Abstract
This work reports the design and evaluation of a rapid loop-mediated isothermal amplification test for detecting Mycobacterium ulcerans DNA based on the multicopy insertion sequence IS2404. The test is robust and specific with a detection limit equivalent to 20 copies of the target sequence (0.01 to 0.1 genome). The test has potential for the diagnosis of Buruli ulcer under field conditions.
TEXT
Buruli ulcer (BU) is a destructive disease of the skin and subcutis caused by the toxin-producing human pathogen Mycobacterium ulcerans. Infection often leads to extensive destruction of skin and soft tissue with associated long-term functional disability. The disease has been reported in tropical and subtropical regions of Africa, the Americas, Asia, and the western Pacific. In poor regions of endemicity of sub-Saharan Africa, the burden of disease is greatest in children under 15 years (2), and in some regions, the disease is more common than tuberculosis and leprosy (4). The precise mode of BU transmission remains unclear; however, research suggests that mosquitoes and some aquatic insects may be either reservoirs or vectors (8, 11). Moreover, activities such as farming near rivers (12), swimming in rivers/marshes (1), and dam construction (10) have been associated with increased risk of BU. The occurrence of the disease in poor rural communities coupled with the lack of simple definitive diagnostic tests has largely limited the collection of accurate epidemiological data.
The incidence of M. ulcerans infection has been increasing while antibiotic treatment regimens and vaccine strategies are still under investigation. The control of BU partly depends on accurate detection of the bacteria in lesions, followed by appropriate treatment of the patient. This has become increasingly important with clear evidence of the benefit of antibiotic treatment (16). The common confirmatory methods include direct detection of acid-fast bacilli (AFB) by smear microscopy, culture of M. ulcerans, and histopathology (21). However, these methods have limitations in that they (i) are not readily available in areas of endemicity, (ii) have low sensitivities, and (iii) have long turnaround times in the case of culture. To improve methods of confirmation of M. ulcerans, several PCR formats, all targeting the multicopy insertion sequence IS2404, have been developed and evaluated, i.e., classical PCR (18), dry reagent-based (DRB) PCR (19), and TaqMan real-time PCR (7). Despite these advances in PCR technologies, early detection of BU remains unsatisfactory in areas of endemicity due to limited facilities and expertise at health care centers; therefore, laboratory diagnosis is usually performed retrospectively at major research centers. Since the best treatment outcome is achieved when the disease is diagnosed early, a test that permits pretreatment diagnosis in primary health care settings would be of benefit.
In recent years, a rapid and sensitive amplification platform for DNA called loop-mediated isothermal amplification (LAMP) has been developed (15). The LAMP technology amplifies DNA with high sensitivity under isothermal conditions by relying on an enzyme with strand displacement activity. Also, the technology uses 4 to 6 specially designed primers recognizing 6 to 8 regions of the target DNA sequence and hence has a high specificity. The autocycling reactions lead to accumulation of large amounts of the target DNA and other reaction by-products such as magnesium pyrophosphate which allow rapid detection using varied formats (14). The technique has been used widely in laboratory settings to detect pathogens of medical and veterinary importance and plant parasitic diseases (6). The platform is robust and specific and shows tolerance to several biological products that inhibit conventional PCR, meaning that extraction of highly purified DNA templates may not be necessary. LAMP tests are rapid (∼1 h) with sensitivity higher than or equal to that of PCR targeting the same gene. Amplification can be achieved using a simple incubator such as a water bath or heating block. In addition, the results can be visually inspected through a color change or use of a chromatographic lateral flow dipstick format (17). LAMP has proved a powerful tool in detection of parasite DNA and is cheaper than other DNA-based tests (20). In this current study, we report the development of a rapid and sensitive LAMP test for M. ulcerans targeting the repetitive insertion sequence element IS2404.
The institutional ethical clearance for the collection of human samples in Ghana was approved by the Noguchi Memorial Institute of Medical Research (NMIMR) Institutional Review Board. In Australia, DNA extracts from human samples submitted for routine PCR diagnosis of BU and stored for reference purposes were deidentified prior to LAMP testing. DNA extracted from the M. ulcerans reference strain Agy99 was used in the development of the BU LAMP test. A total of 136 and 79 DNA extracts from Australia and Ghana, respectively, which had been prepared from swabs, fresh tissue biopsy specimens, and formalin-fixed, paraffin-embedded tissue and previously analyzed with TaqMan real-time PCR or standard PCR (18), were used for the validation. Further, nine cultured isolates were used. DNA extracts from a series of soil, water, algal, and possum fecal samples spiked with M. ulcerans and prepared as part of a WHO-sponsored quality assurance program (QAP) for the PCR detection of M. ulcerans in environmental samples were also included in the analysis. The DNA from the clinical and environmental samples was extracted as previously described (7). In addition, two M. ulcerans-positive tissue specimens from Australia were randomly selected and heat treated. Briefly, the tissues were macerated and homogenized in a bottle containing glass beads and phosphate-buffered saline. Approximately 0.5 ml of the homogenate was incubated at 95°C in a heating block for 30 min, followed by centrifugation at 14,000 rpm for 5 min. The resulting supernatant was used in the analysis.
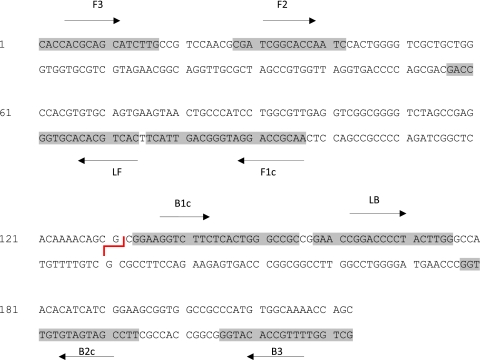
Three sets of LAMP primers consisting of six primers for each set were designed targeting the ketoreductase B domain (KR-B) and IS2404 (accession no. BX649209), respectively, and using the software Primerexplorer. The primer specificity was checked using the basic local alignment search tool (BLAST) against human DNA and other mycobacterial sequences in the nonredundant GenBank database, after which the performance of the primer sets was tested using the standard LAMP conditions for “must-detect samples,” i.e., M. ulcerans DNA from varied specimens, and “must-not-detect samples,” i.e., Mycobacterium intracellulare, Mycobacterium leprae, Mycobacterium chelonae, Mycobacterium lepraemurium, Mycobacterium avium, and Mycobacterium marinum. The set(s) of primers that passed these criteria was then analyzed using a 10-fold serial dilution set of DNA from M. ulcerans Agy99, followed by the selection of the set that was most sensitive (Fig. 1).
Fig 1.
The partial nucleotide sequence of a multicopy insertion sequence, IS2404, showing the most sensitive LAMP primer set. The restriction enzyme HhaI site is shown as a red line. The primers are as follows: F3, forward; B3, backward; LF, loop forward; LB, loop backward; FIP, forward inner (F1c and F2); BIP, backward inner (B2c and B1c).
The 25-μl LAMP reaction mixtures were standardized for optimal temperature and time using M. ulcerans Agy99 DNA, according to the Taguchi method (3) and as previously described (17). The template used for optimization was ∼100 pg of DNA, and the reactions were carried out for 1 h at 62°C using the Rotor-Gene 6000 (Qiagen Inc., Valencia, CA) and terminated by increasing the temperature to 80°C for 4 min. Three methods were used to detect the BU LAMP product, namely, (i) electrophoresis through 2.0% agarose gels stained with SYBR Safe DNA gel stain (Invitrogen, Carlsbad, CA), (ii) visual inspection after addition of a 1:10 dilution of SYBR green I, and (iii) monitoring the fluorescence of double-stranded DNA (dsDNA) using the Rotor-Gene 6000. The real-time fluorescence data were obtained on the 6-carboxyfluorescein (FAM) channel (excitation at 470 nm and detection at 510 nm). Confirmation of the BU LAMP product was done through HhaI restriction enzyme digestion followed by electrophoresis in a 3% agarose gel and postamplification acquisition of melting curves on the FAM channel using 1°C steps, with a hold of 30 s, from 63°C to 96°C (13). A serial 10-fold dilution of M. ulcerans strain Agy99 DNA (∼480 ng/μl) was used to determine the analytical sensitivity of the assay, and results were compared with those of TaqMan real-time PCR testing and classical PCR. Additional assay specificity was determined using DNA extracted from clinical specimens from skin lesions previously shown to contain M. intracellulare, M. leprae, M. chelonae, M. lepraemurium, and M. avium.
The Taguchi method determined the optimal concentration for forward and backward inner primers (FIP and BIP, respectively) at 40 pmol, 2 mM for each deoxynucleotide triphosphate, 1.2 M betaine, and 3 mM extra magnesium sulfate. Concentrations of other reagents were as previously reported (17). The BU LAMP optimum temperature was determined to be 62°C, and the reaction cutoff time was determined to be 35 min. The optimized LAMP conditions were more efficient, showing a reduction in reaction time of ∼6 min per every 10-fold serial dilution compared with the standard LAMP conditions using a real-time PCR machine. The analytical sensitivities of the classical PCR and TaqMan real-time PCR assays as determined through 10-fold serial dilution were 10−6 (2,000 copies) and 10−9 (2 copies), respectively, while those of the KR-B- and IS2404-based LAMP tests were 10−5 and 10−8 (20 copies), respectively. As such, the KR-B test was not further developed. The IS2404 LAMP assay was specific, and no cross-reactivity was recorded with nontarget DNA of closely related Mycobacterium spp. Positive LAMP reactions showed exponential amplification curves (similar to those in Fig. 2B) with cycle threshold values (CT) ranging from 15 to 32 for the M. ulcerans-positive clinical samples tested. Electrophoresis of the same product on a 2.0% agarose gel showed a ladder-like pattern, indicating the formation of stem-loops with inverted repeats. On addition of 1 μl of a 1:10 dilution of SYBR green I to the same samples, all the positive samples turned green while the negatives remained brown/orange (Fig. 2A). The acquired melting curves showed melting temperatures (Tm) of ∼89°C, while the restriction enzyme digestion indicated the predicted products of ∼87 bp and 126 bp. In the analysis of primary specimens, the tests were performed in duplicate, and in cases of discrepancies, the test in question was repeated a third time, the results of which were considered final. Agreement between the tests was determined using the kappa index estimated at the 95% confidence interval (95% CI). The developed assay was further tested in an isothermal amplification and detection unit (ESE-Quant tube scanner; ESE GmbH., Stockach, Germany), which was set to collect fluorescence signals at 1-min intervals. The ESE-Quant tube scanner provides a major advancement toward “electricity-free” technology for LAMP technology and offers a single-step amplification and product detection step. The device is simple to operate, small (74 mm high by 178 mm wide by 188 mm), and lightweight (1 kg).
Fig 2.
(A) The visual appearance of the BU LAMP amplification product after addition of a 1:10 dilution of SYBR green I dye. The dye fluoresces strongly when bound to the double-stranded DNA, and the resulting DNA-dye complex gives a green color, while fluorescence is minimal when the dye is free in the solution and gives an orange/brown color. (B) The amplification curves obtained using the ESE-Quant tube scanner. Results can be read using the LCD panel as either positive or negative and/or in real time using a computer with the appropriate software (9). The graph reports the fluorescence in millivolts (mV) on the y axis and time in minutes on the x axis. Sample 1, M. ulcerans-spiked soil; 2, supernatant from positive specimen; 3, DNA from needle aspirate; 4, M. marinum; control (C), M. ulcerans DNA (Agy99); negative control (NC), water.
The IS2404 LAMP test was able to detect seven M. ulcerans DNAs in the environmental QAP samples (Table 1) and directly from the supernatant derived from two heat-treated tissue specimens (Fig. 2A). Results for the DNA extracts from primary specimens from Australia showed a kappa value of 0.8 (95% CI, 0.70 to 0.98) between TaqMan real-time PCR and LAMP, and those from Ghana showed a kappa value of 0.7 (95% CI, 0.60 to 0.89) between PCR and LAMP, respectively (Table 1). The ESE-Quant tube scanner showed results identical to those of the Rotor-Gene 6000, with the amplification of M. ulcerans DNA showing sigmoid curves while the negative controls had no measurable fluorescence as indicated by flat lines in the plot (Fig. 2B).
Table 1.
Analysis of various DNA extracts using standard PCR, TaqMan real-time PCR, and LAMP assay
| DNA source | No. of samples |
Kappa value | |||
|---|---|---|---|---|---|
| Total | Positive by type of assay: |
||||
| PCR | TaqMan PCR | LAMP | |||
| Mycobacterium ulceransa | 9 | 9 | 9 | 9 | |
| Spiked environmental samplesb | 8 | NDh | 8 | 7 | |
| Heat-treated specimensc | 2 | 2 | 2 | 2 | |
| Australian clinical specimensd | 136 | ND | 18 | 15 (83.3%f) | 0.87 |
| Ghanaian clinical specimense | 79 | 50 | ND | 42 (84%g) | 0.74 |
Cultured isolates (7).
Water, algae, soil, and possum excreta.
Human tissue.
Swabs, tissues (fresh biopsy specimens or scrapings and formalin-fixed, paraffin-embedded tissue), and koala feces (1 sample only).
Swabs and fine-needle aspirate.
Percent ratio of LAMP- to TaqMan PCR-positive samples.
Percent ratio of LAMP- to PCR-positive samples.
ND, not done.
The LAMP technology in its simplest form offers the potential for on-site testing for a pathogen, as an alternative to methodologies requiring sophisticated instrumentation, present only in specialized laboratories. The IS2404 BU LAMP designed in this study has an analytical sensitivity comparable to that of TaqMan real-time PCR, which is equivalent to 0.01 to 0.1 genome (2 to 20 copies of the target sequence) (7). This is an excellent detection limit, taking into account the simplicity and the rapidity of the assay (∼35 min) compared to other DNA tests. Moreover, the large amount of dsDNA formed allows visual inspection of results using varied formats. The potential usefulness of BU LAMP as a point-of-care test is further demonstrated by the ability of the new assay to amplify target DNA from supernatant prepared from heat-treated specimens (tissue), meaning that DNA extraction may not be necessary. We recorded no inhibition of LAMP reactions or formation of nonspecific products with the use of up to 3 μl of supernatant from heat-treated samples. The possibility of heating the specimens directly to obtain template shortens the overall assay time; however, this method requires rigorous evaluation. The BU LAMP assay reported here has a clear advantage over most nucleic acid amplification assays in that the test is robust and simple and requires less instrumentation to make a diagnosis.
It is crucial to ensure that any designed LAMP assay amplifies the target DNA, since most product detection methods do not offer the chance of confirming the end product; e.g., SYBR green I, the dye of choice, binds nonspecifically to any dsDNA such as primer-dimers and spurious amplicons, leading to errors in interpretation, (17). We confirmed that our BU LAMP assay amplifies the correct product through HhaI restriction enzyme digestion, which gave the predicted product sizes of ∼87 bp and 126 bp. In addition, the postamplification acquisition of the melting curves of products amplified from M. ulcerans from both Ghana and Australia revealed a consistent Tm of ∼89°C, indicating similar sequence compositions. The combination of these results and the use of three negative controls per test run increases our confidence when using SYBR green I to detect the BU LAMP product. The LAMP detection methods are still in their infancy; however, it is worth noting that higher test specificity can be achieved by targeting an internal sequence of the amplicon through incorporating a fluorescent molecular beacon probe and thus minimizing nonspecific signal (5) or using a lateral flow dipstick format. Although lateral flow matrix is slightly more expensive than nonspecific fluorescence dyes, it shows improved LAMP test specificity and presents one way of developing the assay into a field-applicable format (17), especially when the heat treatment method is used to prepare the LAMP template. In general, an ideal LAMP detection format should be specific and include a closed amplification and detection unit to limit amplicon contamination, an inherent problem with many assays involving nucleic acid amplification and especially in less sophisticated settings.
The final components that will constitute a point-of-care test for BU should be affordable and simple to perform without compromising sensitivity and specificity. The ESE-Quant tube scanner tested here is a major advancement toward this goal because the device permits single-step amplification and product detection. The device uses a customized rechargeable battery, and results are obtained directly using the device liquid crystal display (LCD) window or software installed on a laptop computer, thus avoiding post-DNA manipulation (Fig. 2B). Our preliminary data suggest that the device can reliably be used to detect various pathogens of medical and veterinary importance (data not shown) with sensitivity identical to that of the Rotor-Gene 6000. Therefore, the ESE-Quant tube scanner can potentially allow detection of BU in resource-poor areas of endemicity. A good performance of the device has also been reported (9) in detection of malaria parasites. The downside of the device is that it can accommodate only eight samples and uses nonspecific dye; therefore, modification is required to expand the device to accommodate more samples.
The development of the BU LAMP assay in this work, based on the target that is currently used for the diagnosis of BU, may potentially improve the detection of early disease in the field. Our results indicate that the LAMP assay was able to detect ∼83% of PCR-positive DNA extracts from Australia and Ghana and initially prepared from varied primary specimens (Table 1). However, the BU LAMP failed to detect three of the TaqMan PCR-positive samples and eight PCR-positive samples (Table 1). It is possible that (i) the pathogen DNA concentration in the three samples was below the LAMP detection level, as our results indicate that TaqMan PCR was more sensitive than BU LAMP, and/or that (ii) DNA degradation may have occurred in the eight samples from Ghana after a long storage period (Table 1). The latter idea is supported by the repeat of PCR testing using the same aliquot, in which no positive signals were recorded. Among the samples from Australia and Ghana found to be negative by TaqMan and classical PCR, respectively, the LAMP test showed positive signals from two samples in each group. It was not possible to determine whether the two archived Australian samples were false positives; however, the two samples from patients in Ghana are being followed to ascertain the patients' disease status, since these are recent cases.
In summary, this study has shown that the BU LAMP has good sensitivity that is comparable to that of PCR tests (kappa values, 0.7 to 0.8); therefore, the LAMP assay may be used to reliably detect M. ulcerans DNA. It is unlikely that the PCR-based methods will soon be integrated into routine point-of-care diagnosis of BU disease in areas of endemicity, largely due to the lack of sophisticated laboratories; rather, they will remain invaluable in reference laboratories. The BU LAMP assay developed here has potential to contribute toward the early detection of BU and confirmation of clinically diagnosed cases. Furthermore, the assay may contribute toward a better understanding of the epidemiology, ecology, and mode of transmission of M. ulcerans. Our next step is to lyophilize the BU LAMP assay, develop a dipstick detection matrix, and carry out extensive field evaluations.
ACKNOWLEDGMENTS
This project has been funded by UBS Optimus Foundation.
We acknowledge the help of Adwoa Asante-Poku and Kobina Ampah, NMIMR, for PCR work.
The views expressed by the authors do not necessarily reflect the views of their respective institutes.
Footnotes
Published ahead of print 22 February 2012
REFERENCES
- 1. Aiga H, et al. 2004. Assessing water-related risk factors for Buruli ulcer: a case-control study in Ghana. Am. J. Trop. Med. Hyg. 71:387–392 [PubMed] [Google Scholar]
- 2. Asiedu K, Etuaful S. 1998. Socioeconomic implications of Buruli ulcer in Ghana: a three-year review. Am. J. Trop. Med. Hyg. 59:1015–1022 [DOI] [PubMed] [Google Scholar]
- 3. Cobb B, Clarkson JM. 1994. A simple procedure for optimising the polymerase chain reaction (PCR) using modified Taguchi methods. Nucleic Acids Res. 22:3301–3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Debacker M, et al. 2004. Mycobacterium ulcerans disease (Buruli ulcer) in rural hospital, Southern Benin, 1997–2001. Emerg. Infect. Dis. 10:1391–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dominguez M, et al. 2010. The MIT D-lab electricity-free PortaTherm incubator for remote testing with the QuantiFERON-TB Gold In-Tube assay. Int. J. Tuberc. Lung. Dis. 14:1468–1474 [PMC free article] [PubMed] [Google Scholar]
- 6. Fu S, et al. 2011. Applications of loop-mediated isothermal DNA amplification. Appl. Biochem. Biotechnol. 163:845–850 [DOI] [PubMed] [Google Scholar]
- 7. Fyfe JA, et al. 2007. Development and application of two multiplex real-time PCR assays for the detection of Mycobacterium ulcerans in clinical and environmental samples. Appl. Environ. Microbiol. 73:4733–4740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson PD, et al. 2007. Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg. Infect. Dis. 13:1653–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lucchi NW, et al. 2010. Real-time fluorescence loop mediated isothermal amplification for the diagnosis of malaria. PLoS One 5:e13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marion E, et al. 2011. Geographic expansion of Buruli ulcer disease, Cameroon. Emerg. Infect. Dis. 17:551–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marsollier L, et al. 2002. Aquatic insects as a vector for Mycobacterium ulcerans. Appl. Environ. Microbiol. 68:4623–4628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marston BJ, Diallo, et al. 1995. Emergence of Buruli ulcer disease in the Daloa region of Côte d'Ivoire. Am. J. Trop. Med. Hyg. 52:219–224 [DOI] [PubMed] [Google Scholar]
- 13. Monis PT, Giglio S, Saint CP. 2005. Comparison of SYTO-9 and SYBR Green I for real-time polymerase chain and investigation of the effect of dye concentration on amplification and DNA melting curve analysis. Anal. Biochem. 30:24–34 [DOI] [PubMed] [Google Scholar]
- 14. Mori Y, Nagamine K, Tomita N, Notomi T. 2001. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 289:150–154 [DOI] [PubMed] [Google Scholar]
- 15. Mori Y, Notomi T. 2009. Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J. Infect. Chemother. 15:62–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nienhuis WA, et al. 2010. Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. Lancet 375:664–672 [DOI] [PubMed] [Google Scholar]
- 17. Njiru ZK. 2011. Rapid and sensitive detection of human African trypanosomiasis by loop-mediated isothermal amplification combined with a lateral-flow dipstick. Diagn. Microbiol. Infect. Dis. 69:205–209 [DOI] [PubMed] [Google Scholar]
- 18. Ross BC, et al. 1997. Development of a PCR assay for the rapid diagnosis of Mycobacterium ulcerans infection. J. Clin. Microbiol. 35:1696–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siegmund V, et al. 2007. Dry reagent-based polymerase chain reaction compared with other laboratory methods available for the diagnosis of Buruli ulcer disease. Clin. Infect. Dis. 45:68–75 [DOI] [PubMed] [Google Scholar]
- 20. Wastling SL, Picozzi K, Kakembo AS, Welburn SC. 2010. LAMP for human African trypanosomiasis: a comparative study of detection formats. PLoS Negl. Trop. Dis. 4:e865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization 2004. Surveillance and control of Mycobacterium ulcerans disease (Buruli ulcer). Fifty-Seventh World Health Assembly, document A57/5. World Health Organization, Geneva, Switzerland [Google Scholar]