Abstract
The subgenomic promoter of a (+)-stranded RNA virus, brome mosaic virus (BMV) controlling synthesis of subgenomic RNA4 has been defined in vitro. Truncated and mutant (-)-strand RNA templates were produced by in vitro transcription of cloned RNA3 cDNA. Subgenomic (+)-sense RNA was synthesized in vitro from these templates by a replicase (RNA-dependent RNA polymerase) preparation extracted from infected barley leaves. The activities of templates with truncations and deletions surrounding the RNA4 initiation site revealed a promoter of approximately 62 bases grouped into four functional domains. The core sequence consists of about twenty bases immediately upstream of, and including, the initiation nucleotide. In addition to the core sequence, a domain overlapping the 5' untranslated end of RNA4 apparently determines correct initiation. Two domains immediately upstream of the promoter core consist of the internal poly(A) tract of RNA3, which probably serves as an non base-paired spacer facilitating access of the replicase to the promoter, and a sequence, UUAUUAUU, that is required for high levels of promoter activity. Homologies to sequences surrounding the initiation sites of subgenomic RNAs from several plant RNA viruses, and from alphaviruses, have been detected.
Full text
PDF


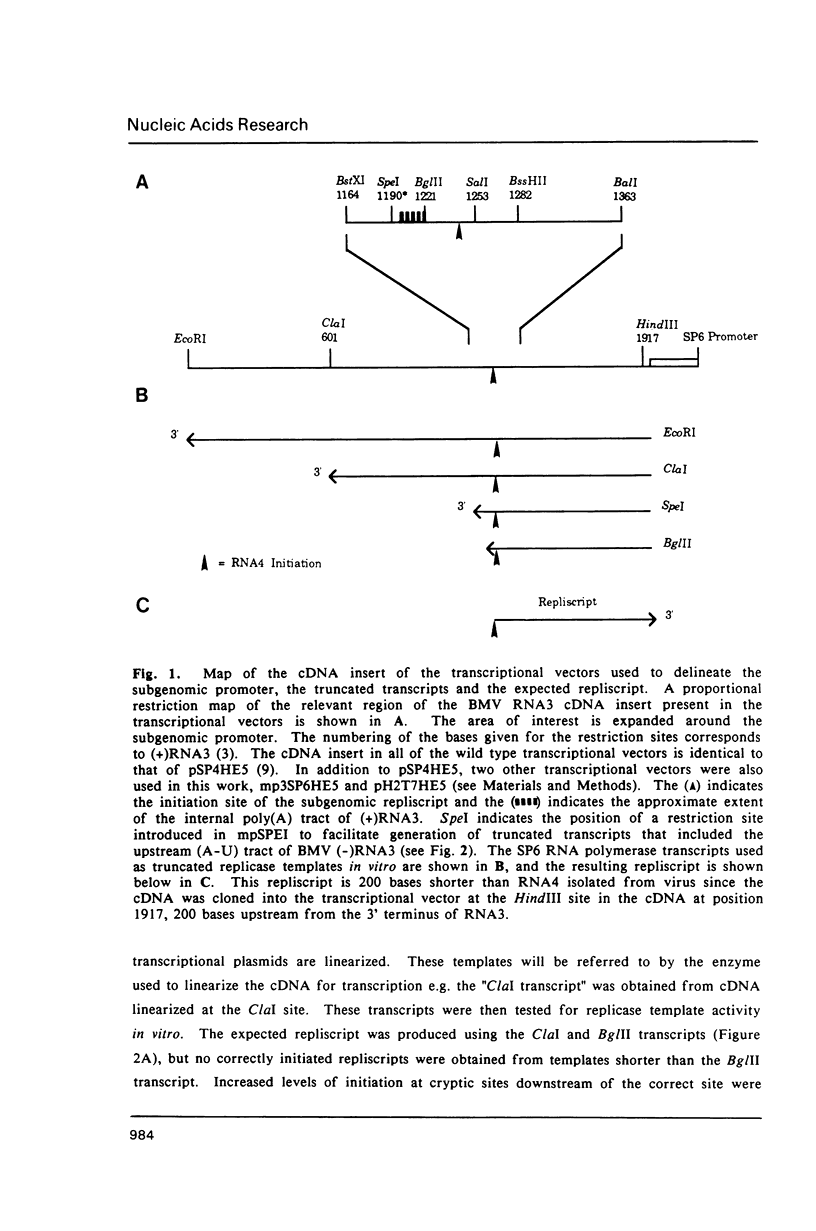
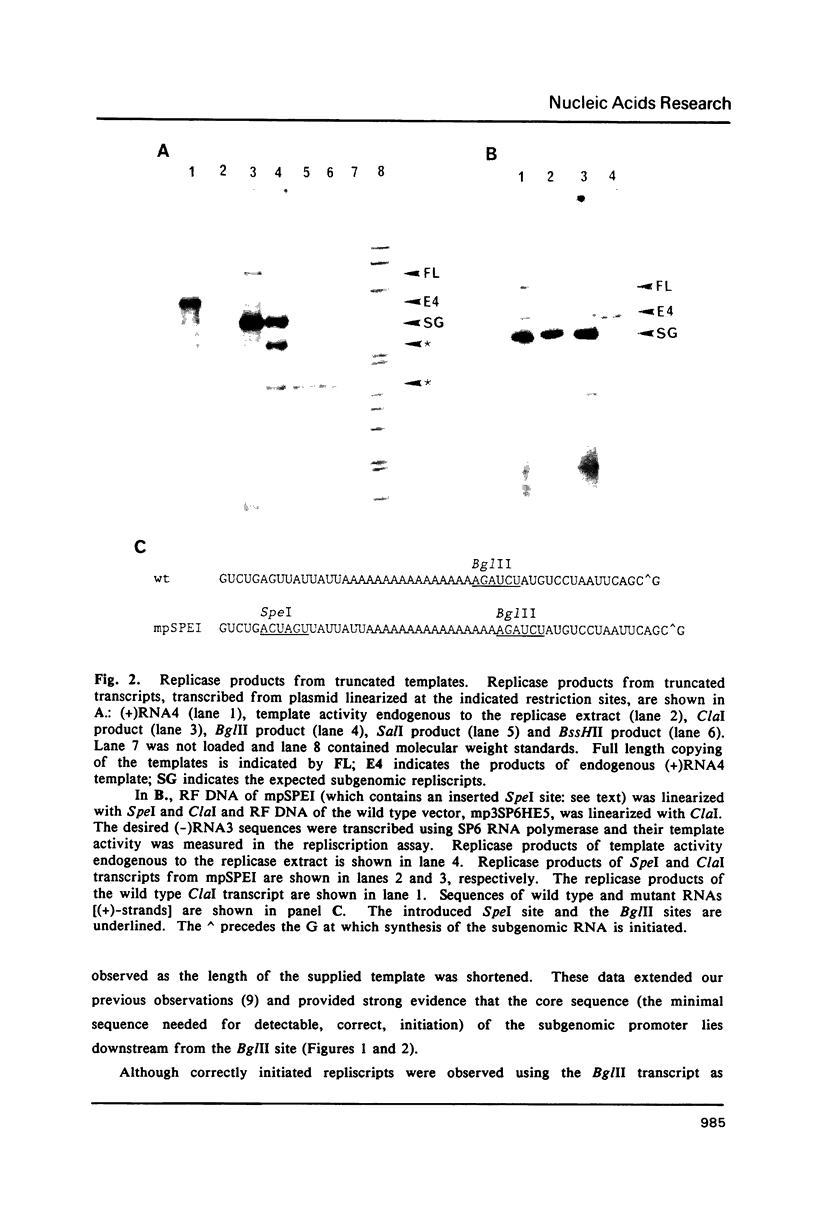
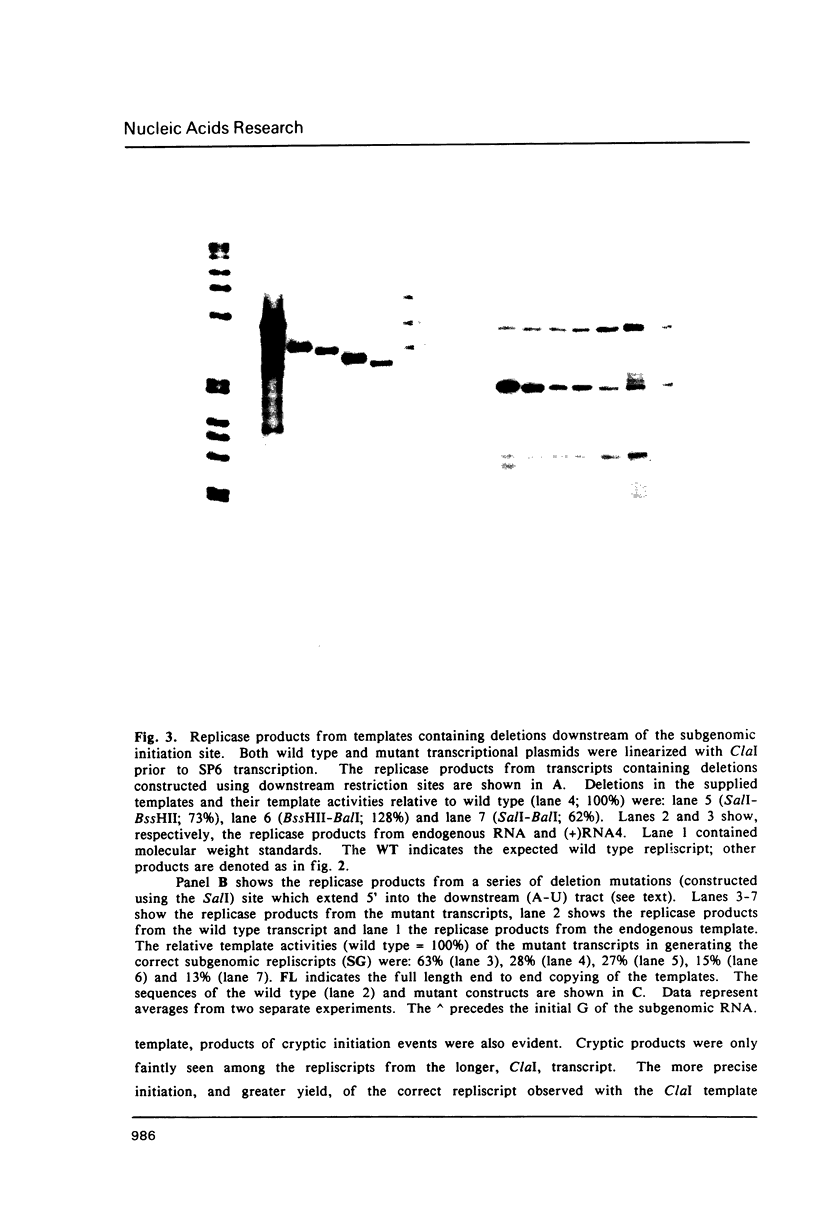

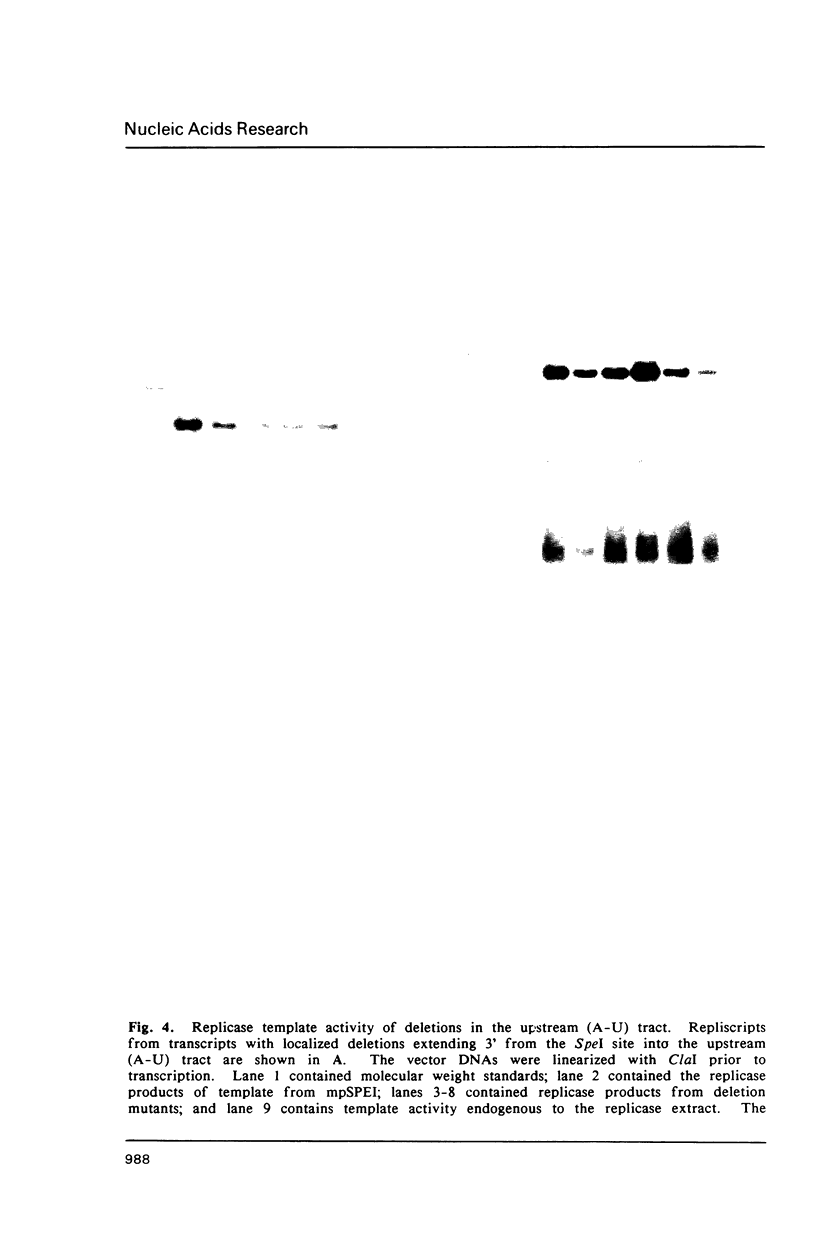







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Kaesberg P. Determination of the length distribution of poly(A) at the 3' terminus of the virion RNAs of EMC virus, poliovirus, rhinovirus, RAV-61 and CPMV and of mouse globin mRNA. Nucleic Acids Res. 1979 Nov 10;7(5):1195–1204. doi: 10.1093/nar/7.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlquist P., Luckow V., Kaesberg P. Complete nucleotide sequence of brome mosaic virus RNA3. J Mol Biol. 1981 Nov 25;153(1):23–38. doi: 10.1016/0022-2836(81)90524-6. [DOI] [PubMed] [Google Scholar]
- Barker R. F., Jarvis N. P., Thompson D. V., Loesch-Fries L. S., Hall T. C. Complete nucleotide sequence of alfalfa mosaic virus RNA3. Nucleic Acids Res. 1983 May 11;11(9):2881–2891. doi: 10.1093/nar/11.9.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh S. T., Koziel M. G., Huang S. C., Thomas R. A., Gilley D. P., Siegel A. The nucleotide sequence of tobacco rattle virus RNA-2 (CAM strain). Nucleic Acids Res. 1985 Dec 9;13(23):8507–8518. doi: 10.1093/nar/13.23.8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccara M., Hamilton W. D., Baulcombe D. C. The organisation and interviral homologies of genes at the 3' end of tobacco rattle virus RNA1. EMBO J. 1986 Feb;5(2):223–229. doi: 10.1002/j.1460-2075.1986.tb04202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski J. J., Dreher T. W., Hall T. C. Deletions in the 3'-terminal tRNA-like structure of brome mosaic virus RNA differentially affect aminoacylation and replication in vitro. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5636–5640. doi: 10.1073/pnas.82.17.5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta R., Kaesberg P. Complete nucleotide sequences of the coat protein messenger RNAs of brome mosaic virus and cowpea chlorotic mottle virus. Nucleic Acids Res. 1982 Jan 22;10(2):703–713. doi: 10.1093/nar/10.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R., Ahlquist P. Intercistronic as well as terminal sequences are required for efficient amplification of brome mosaic virus RNA3. J Virol. 1987 May;61(5):1457–1465. doi: 10.1128/jvi.61.5.1457-1465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R., Janda M., Ahlquist P. Bacterial gene inserted in an engineered RNA virus: efficient expression in monocotyledonous plant cells. Science. 1986 Mar 14;231(4743):1294–1297. doi: 10.1126/science.231.4743.1294. [DOI] [PubMed] [Google Scholar]
- Goelet P., Karn J. Tobacco mosaic virus induces the synthesis of a family of 3' coterminal messenger RNAs and their complements. J Mol Biol. 1982 Jan 25;154(3):541–550. doi: 10.1016/s0022-2836(82)80013-2. [DOI] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson G., Armour S. L. The complete nucleotide sequence of RNA beta from the type strain of barley stripe mosaic virus. Nucleic Acids Res. 1986 May 12;14(9):3895–3909. doi: 10.1093/nar/14.9.3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S. F., German T. L., Loesch-Fries L. S., Hall T. C. Highly active template-specific RNA-dependent RNA polymerase from barley leaves infected with brome mosaic virus. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4956–4960. doi: 10.1073/pnas.76.10.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keränen S., Käriäinen L. Functional defects of RNA-negative temperature-sensitive mutants of Sindbis and Semliki Forest viruses. J Virol. 1979 Oct;32(1):19–29. doi: 10.1128/jvi.32.1.19-29.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane L. C., Kaesberg P. Multiple genetic components in bromegrass mosaic virus. Nat New Biol. 1971 Jul 14;232(28):40–43. doi: 10.1038/newbio232040a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller W. A., Dreher T. W., Hall T. C. Synthesis of brome mosaic virus subgenomic RNA in vitro by internal initiation on (-)-sense genomic RNA. Nature. 1985 Jan 3;313(5997):68–70. doi: 10.1038/313068a0. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Rice C. M., Dalgarno L., Strauss E. G., Strauss J. H. Sequence studies of several alphavirus genomic RNAs in the region containing the start of the subgenomic RNA. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5235–5239. doi: 10.1073/pnas.79.17.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Ravelonandro M., Pinck M., Pinck L. Complete nucleotide sequence of RNA 3 from alfalfa mosaic virus, strain S. Biochimie. 1984 May;66(5):395–402. doi: 10.1016/0300-9084(84)90023-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Kaesberg P. Translation of brome mosaic viral ribonucleic acid in a cell-free system derived from wheat embryo. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1799–1803. doi: 10.1073/pnas.70.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Meshi T., Okada Y. The initiation site for transcription of the TMV 30-kDa protein messenger RNA. FEBS Lett. 1984 Jul 23;173(1):247–250. doi: 10.1016/0014-5793(84)81056-x. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]