 4 independent cultures, and standard deviations are denoted by error bars. (C) Map of the pRY112-derived vector utilized to investigate RacR-dependent transcriptional regulation in E. coli. The luciferase operon was fused to PdnaJ in a plasmid with racR under the transcriptional regulation of the arabinose-inducible PBAD promoter. The transcriptional expression of araC was under regulation of the constitutive promoter (Pc). (D) Light produced by E. coli cultures carrying thePdnaJ-luxCDABE/araC or PdnaJ-luxCDABE/araC/PBAD-racR vector in the presence (+) or absence (−) of arabinose. Error bars denote standard deviations. ns, not significant.
We next investigated whether RacR directly binds the intergenic region between racRS and dnaJ. Electrophoretic mobility shift assays (EMSAs) were performed by using the racRS-dnaJ intergenic DNA sequence and recombinant RacR purified from E. coli. However, we were unable to establish specific binding using this strategy, potentially due to a lack of or unstable RacR phosphorylation, which has hindered EMSA approaches in other systems (59). As an alternative, we developed a synthetic system to study C. jejuni transcriptional regulation in E. coli. It was previously found that E. coli RNA polymerase can recognize C. jejuni promoters and initiate the transcription of downstream genes (69). As repression frequently involves direct regulator-DNA binding whereas activation can be more complicated (i.e., requiring additional variables such as regulator interactions with RNA polymerase), we used negative regulation of dnaJ as a readout for the direct interaction of RacR with the racRS-dnaJ intergenic region. araC and racR were cloned under the control of constitutive (Pc) and arabinose-inducible (PBAD) promoters, respectively, into plasmid pRY112, described above, harboring the PdnaJ-luxCDABE luciferase-reporter fusion (Fig. 2C). The addition of arabinose to E. coli harboring this plasmid will induce racR expression and RacR protein production, allowing the determination of whether the transcription of the exogenous promoter-luciferase fusion is directly regulated by RacR. A control plasmid harboring all components except PBAD-racR was also constructed to ensure that any significant differences seen with or without arabinose were due to the presence or absence of RacR. Upon arabinose induction, the amount of light produced by E. coli containing PdnaJ-luxCDABE/araC/PBAD-racR decreased approximately 2-fold compared to that produced by uninduced samples (P < 0.0001) (Fig. 2D). E. coli harboring the control plasmid (PdnaJ-luxCDABE/araC) did not exhibit statistically significant changes in light production following arabinose induction. This finding suggests that RacR interacts with the DNA region between the dnaJ and racRS operons to modulate their expression.
The ΔracR and ΔracS mutants are sensitive to elevated temperatures and hyperosmotic stress.
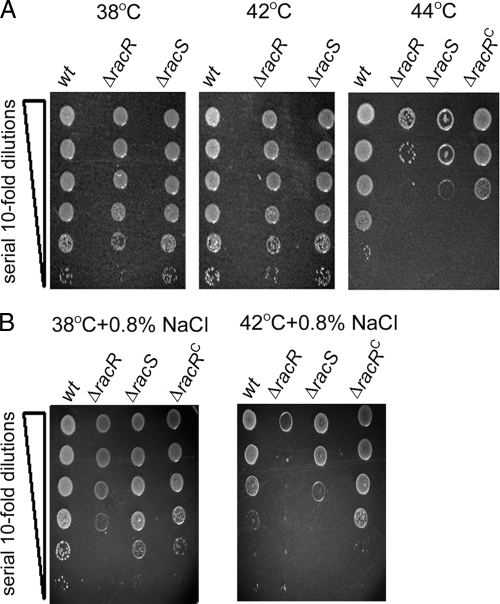
Heat shock proteins (HSPs) have an important function in allowing organisms to overcome physiologically stressful conditions such as elevated temperatures and osmotic stress. As RacR regulates dnaJ expression, we first investigated survival and colony formation by the ΔracR and ΔracS mutants at above-optimal temperatures. Serial dilutions of log-phase C. jejuni wt strain 81-176 and mutant strains were inoculated onto solid medium and incubated at 38°C, 42°C, 44°C, and 46°C. While the ΔracR and ΔracS strains did not yield defects in colony formation at 38°C or 42°C, there was a significant defect in colony formation for both mutants when propagated at 44°C (Fig. 3A). The complementation of racR in trans (ΔracRC) partially rescued this defect. At 46°C, all strains, including the wt, failed to form colonies after 48 h (data not shown).
Fig 3.
Survival and colony formation of C. jejuni exposed to elevated growth temperatures or osmotic stress. The 81-176 wt, mutant (ΔracR and ΔracS), and complemented (ΔracRC) strains were diluted to an OD600 of 0.1 and spot plated onto solid medium at a serial dilution ranging from 100 to 10−5 (denoted by the vertical wedge). Images were captured after 48 h of incubation at the indicated temperatures on unsupplemented MH plates (A) or on MH plates supplemented with 0.8% NaCl at the indicated temperatures (B) and are representative of three independent experiments.
Osmotolerance is also influenced by HSPs in numerous bacterial species (7), and C. jejuni is likely to encounter hyperosmotic conditions during the gastrointestinal colonization of vertebrate hosts (23, 38). On plates supplemented with 0.8% NaCl, the ΔracR strain was modestly defective for growth and colony formation at 38°C and severely defective at 42°C, with the ΔracS strain exhibiting a milder phenotype (Fig. 3B). Complementation of racR restored osmotolerance to wt levels. These findings indicate that RacRS provides resistance during temperature and osmotic stresses and identify an additive detrimental effect on ΔracR and ΔracS mutant survival when the stressors are present simultaneously.
dnaJ disregulation is not the sole factor contributing to the temperature sensitivity of the ΔracR and ΔracS mutants, with other heat shock genes also exhibiting dampened induction upon temperature upshift.
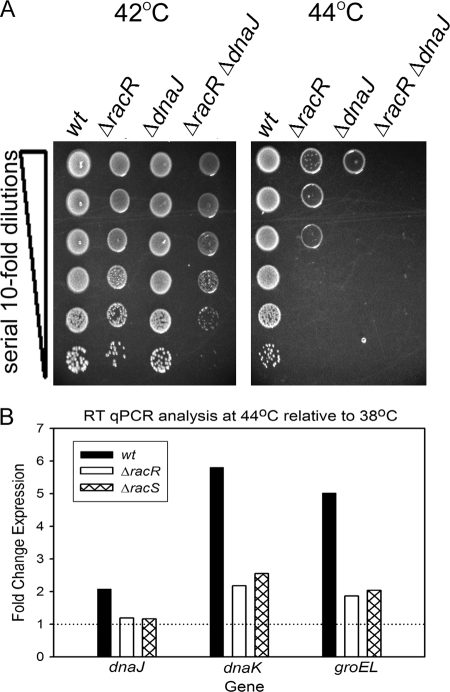
dnaJ is not essential for C. jejuni under nonstressful laboratory conditions but has a role in temperature resistance (39). We next tested if the temperature sensitivity of the ΔracR mutant was caused by a disregulation of dnaJ. As no system currently exists for the inducible expression/repression of genes in C. jejuni, we generated a ΔracR ΔdnaJ double mutant to investigate whether disregulation of dnaJ might account for the observed ΔracR defects. Neither the ΔdnaJ mutant nor a ΔdnaJ ΔracR double mutant was defective in colony formation at 42°C, although the ΔdnaJ ΔracR mutant formed smaller and fewer colonies than the wt or either of the single mutants. At 44°C, the ΔdnaJ mutant had a substantial defect in colony formation, which was more severe than that of the ΔracR mutant, and the ΔdnaJ ΔracR double mutant was unable to form colonies after 48 h of incubation (Fig. 4A). These data indicate that dnaJ disregulation is not the sole factor contributing to the temperature sensitivity of the ΔracR strain.
Fig 4.
Survival and colony formation of C. jejuni at elevated growth temperatures and RT-qPCR analysis of C. jejuni exposed to a temperature increase from 38°C to 44°C. (A) wt strain 81-176 and mutant strains (ΔracR, ΔdnaJ, and ΔdnaJ ΔracR) were diluted to an OD600 of 0.1 and spot plated onto solid medium at a serial dilution ranging from 100 to 10−5 (denoted by the vertical wedge). Images were captured after incubation at the indicated temperatures and are representative of three independent experiments. (B) wt C. jejuni 81-176 and mutant strains (ΔracR and ΔracS) were grown to the early log phase in MH broth at 38°C and shifted to 44°C for 15 min. The expression of dnaJ, dnaK, and groEL was analyzed by RT-qPCR. Bars represent the expression ratios from strains shifted to 44°C relative to those that remained at 38°C, with rpoA used as an internal control. The horizontal line indicates no difference in expression levels between the experimental conditions. Data are representative of three independent experiments.
C. jejuni exhibits a transient increase in expression of multiple HSPs upon temperature upshift (57). To expand our understanding of potential mechanisms underlying the temperature sensitivity of the ΔracR and ΔracS mutants, we tested whether this response might be generally defective in our mutant strains. C. jejuni cells were grown to early log phase at 38°C and then shifted to 44°C for 15 min or allowed to remain at 38°C. Levels of expression of dnaJ, dnaK, and groEL at each temperature were quantified by RT-qPCR. Data are presented as the fold induction of each gene at 44°C relative to that at 38°C in the wt, ΔracR, and ΔracS strains. As expected, all three heat shock genes were induced in the wt following a temperature upshift (Fig. 4B). In the ΔracR and ΔracS mutants, induction at 44°C was negligible for dnaJ (Fig. 4B), as might be predicted given RacR's likely direct effect on dnaJ expression (Fig. 2). However, induction at 44°C was also significantly dampened for dnaK and groEL (Fig. 4B), neither of which was affected in the ΔracR strain at 38°C (data not shown). For instance, while the dnaK expression level increased 5.2-fold in wt C. jejuni, the increases were only 2- and 2.5-fold in the ΔracR and ΔracS mutants, respectively. A similar trend was observed for groEL. Thus, in addition to directly regulating dnaJ, RacRS is also required for the proper upregulation of other HSP genes under heat shock conditions (Fig. 4B).
The ΔracR and ΔracS mutants have increased cell length heterogeneity resulting from filamented bacteria in the population.
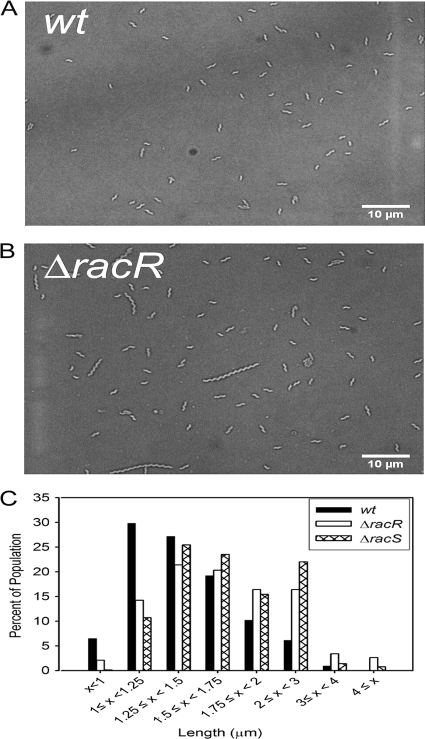
HSP disregulation has been linked to an altered bacterial length in C. jejuni and other bacterial species (5, 11, 43, 63). To determine if loss of racR or racS affects cell length, we quantified the length of individual bacteria in the population using light micrographs and imaging software (Fig. 5 and Table 2). While the wt population was composed primarily of short cells, the ΔracR mutant exhibited an increased mean cell length relative to that of the wt, with the population being distributed broadly over multiple lengths. For instance, ∼63% of the wt population was less than 1.5 μm in length, while only ∼38% of the ΔracR mutant population was less than 1.5 μm (Fig. 5C and Table 2). The degree of cell length heterogeneity within the wt, ΔracR, and ΔracS populations was quantified by calculating the coefficient of variation (Cv), which is a ratio of the standard deviation to the mean and, in our case, provides a normalized measure of the length distribution in a population when strains with different mean cell lengths are compared. The Cv was two times higher for the ΔracR mutant than for the wt, further supporting cell length heterogeneity of the ΔracR mutant strain (Table 2). The ΔracRC strain exhibited cell length and population distribution characteristics similar to those of the wt (Table 2), while the ΔracS mutant exhibited phenotypes intermediate to those of the wt and ΔracR strains (Fig. 5C and Table 2).
Fig 5.
Analysis and quantification of C. jejuni cell length by light microscopy of bacterial populations. Shown are representative images of wt strain 81-176 (A) and the ΔracR strain (B) and the distribution of the length of individual cells of the wt and the ΔracR and ΔracS mutants cultured at 38°C (C). Over 2,000 individual bacteria of each strain from two individual experiments were examined.
Table 2.
Bacterial length characteristics of C. jejuni wt and ΔracR and ΔracS mutant strains propagated at 38°C
| Strain | No. of bacteria | Mean length (μm) | SD | Cva | % of population <1.50 μmb |
|---|---|---|---|---|---|
| wt | 2,121 | 1.44 | 0.39 | 0.27 | 63.47 |
| ΔracR mutant | 2,228 | 1.84 | 0.99 | 0.54 | 37.7 |
| ΔracS mutant | 2,065 | 1.74 | 0.53 | 0.31 | 36.26 |
| ΔracRC mutant | 2,024 | 1.48 | 0.37 | 0.25 | 62.4 |
The coefficient of variation (Cv) is the ratio of the standard deviation to the mean and provides a normalized measure of the length distribution in the population when strains with different mean cell lengths are compared.
Refers to the percentage of the isogenic bacterial population that is less than 1.50 μm.
Filamentation of ΔracR cells causes motility and epithelial cell invasion defects.
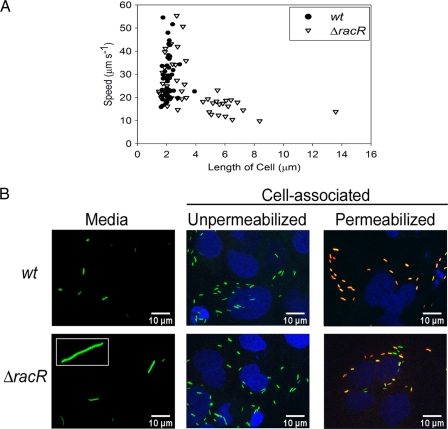
We next determined whether the filamentation of ΔracR cells affected other attributes linked to C. jejuni colonization and pathogenesis. A previous report suggested that RacR was not required for C. jejuni motility (12); however, we hypothesized that elongated bacteria may exhibit motility defects because of their increased size. Time-lapse microscopy was used to assess the swimming speed of individual C. jejuni bacteria in broth cultures as a function of cell length in the wt and ΔracR populations (Fig. 6A). To normalize for Brownian motion, ethanol-treated bacteria were imaged and tracked to provide a baseline for subsequent analyses of swimming speed. In live cultures, only bacteria that were moving at a speed greater than 4 times that of ethanol-treated cultures were analyzed. The mean swimming speeds of wt and ΔracR C. jejuni bacteria were 28 ± 10 μm s−1 and 24 ± 10 μm s−1, respectively. Shorter ΔracR cells (<3.5 μm) had an average swimming rate of 29 ± 10 μm s−1; however, ΔracR cells that were >3.5 μm in length had an average swimming speed of 15.0 ± 3.5 μm s−1 (P < 0.0001). In addition, short (<3.5 μm) ΔracR cells were capable of reaching speeds up to 55 μm s−1, similar to the wt, while ΔracR cells >3.5 μm in length did not exceed a speed of 25 μm s−1. The only tracked wt cell >3.5 μm in length (3.9 μm) likewise swam at 22.6 μm s−1. Filamented cells also changed direction more slowly than did shorter cells (data not shown). Transmission electron microscopy indicated that the position and length of flagella were unaltered in the ΔracR and ΔracS mutants as well as among individual bacteria of different lengths in the ΔracR and ΔracS populations (data not shown). Single-cell tracking also demonstrated that the elongated cells were viable, as their swimming speed was more than 4 times higher than that of ethanol-treated bacteria in all but one examined individual bacterium (data not shown). Collectively, these findings demonstrate that while shorter cells in both the wt and ΔracR mutant populations swam at a variety of speeds and at similar average rates in broth medium, elongated ΔracR cells swam significantly more slowly.
Fig 6.
Investigation of the impact of C. jejuni cell length on swimming speed and invasion of Caco-2 epithelial cells. (A) The swimming speed of 50 individual wt and ΔracR cells was examined by tracking swimming C. jejuni cells through MH broth by time-lapse microscopy. (B) wt and ΔracR strains expressing GFP were used to infect Caco-2 cells. The bacterium shown in the inset of the ΔracR mutant “Media” panel is ∼30 μm long. To differentiate between extracellular and intracellular bacteria in the cell-associated fractions, those samples were exposed to an anti-C. jejuni antibody either without permeabilization (only extracellular bacteria will be antibody accessible) or following permeabilization with Triton X-100 (extracellular and intracellular bacteria will be antibody accessible) and visualized by using a secondary antibody conjugated to Alexa 568, which appears red. Depending on the relative ratio of GFP expression to antibody reactivity, antibody-accessible bacteria will appear red to yellow-orange. Caco-2 cell nuclei were stained with DAPI and are depicted in blue.
The ability of C. jejuni to invade epithelial cells in vitro is frequently used as a marker for virulence (16, 21). Using a gentamicin protection assay and enumerations of CFU recovery, we did not observe a general defect for adherence to or invasion of Caco-2 (epithelial) tissue culture cells for the ΔracR or ΔracS mutant (data not shown). However, we were intrigued by the possibility that elongated bacteria may exhibit a defect in the adherence to and/or invasion of epithelial cells. To visualize cell-associated bacteria, we utilized a combination of GFP-expressing C. jejuni cells and immunofluorescence. Caco-2 cells were infected with C. jejuni for 3 h, at which time bacteria in the medium above the cells were removed, cells and cell-associated bacteria were washed, and all samples were fixed and processed for confocal microscopy. Within the medium fraction, both wt and ΔracR C. jejuni bacteria were generally more elongated than when the same strains were propagated in MH broth. However, ΔracR mutant bacteria in the medium fraction were highly variable in length, with some individuals being substantially longer than bacteria of the wt strain and measuring up to 30 μm in length (a representative example is shown in Fig. 6B). The cell-associated bacteria were more homogeneous in length for both the wt and the ΔracR mutant and were always short (<3.5 μm) (Fig. 6B). Consistent with CFU recovery-based experiments, there was no striking difference between the wt and ΔracR strains with respect to the total number of cell-associated bacteria. The application of a C. jejuni antibody to unpermeabilized versus permeabilized cells also suggested that cell-associated bacteria of both strains were predominantly intracellular, as the red antibody signal seldom colocalized with the green GFP-expressing bacteria when epithelial membranes remained intact but readily colocalized when Caco-2 cell membranes were permeabilized prior to staining. Together, these results suggest an advantage for short bacteria in the invasion of epithelial cells and demonstrate that elongated individuals in the ΔracR population are defective for cell association.
The ΔracR and ΔracS mutant populations are defective in motility through semisolid agar.
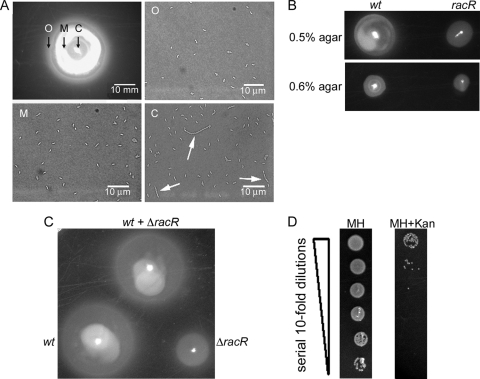
The single-cell analyses showing motility defects for filamented bacteria led us to revisit whether RacRS might also be important for general motility in semisolid agar medium, a substance of higher viscosity than liquid broth. Strains were inoculated into MH plates with 0.4% agar and grown at 38°C or 42°C, and halo diameters were documented and quantified. Differences in halo diameters between the mutant and wt strains were similar at both temperatures; thus, only data for 38°C are shown (Fig. 7). Both ΔracR and ΔracS mutants were impaired for motility in soft agar, exhibiting halos 50% and 58% of the diameter of those of wt cells respectively (Fig. 7A and B). As these findings are inconsistent with a previous report describing no motility defect for a C. jejuni 81116 ΔracR mutant through semisolid medium or by microscopy, and as discrepancies between C. jejuni strains are frequently observed (19, 25), we constructed ΔracR mutants from two other commonly utilized C. jejuni strains, 11168 and 81116, and assessed motility in soft agar relative to that of the wt. Although there were significant differences between the halo diameters of the three wt isolates, the ΔracR mutants had similar motility defects irrespective of the parent strain (Fig. 7C). These findings demonstrate a role for RacRS in motility through soft agar in at least three C. jejuni strains.
Fig 7.
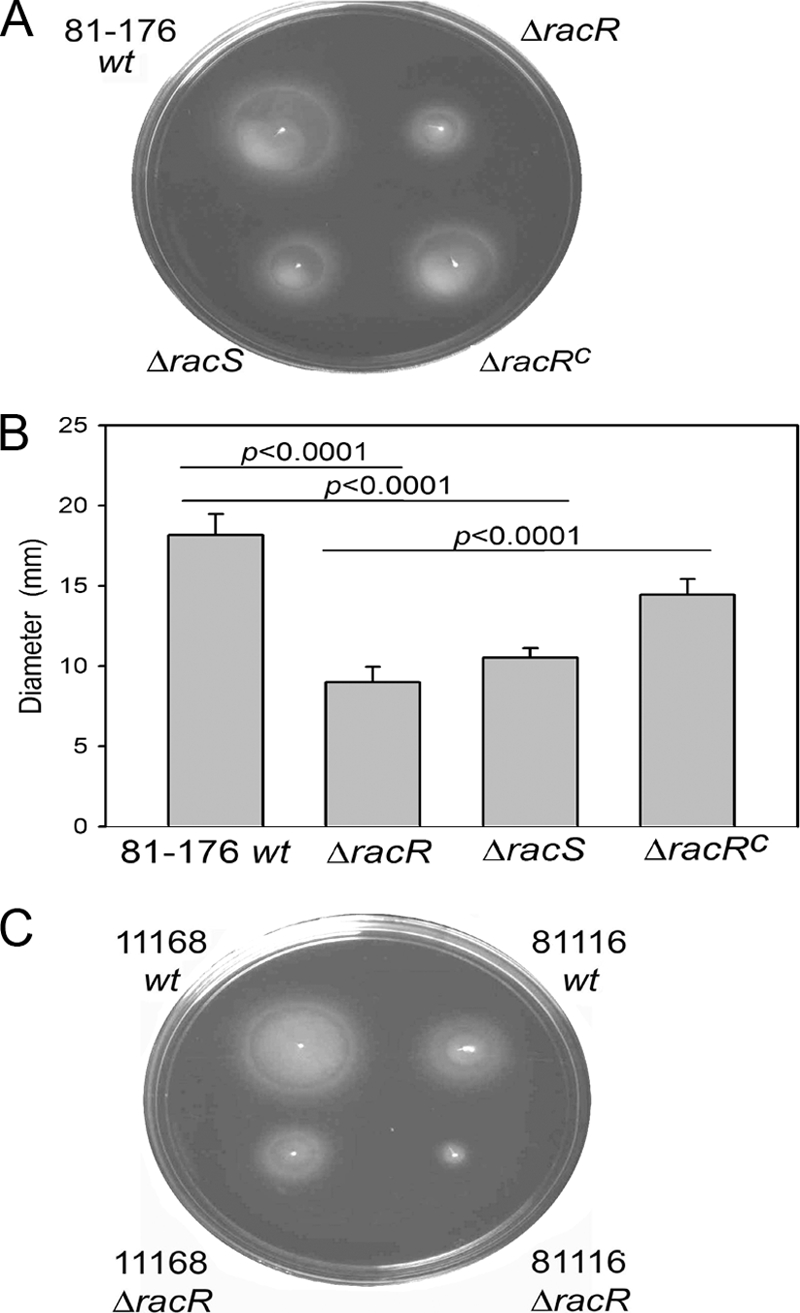
Motility analysis of wt C. jejuni and the ΔracR and ΔracS mutants. (A and B) Representative images of semisolid motility plates incubated for 28 h at 38°C with the 81-176 wt, ΔracR, ΔracS, and ΔracRC strains (A) and quantification of halo diameters (B). Values are averages of data from at least six independent experiments, and the standard deviations are represented by error bars. (C) Representative images of motility plates of wt and ΔracR mutant strains of C. jejuni 11168 and 81116.
Short bacteria in the ΔracR mutant population are defective in motility through semisolid agar.
Consistent with the broth motility data described above (Fig. 6A), we also found that filamented ΔracR bacteria were more defective for migration through semisolid agar than short ΔracR bacteria, as assessed by a microscopic examination of ΔracR cells taken from the center (C), middle (M), and outer (O) zones of a motility halo (Fig. 8A). However, we still needed to reconcile why the general motility of three independent ΔracR mutant strains consistently appeared to be defective compared to that of the parent strain in soft agar despite no apparent motility differences between short bacteria of the wt and ΔracR strains in broth medium (Fig. 6A). Several hypotheses were considered. First, to test whether the soft-agar motility defect might reflect a decreased growth rate of the ΔracR strain in soft agar, the wt and ΔracR strains were inoculated into semisolid medium with a range of agar concentrations (0.4% to 0.6%). As the largest halo diameter in this range occurs in 0.4% agar, halo diameters in higher-concentration agar should become less dependent on swimming speed and more reflective of growth. Increasing the agar concentration reduced the relative halo size difference between wt and ΔracR mutant cells (Fig. 8B), suggesting that the ΔracR bacteria are not defective for growth in semisolid media. We also investigated if elongated bacteria in the ΔracR mutant population might be impeding the outward migration of shorter cells. To test this, we coinoculated wt and ΔracR mutant bacteria into a motility plate alongside singly inoculated wt and ΔracR strains. If elongated bacteria in the ΔracR mutant population impeded the outward migration of shorter cells, the motility halo of the culture coinoculated with the wt and ΔracR strains should appear smaller than that of the wt alone. The motility halo of the culture coinoculated with the wt and ΔracR strains was similar to that of the wt inoculated alone (Fig. 8C); thus, blocking of short cells by elongated bacteria was unlikely. These findings led us finally to hypothesize that short cells in the ΔracR mutant population have reduced motility through semisolid agar despite having no obvious swimming defect in liquid broth. To test this, we sampled bacteria from the migration front of a motility plate which had been coinoculated with the wt and ΔracR strains and enumerated the CFU on MH agar (allows the growth of both strains) or MH agar supplemented with kanamycin (selective for the ΔracR mutant). The numbers of recovered CFU of the wt outnumbered those of the ΔracR strain by a factor of 103 to 105 (Fig. 8D), suggesting that wt bacteria were able to swim faster than the short ΔracR mutant cells. Together, these data indicate that while filamented bacteria swim more slowly than short bacteria, the short ΔracR bacteria and, indeed, the entire ΔracR population are defective in motility through semisolid media.
Fig 8.
Population-level analysis of C. jejuni swimming speed through semisolid medium. (A) The ΔracR mutant bacteria were sampled from either the outer (O), middle (M), or center (C) locations of the migration front of semisolid motility agar. Elongated bacteria were localized at or near the zone of inoculation at the center of the motility halo and are indicated by white arrows. (B) Migration of wt and ΔracR strains through 0.5% and 0.6% agar. (C) wt and ΔracR strains were coinoculated at a 1:1 ratio (wt + ΔracR) in a 0.4% agar motility plate, and the halo diameter was compared to that of either strain inoculated alone. (D) Bacteria from the migration front of a wt- and ΔracR-coinoculated culture were harvested and spot plated onto solid medium with a serial dilution ranging from 100 to 10−5 (denoted by the vertical wedge) on MH medium or MH medium supplemented with kanamycin. In all cases, a representative image is shown.
DISCUSSION
C. jejuni is a significant burden to human health. In developed countries, where infection is believed to be primarily the result of the consumption of contaminated poultry products, our understanding of bacterial colonization of chickens is paramount to the implementation of control strategies. Within poultry, C. jejuni is found at high numbers in the cecum and large intestine (6, 28) and localized within the deep crypts in the mucus layer in close proximity to epithelial cells (40). To reach its preferred niche, C. jejuni encounters stresses (e.g., osmotic, oxidative, acid, etc.) that pose survival challenges. The bacteria must also be able to migrate (swim) to an area of the gastrointestinal tract more suitable for growth and replication. C. jejuni likely utilizes highly evolved strategies to overcome these potential stressors, reach its preferred niche, and successfully colonize the avian host.
C. jejuni has a relatively limited number of two-component systems (48). Although genes encoding the response regulators CbrR, CheY, FlgR, RacR, and DccR and the sensor kinase CprS are each important for animal colonization (12, 50, 64, 70, 73), the unique phenotypic profile of each mutant indicates a lack of functional redundancy between individual systems. In this work, we have also established the importance of racS in colonization and found that both ΔracR and ΔracS mutants of strain 81-176 exhibit a ∼100-fold defect in colonizing 1-day-old chicks. Our ΔracR chick data are consistent with the ΔracR colonization defect noted in a previous study (12); however, some discrepancies were observed regarding the fitness of the ΔracR mutant under nonstressful laboratory conditions. These results are puzzling but may stem from the fact that the previous experiments were performed in an 81116 strain background, whereas this study primarily utilized the highly invasive strain 81-176. Nonetheless, it is now evident that C. jejuni requires not only RacR but also RacS for optimal chick colonization.
Given the modest growth defects (ΔracR mutant) or lack of growth defects (ΔracS mutant) at 42°C, we reasoned that another factor(s) important for colonization may be influenced by RacRS. Our observation of severe growth defects for both mutants at a true temperature stress of 44°C suggested a potential defect in the induction of the heat shock response. This response is modulated by HSPs, which are of two general classes: molecular chaperones that facilitate protein folding and ATP-dependent proteases that degrade misfolded polypeptides (20, 42, 67). HSPs are especially important during sudden exposure to stressful conditions, where they act posttranslationally to prevent protein misfolding, denaturation, and aggregation (61, 63, 67). The DnaK/DnaJ/GrpE complex plays a role in cell division, chromosome segregation, and resistance to extracellular stressors (13, 61, 62). In C. jejuni, dnaJ is monocistronic, while dnaK and grpE are cotranscribed. A C. jejuni ΔdnaJ mutant was unable to colonize chickens and was severely defective in colony formation at elevated temperatures (39). As dnaJ is also divergently transcribed from racRS, we hypothesized that RacR may directly regulate both its own operon and dnaJ and that dnaJ disregulation might account for the temperature sensitivity of the ΔracR and ΔracS mutants. Using RT-qPCR, promoter-lux reporters in C. jejuni, and a new synthetic luciferase reporter system in E. coli, we established that RacR activates racRS and represses dnaJ. However, analysis of a ΔracR ΔdnaJ double mutant indicated that dnaJ disregulation may contribute to but is not exclusively responsible for the temperature sensitivity of a ΔracR mutant at 44°C, suggesting a role for other factors as well. Unlike dnaJ, other HSPs were not significantly disregulated in the ΔracR mutant at 38°C and did not appear to be under direct RacR regulation. However, the upregulation of other chaperone genes (dnaK and groEL) was dampened in the ΔracR and ΔracS mutants upon a temperature upshift, suggesting a complex influence of RacRS on the heat shock response. The effect of RacRS on the heat shock response may also contribute to the ΔracR growth defect in MH medium supplemented with 0.8% NaCl. Hyperosmotic conditions also cause accumulation of misfolded proteins, and in other bacteria, the heat shock response is required for survival. Of note, MH medium with added 0.8% NaCl more closely approximates the osmolarity of the chicken cecum than unsupplemented MH medium (23, 38). Collectively, the stress survival attributes to which RacRS contributes and its pleiotropic effects on the heat shock response all likely contribute to the fitness defects of the ΔracR and ΔracS mutants in vivo.
Bacterial cell length is influenced by the timing of cell division, specifically the regulation and activity of the bacterial septation apparatus (74, 75). The disregulation of heat shock gene expression can affect this process, leading to an increase in cell length (11, 43, 60). For instance, C. jejuni mutants deleted for HspR, a transcriptional repressor of multiple heat shock genes, have increased cell lengths and elevated temperature sensitivity similar to those observed for the ΔracR and ΔracS mutants (5). We initially considered that filamentation in the ΔracR and ΔracS mutants may be the result of dnaJ overexpression; however, a ΔracR ΔdnaJ mutant displayed the same population heterogeneity as the ΔracR mutant (data not shown). It is possible that very minor disregulation of other HSPs, undetectable by our analysis methods, may occur at 38°C and contribute to filamentation. As filamentation can also be caused by numerous other factors (i.e., DNA damage and nutritional limitation, etc.), this may be a highly complex phenomenon influenced by multiple other genes. Reasons underlying the variability in cell length associated with the disruption of RacRS are also likely to be complex. A contributing factor may be the degree of synchronization of the bacterial culture. C. jejuni does not have the classic stationary phase typically found in many other eubacteria (36); thus, even carefully propagated and seemingly exponentially growing cultures may actually be composed of a diverse population of individuals in distinct growth phases. Our data suggest that RacRS may be important for ensuring the proper timing of septation kinetics within a physiologically variable population. Confocal microscopic examination using several DNA and peptidoglycan stains did not provide enough resolution to determine if filaments contained multiple genome copies or lacked complete division septa (not shown). Future work investigating DNA replication and septation apparatus formation should yield additional insights into this aspect of RacRS function.
Using single-cell analyses, we demonstrated that filamented ΔracR bacteria were more defective than their shorter counterparts for two key pathogenic attributes: motility and host cell association. Motility and chemotaxis defects are associated with decreased colonization in multiple animal models (28, 33, 46, 66, 73). Chemotaxis is important for the affinity of C. jejuni for the lower intestinal tract, where attractants such as carbon sources and electron donors and acceptors are abundant (31, 68). Motility may also be required to overcome the peristaltic action of the gastrointestinal tract, preventing the rapid shedding of intestinal bacteria before successful colonization is established (28). In all organisms, body (or cell) size sets an energetic limit for movement (53), and alterations in size without a parallel differentiation of the movement apparatus will have a dramatic effect on movement energetics. For instance, theoretical calculations have intimated that a 0.1-μm difference in bacterial diameter results in a 105-fold change in the energetic cost of chemotaxis (45). In the ΔracR and ΔracS mutants, flagellar structures appeared to be unaffected, with all of the bacteria displaying two polar flagella, which presumably generate the same motive force irrespective of bacterial size. Elongated bacteria were the slowest-swimming cells in broth cultures, were unable to migrate from the zone of inoculation in semisolid agar, and were also impaired in their ability to change direction. The elongated subset was also defective for invasion of tissue culture cells, an established in vitro marker for virulence. Several reasons for this can be envisaged. Increased swimming speed was previously correlated with an elevated ability of C. jejuni to invade Caco-2 epithelial cells (65). While the exact mechanism remains to be elucidated, Szymanski et al. postulated that this may be the result of increases in impact during contact between the rapidly swimming bacteria and the host cell surface (65). It is also possible that the invasion of elongated bacteria is physically impeded simply by virtue of their larger size, with increased eukaryotic cell membrane remodeling being required to take up larger organisms, potentially biasing toward the invasion of primarily shorter cells.
Given our microscopy data and previously reported findings (12), we were surprised that the 81-176 ΔracR mutant produced halo diameters approximately half the size of those of the wt in semisolid agar. It was important to establish whether this defect was strain dependent and also whether this reflected the swimming behavior of the entire population or was an indirect effect, such as the decreased growth rate in viscous media, or an effect of elongated bacteria “blocking” the outward migration of shorter cells. We first established that this defect was not specific to the growth temperature or strain 81-176, as ΔracR mutants of strains 11168 and 81116 exhibited similar defects relative to the parent strain at both 38°C and 42°C, and a comparison of halo diameters with various agar concentrations suggested motility rather than growth defects. Coinoculation experiments also showed that elongated bacteria in the ΔracR mutant population did not inhibit the outward migration of motile wt (short) organisms in semisolid motility agar. Quantification established that wt individuals have a clear advantage in swimming speed over ΔracR bacteria. It is of note that in several bacterial species, altered levels of heat shock proteins can lead to a motility defect (5, 51, 54). Hypotheses to explain these findings have been proposed but await experimental validation. In support of the idea that chaperones influence motility, we observed that a ΔdnaJ mutant exhibited a modest decrease in motility at 38°C relative to that of the parent strain in semisolid agar (data not shown). Also of note, 3 of the 11 proteins previously identified as being disregulated in the ΔracR mutant were sequenced and found to be RacR and two isoforms of a cytochrome c peroxidase (12). A different group previously showed that two periplasmic C. jejuni cytochrome c peroxidases are important for chicken colonization, but no in vitro roles have been identified (8). Future studies will investigate if the disregulation of dnaJ or other genes in the RacR regulon has an impact on motility and other aspects important for colonization.
In our study, the ΔracR but not the ΔracS mutant had a modest growth defect at 42°C; however, similar chicken colonization defects in the absence of either racR or racS suggested that physiological defects other than the ability to grow at 42°C are associated with these mutants. Our findings highlight roles for the RacRS two-component system in overcoming stresses associated with the heat shock response (i.e., elevated temperatures, osmolarity, and the ability to modulate heat shock gene expression), influencing bacterial length, and maintaining motility. We hypothesize that a significant proportion of ΔracR and ΔracS mutant populations that are orally administered to chickens are unable to traverse the viscous mucus covering the intestinal epithelium and are consequently both more subject to peristaltic expulsion and exposed for a longer period to stressors within the intestinal/cecal lumen, which they are likewise compromised for surviving. In sum, this work shows the importance of RacRS in previously unidentified physiological aspects that likely collectively contribute to C. jejuni fitness in vivo.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. E. Davies for providing plasmid pCS26, S. J. Hallam for access to the Varioskan Flash luminometer, C. M. Szymanski for providing C. jejuni strain 81116, and P. St-Pierre and I. R. Nabi for expert technical advice for confocal microscopy.
D.A. is a recipient of Frederick Banting and Charles Best graduate scholarships from the Canadian Institute of Health Research (CIHR). J.E. was supported by USDA fellowship number 2010-65201-20594. E.C.G. is supported by a Canada Research Chair award. This work was funded by a Burroughs Wellcome career development award and CIHR operating grant MOP-68981 to E.C.G. and by NIAID grant AI069383 to V.J.D.
Footnotes
Published ahead of print 17 February 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Allen KJ, Griffiths MW. 2001. Effect of environmental and chemotactic stimuli on the activity of the Campylobacter jejuni flaA sigma(28) promoter. FEMS Microbiol. Lett. 205:43–48 [DOI] [PubMed] [Google Scholar]
- 2. Allos BM. 1998. Campylobacter jejuni infection as a cause of the Guillain-Barre syndrome. Infect. Dis. Clin. North. Am. 12:173–184 [DOI] [PubMed] [Google Scholar]
- 3. Allos BM. 2001. Campylobacter jejuni infections: update on emerging issues and trends. Clin. Infect. Dis. 32:1201–1206 [DOI] [PubMed] [Google Scholar]
- 4. Altekruse SF, Stern NJ, Fields PI, Swerdlow DL. 1999. Campylobacter jejuni—an emerging foodborne pathogen. Emerg. Infect. Dis. 5:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andersen MT, et al. 2005. Diverse roles for HspR in Campylobacter jejuni revealed by the proteome, transcriptome and phenotypic characterization of an hspR mutant. Microbiology 151:905–915 [DOI] [PubMed] [Google Scholar]
- 6. Beery JT, Hugdahl MB, Doyle MP. 1988. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl. Environ. Microbiol. 54:2365–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bianchi AA, Baneyx F. 1999. Hyperosmotic shack induces the sigma(32) and sigma(E) stress regulons of Escherichia coli. Mol. Microbiol. 34:1029–1038 [DOI] [PubMed] [Google Scholar]
- 8. Bingham-Ramos LK, Hendrixson DR. 2008. Characterization of two putative cytochrome c peroxidases of Campylobacter jejuni involved in promoting commensal colonization of poultry. Infect. Immun. 76:1105–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bjarnason J, Southward CM, Surette MG. 2003. Genomic profiling of iron-responsive genes in Salmonella enterica serovar Typhimurium by high-throughput screening of a random promoter library. J. Bacteriol. 185:4973–4982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blaser MJ. 1997. Epidemiologic and clinical features of Campylobacter jejuni infections. J. Infect. Dis. 176(Suppl 2):S103–S105 [DOI] [PubMed] [Google Scholar]
- 11. Blum P, Ory J, Bauernfeind J, Krska J. 1992. Physiological consequences of DnaK and DnaJ overproduction in Escherichia coli. J. Bacteriol. 174:7436–7444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bras AM, Chatterjee S, Wren BW, Newell DG, Ketley JM. 1999. A novel Campylobacter jejuni two-component regulatory system important for temperature-dependent growth and colonization. J. Bacteriol. 181:3298–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brehmer D, Gassler C, Rist W, Mayer MP, Bukau B. 2004. Influence of GrpE on DnaK-substrate interactions. J. Biol. Chem. 279:27957–27964 [DOI] [PubMed] [Google Scholar]
- 14. Butzler JP. 2004. Campylobacter, from obscurity to celebrity. Clin. Microbiol. Infect. 10:868–876 [DOI] [PubMed] [Google Scholar]
- 15. Butzler JP, Skirrow MB. 1979. Campylobacter enteritis. Clin. Gastroenterol. 8:737–765 [PubMed] [Google Scholar]
- 16. Carvalho AC, et al. 2001. Molecular characterization of invasive and noninvasive Campylobacter jejuni and Campylobacter coli isolates. J. Clin. Microbiol. 39:1353–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cormack BP, Valdivia RH, Falkow S. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33–38 [DOI] [PubMed] [Google Scholar]
- 18. Datta S, Niwa H, Itoh K. 2003. Prevalence of 11 pathogenic genes of Campylobacter jejuni by PCR in strains isolated from humans, poultry meat and broiler and bovine faeces. J. Med. Microbiol. 52:345–348 [DOI] [PubMed] [Google Scholar]
- 19. Dorrell N, et al. 2001. Whole genome comparison of Campylobacter jejuni human isolates using a low-cost microarray reveals extensive genetic diversity. Genome Res. 11:1706–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dougan DA, Mogk A, Bukau B. 2002. Protein folding and degradation in bacteria: to degrade or not to degrade? That is the question. Cell. Mol. Life Sci. 59:1607–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Everest PH, et al. 1992. Differentiated Caco-2 cells as a model for enteric invasion by Campylobacter jejuni and C. coli. J. Med. Microbiol. 37:319–325 [DOI] [PubMed] [Google Scholar]
- 22. Ferrero RL, Lee A. 1988. Motility of Campylobacter jejuni in a viscous environment: comparison with conventional rod-shaped bacteria. J. Gen. Microbiol. 134:53–59 [DOI] [PubMed] [Google Scholar]
- 23. Fordtran JS, Locklear TW. 1966. Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am. J. Dig. Dis. 11:503–521 [DOI] [PubMed] [Google Scholar]
- 24. Fouts DE, et al. 2005. Major structural differences and novel potential virulence mechanisms from the genomes of multiple Campylobacter species. PLoS Biol. 3:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gaynor EC, et al. 2004. The genome-sequenced variant of Campylobacter jejuni NCTC 11168 and the original clonal clinical isolate differ markedly in colonization, gene expression, and virulence-associated phenotypes. J. Bacteriol. 186:503–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gaynor EC, Wells DH, MacKichan JK, Falkow S. 2005. The Campylobacter jejuni stringent response controls specific stress survival and virulence-associated phenotypes. Mol. Microbiol. 56:8–27 [DOI] [PubMed] [Google Scholar]
- 27. Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hendrixson DR, DiRita VJ. 2004. Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol. Microbiol. 52:471–484 [DOI] [PubMed] [Google Scholar]
- 29. Hendrixson DR, DiRita VJ. 2003. Transcription of sigma54-dependent but not sigma28-dependent flagellar genes in Campylobacter jejuni is associated with formation of the flagellar secretory apparatus. Mol. Microbiol. 50:687–702 [DOI] [PubMed] [Google Scholar]
- 30. Hofreuter D, et al. 2006. Unique features of a highly pathogenic Campylobacter jejuni strain. Infect. Immun. 74:4694–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hugdahl MB, Beery JT, Doyle MP. 1988. Chemotactic behavior of Campylobacter jejuni. Infect. Immun. 56:1560–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jagannathan A, Constantinidou C, Penn CW. 2001. Roles of rpoN, fliA, and flgR in expression of flagella in Campylobacter jejuni. J. Bacteriol. 183:2937–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jones MA, et al. 2004. Adaptation of Campylobacter jejuni NCTC11168 to high-level colonization of the avian gastrointestinal tract. Infect. Immun. 72:3769–3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karlyshev AV, Wren BW. 2005. Development and application of an insertional system for gene delivery and expression in Campylobacter jejuni. Appl. Environ. Microbiol. 71:4004–4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kassem II, et al. 2010. Use of bioluminescence imaging to monitor Campylobacter survival in chicken litter. J. Appl. Microbiol. 109:1988–1997 [DOI] [PubMed] [Google Scholar]
- 36. Kelly AF, Park SF, Bovill R, Mackey BM. 2001. Survival of Campylobacter jejuni during stationary phase: evidence for the absence of a phenotypic stationary-phase response. Appl. Environ. Microbiol. 67:2248–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khlebnikov A, Datsenko KA, Skaug T, Wanner BL, Keasling JD. 2001. Homogeneous expression of the P(BAD) promoter in Escherichia coli by constitutive expression of the low-affinity high-capacity AraE transporter. Microbiology 147:3241–3247 [DOI] [PubMed] [Google Scholar]
- 38. Klasing KC, Adler KL, Remus JC, Calvert CC. 2002. Dietary betaine increases intraepithelial lymphocytes in the duodenum of coccidia-infected chicks and increases functional properties of phagocytes. J. Nutr. 132:2274–2282 [DOI] [PubMed] [Google Scholar]
- 39. Konkel ME, Kim BJ, Klena JD, Young CR, Ziprin R. 1998. Characterization of the thermal stress response of Campylobacter jejuni. Infect. Immun. 66:3666–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee A, O'Rourke JL, Barrington PJ, Trust TJ. 1986. Mucus colonization as a determinant of pathogenicity in intestinal infection by Campylobacter jejuni: a mouse cecal model. Infect. Immun. 51:536–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lertsethtakarn P, Ottemann KM, Hendrixson DR. 2011. Motility and chemotaxis in Campylobacter and Helicobacter. Annu. Rev. Microbiol. 65:389–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lund PA. 2001. Microbial molecular chaperones. Adv. Microb. Physiol. 44:93–140 [DOI] [PubMed] [Google Scholar]
- 43. McCarty JS, Walker GC. 1994. DnaK mutants defective in ATPase activity are defective in negative regulation of the heat shock response: expression of mutant DnaK proteins results in filamentation. J. Bacteriol. 176:764–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miller WG, et al. 2000. Detection on surfaces and in Caco-2 cells of Campylobacter jejuni cells transformed with new gfp, yfp, and cfp marker plasmids. Appl. Environ. Microbiol. 66:5426–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mitchell JG. 2002. The energetics and scaling of search strategies in bacteria. Am. Nat. 160:727–740 [DOI] [PubMed] [Google Scholar]
- 46. Nachamkin I, Yang XH, Stern NJ. 1993. Role of Campylobacter jejuni flagella as colonization factors for three-day-old chicks: analysis with flagellar mutants. Appl. Environ. Microbiol. 59:1269–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ottemann KM, Miller JF. 1997. Roles for motility in bacterial-host interactions. Mol. Microbiol. 24:1109–1117 [DOI] [PubMed] [Google Scholar]
- 48. Parkhill J, et al. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665–668 [DOI] [PubMed] [Google Scholar]
- 49. Pearson BM, et al. 2007. The complete genome sequence of Campylobacter jejuni strain 81116 (NCTC11828). J. Bacteriol. 189:8402–8403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Raphael BH, et al. 2005. The Campylobacter jejuni response regulator, CbrR, modulates sodium deoxycholate resistance and chicken colonization. J. Bacteriol. 187:3662–3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roncarati D, Danielli A, Spohn G, Delany I, Scarlato V. 2007. Transcriptional regulation of stress response and motility functions in Helicobacter pylori is mediated by HspR and HrcA. J. Bacteriol. 189:7234–7243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 53. Schmidt-Nielsen K. 1972. Locomotion: energy cost of swimming, flying, and running. Science 177:222–228 [DOI] [PubMed] [Google Scholar]
- 54. Shi W, Zhou Y, Wild J, Adler J, Gross CA. 1992. DnaK, DnaJ, and GrpE are required for flagellum synthesis in Escherichia coli. J. Bacteriol. 174:6256–6263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shigematsu M, Umeda A, Fujimoto S, Amako K. 1998. Spirochaete-like swimming mode of Campylobacter jejuni in a viscous environment. J. Med. Microbiol. 47:521–526 [DOI] [PubMed] [Google Scholar]
- 56. Stern NJ, Cox NA, Musgrove MT, Park CM. 2001. Incidence and levels of Campylobacter in broilers after exposure to an inoculated seeder bird. J. Appl. Poult. Res. 10:315–318 [Google Scholar]
- 57. Stintzi A. 2003. Gene expression profile of Campylobacter jejuni in response to growth temperature variation. J. Bacteriol. 185:2009–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stock AM, Robinson VL, Goudreau PN. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183–215 [DOI] [PubMed] [Google Scholar]
- 59. Stock JB, Ninfa AJ, Stock AM. 1989. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol. Rev. 53:450–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sugimoto S, Saruwatari K, Higashi C, Sonomoto K. 2008. The proper ratio of GrpE to DnaK is important for protein quality control by the DnaK-DnaJ-GrpE chaperone system and for cell division. Microbiology 154:1876–1885 [DOI] [PubMed] [Google Scholar]
- 61. Suh WC, et al. 1998. Interaction of the Hsp70 molecular chaperone, DnaK, with its cochaperone DnaJ. Proc. Natl. Acad. Sci. U. S. A. 95:15223–15228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Suh WC, Lu CZ, Gross CA. 1999. Structural features required for the interaction of the Hsp70 molecular chaperone DnaK with its cochaperone DnaJ. J. Biol. Chem. 274:30534–30539 [DOI] [PubMed] [Google Scholar]
- 63. Susin MF, Baldini RL, Gueiros-Filho F, Gomes SL. 2006. GroES/GroEL and DnaK/DnaJ have distinct roles in stress responses and during cell cycle progression in Caulobacter crescentus. J. Bacteriol. 188:8044–8053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Svensson SL, et al. 2009. The CprS sensor kinase of the zoonotic pathogen Campylobacter jejuni influences biofilm formation and is required for optimal chick colonization. Mol. Microbiol. 71:253–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Szymanski CM, King M, Haardt M, Armstrong GD. 1995. Campylobacter jejuni motility and invasion of Caco-2 cells. Infect. Immun. 63:4295–4300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Takata T, Fujimoto S, Amako K. 1992. Isolation of nonchemotactic mutants of Campylobacter jejuni and their colonization of the mouse intestinal tract. Infect. Immun. 60:3596–3600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Teixeira-Gomes AP, Cloeckaert A, Zygmunt MS. 2000. Characterization of heat, oxidative, and acid stress responses in Brucella melitensis. Infect. Immun. 68:2954–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vegge CS, Brondsted L, Li YP, Bang DD, Ingmer H. 2009. Energy taxis drives Campylobacter jejuni toward the most favorable conditions for growth. Appl. Environ. Microbiol. 75:5308–5314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wosten MM, Boeve M, Koot MG, van Nuenen AC, van der Zeijst BA. 1998. Identification of Campylobacter jejuni promoter sequences. J. Bacteriol. 180:594–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wosten MM, Wagenaar JA, van Putten JP. 2004. The FlgS/FlgR two-component signal transduction system regulates the fla regulon in Campylobacter jejuni. J. Biol. Chem. 279:16214–16222 [DOI] [PubMed] [Google Scholar]
- 71. Yamamoto K, et al. 2005. Functional characterization in vitro of all two-component signal transduction systems from Escherichia coli. J. Biol. Chem. 280:1448–1456 [DOI] [PubMed] [Google Scholar]
- 72. Yao R, Alm RA, Trust TJ, Guerry P. 1993. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene 130:127–130 [DOI] [PubMed] [Google Scholar]
- 73. Yao R, Burr DH, Guerry P. 1997. CheY-mediated modulation of Campylobacter jejuni virulence. Mol. Microbiol. 23:1021–1031 [DOI] [PubMed] [Google Scholar]
- 74. Young KD. 2007. Bacterial morphology: why have different shapes? Curr. Opin. Microbiol. 10:596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Young KD. 2010. Bacterial shape: two-dimensional questions and possibilities. Annu. Rev. Microbiol. 64:223–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Young KD. 2006. The selective value of bacterial shape. Microbiol. Mol. Biol. Rev. 70:660–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Young KT, Davis LM, DiRita VJ. 2007. Campylobacter jejuni: molecular biology and pathogenesis. Nat. Rev. Microbiol. 5:665–679 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.