Abstract
The common properties of broadly cross-reactive HIV-1 neutralization antibodies found in certain HIV-1-infected individuals holds significant value for understanding natural and vaccine-mediated anti-HIV immunity. Recent efforts have addressed this question by deriving neutralizing monoclonal anti-envelope antibodies from memory B cell pools of selected subjects. However, it has been more difficult to identify whether broadly neutralizing antibodies circulating in plasma possess shared characteristics among individuals. To address this question, we used affinity chromatography and isoelectric focusing to fractionate plasma immunoglobulin from 10 HIV-1-infected subjects (5 subjects with broad HIV-1 neutralizing activity and 5 controls). We find that plasma neutralizing activity typically partitions into at least two subsets of antibodies. Antibodies with restricted neutralization breadth have relatively neutral isoelectric points and preferentially bind to envelope monomers and trimers versus core antigens from which variable loops and other domains have been deleted. In comparison, broadly neutralizing antibodies account for a minor fraction of the total anti-envelope response. They are consistently distinguished by more basic isoelectric points and specificity for epitopes shared by monomeric gp120, gp120 core, or CD4-induced structures. Such biochemical properties might be exploited to reliably predict or produce broad anti-HIV immunity.
INTRODUCTION
A limited number of persons infected with HIV-1 develop circulating plasma antibodies that are able to potently neutralize a wide variety of HIV-1 isolates representing different genetic subtypes. It is widely held that the characteristics and specificities of such antibodies can be used to guide the development of HIV-1 vaccine candidates capable of eliciting protective humoral immunity in a target population. It seems particularly important to define antibody qualities that commonly occur in addition to those that arise in rare subjects; such qualities should reflect a general capacity of the human immune system. Consequently, intensive efforts are under way to derive broadly neutralizing monoclonal antibodies (MAbs) from the memory B cell pools of selected HIV-1-infected persons (32, 43, 52, 53, 62). While clearly important, these efforts may significantly underestimate the components of circulating plasma neutralizing activity (18, 43). For example, we reported that there is often discordance between memory B cell and circulating anti-envelope antibody responses (18). In agreement, others have demonstrated that pools of neutralizing MAbs derived from memory B cells often fail to fully recapitulate the neutralizing activity of the source subject (43). Dissection of neutralizing plasma responses by depletion with mutant HIV-1 envelope antigens has been attempted with some success (9, 28, 41, 42), but such antigen-specific approaches have a limited capacity to elucidate the range of anti-envelope properties that might contribute to plasma neutralizing activity.
We have pursued a comprehensive approach toward addressing this question that uses preparative isoelectric focusing (IEF) to fractionate whole or affinity-purified plasma IgG into separate species, which can then be screened for neutralizing breadth or for binding preferences against a variety of HIV-1 envelope-based antigens. Here we report that among individuals, anti-envelope antibodies display a consistent relationship between isoelectic point (pI), antigen binding specificity, and neutralizing breadth. Furthermore, the most potent neutralizing antibodies consistently manifest signature characteristics with respect to immunological and biochemical properties. Below we will demonstrate that antibodies with restricted neutralization breadth have relatively neutral isoelectric points and bind to native envelope monomers and trimers versus core antigens from which variable loops and other domains have been deleted. In comparison, broadly neutralizing antibodies account for a minor fraction of the total anti-envelope response, are marked by more-basic isoelectric points, and exist within the pool of antibodies that exhibit reactivity with epitopes present on monomeric gp120, gp120 core, or CD4-induced structures.
MATERIALS AND METHODS
Subjects and samples.
We previously described a collection of 10 HIV-infected patients with broad HIV-1 neutralization activity and small amounts of circulating HIV (<10,000 HIV-1 RNA copies/ml) (38). These 10 individuals demonstrated broad plasma neutralization (75% of 12 tier 2 clade B viruses tested), which was confirmed with IgG testing and cross-clade testing (clades A, C, and CRF02_AG). Of these, 5 individuals with the highest and broadest 80% inhibitory dilution titers (ID80) for multiple HIV-1 viruses and adequate sample availability were chosen for the current study. In this study, the individuals are designated subjects 1, 2, 6, 8, and 9 from the previously described study (38). These subjects had a median age of 49 years (range, 34 to 51) and were all male. In addition, 5 HIV-1-positive subjects (not highly active antiretroviral therapy [HAART] treated) with restricted HIV-1 neutralization activity, chosen randomly from a cohort of HIV-1-infected patients, were selected for making comparisons (38). These subjects had a median age of 52 years (range, 44 to 58); 4 were male, and 1 was female. The demographic details of all subjects are given in Table 1. All subjects are African-Americans residing in Baltimore, MD, and have presumed clade B virus infection (confirmed in 5 of the 10 individuals in this study by proviral sequencing). This study was institutional review board (IRB) approved, and all individuals provided informed consent.
Table 1.
Demographics of subjects in this studya
| Subject | Age (yrs) | Sex | Race | Yrs since HIV diagnosis | CD4 count (cells/μl) | No. of HIV-1 RNA copies/ml | Broad HIV-1 neutralization |
|---|---|---|---|---|---|---|---|
| Subject 1 | 50 | M | AA | 1 | 927 | 202 | + |
| Subject 2 | 51 | M | AA | 17 | 675 | 104 | + |
| Subject 6 | 49 | M | AA | 20 | 667 | 2,290 | + |
| Subject 8 | 34 | M | AA | 15 | 410 | 3,610 | + |
| Subject 9 | 46 | M | AA | 4 | 202 | 2,194 | + |
| NVS 7 | 58 | M | AA | 12 | 1,280 | <50 | − |
| NVS 14 | 44 | M | AA | 15 | 397 | 19,100 | − |
| NVS 203 | 56 | F | AA | 11 | 63 | 63,949 | − |
| LT 8 | 45 | M | AA | 2 | 701 | 9,462 | − |
| J5 | 52 | M | AA | 2 | 536 | 94,379 | − |
All subjects are HAART naive. M, male; F, female; AA, African-American.
Proteins and reagents.
Recombinant HIV-1 antigens were generated as described previously (31). Test antigens included the YU2 gp120 core, from which V1, V2, and V3 have been deleted (60), the YU2 gp120 core containing the V3 loop (YU2 gp120 core +V3) (60), monomeric HIV-1 Ba-L gp120 (12), a single-chain gp120-CD4 complex (FLSC) presenting a full-length CD4-induced gp120 structure in which the CD4 binding site is occupied (12), and a stabilized, cleaved HIV-1 Ba-L SOSIP trimer (herein designated gp140) (8). An affinity-purified goat antibody (Ab) (D7324) specific for the C-terminal peptide of HIV-1 gp120 was purchased from Cliniqa (San Marcos, CA). All proteins were expressed by transient transfection of 293T cells as previously described, purified by lectin affinity chromatography as previously described, and dialyzed against phosphate-buffered saline (PBS) prior to use (12).
The isoelectric points of 10 HIV-1 monoclonal antibodies (PG9, PG16, VRC01, b12, 2G12, 17b, E51, A32, C11, and 19e) were compared (6, 23, 25, 29, 52, 53, 60, 62–64). These included five broadly neutralizing MAbs (PG9, PG16, VRC01, b12, and 2G12), two narrowly neutralizing MAbs (17b and E51), and three nonneutralizing MAbs (A32, C11, and 19e). HIV-1 MAbs were purified by protein A affinity chromatography (GE Healthcare, Piscataway, NJ) from 293T cell supernatants prepared by transfecting heavy- and light-chain genes encoding the antibodies, as previously described (18). All MAbs contained an identical IgG1 Fc sequence and were expressed in 293T cells (59).
IgG purification.
Whole-plasma IgG was purified on a protein A affinity chromatography column (GE Healthcare, Piscataway, NJ) according to the manufacturer's instructions and dialyzed against PBS prior to use. For selected subjects, material that passed through the protein A column was sequentially applied to a protein G column (GE Healthcare, Piscataway, NJ) according to the manufacturer's instructions. Material recovered from the protein G column was dialyzed against PBS prior to use. Affinity chromatography columns were made with activated CH Sepharose beads (GE Healthcare, Piscataway, NJ) coupled to 2 mg of recombinant HIV-1 Ba-L gp120 (12), a single-chain gp120-CD4 complex (FLSC) derived from HIV-1 Ba-L gp120 and human CD4 D1D2 (12), or HIV-1 YU2 core (60), as described previously (18). The columns were used to purify antigen-specific IgG from whole IgG as previously described (18, 38). Briefly, IgG was incubated with beads at 37°C for 1 h prior to extensive washing with PBS. Columns were eluted with 0.2 M glycine (pH 2.8) at room temperature and dialyzed against 4 liters PBS 3 times (a minimum of 24 h total) prior to testing. Dedicated columns were used for each subject and antigen. The IgG concentration was measured using an in-house quantitative enzyme-linked immunosorbent assay (ELISA) as previously described (18).
Isoelectric focusing.
Purified IgG was separated by preparative liquid-phase IEF using the Mini Rotofor focusing system (Bio-Rad, Hercules, CA), which has 20 chambers and a 20-ml-volume capacity. The procedure was performed with 10 to 20 mg whole plasma or 2 to 5 mg affinity-purified IgG. Fractionation was accomplished at a 12-W constant power in the presence of 1.5% Servalyt carrier ampholytes (pH 7 to 9; Serva Heidelberg, Germany). The Mini Rotofor instrument is designed to yield 20 fractions. Among them, fraction 1 always contained no detectable antibody. For most subjects, fractions 2 and 20 contained insufficient amounts of antibody to enable detailed analyses. For all subjects, fractions 3 to 19 (spanning a pH range of 6.5 to 9.5) yielded abundant antibody. Accordingly, these fractions were used for comparative analyses. Fraction pH was verified by direct pH testing and/or IEF gel. Undialyzed Mini Rotofor fractions (10 μl, containing 1 to 10 μg IgG) were analyzed on solid-phase IEF gels (Gel Company, San Francisco, CA). A pH 6 to 11 gradient under constant voltage was developed as follows: 700-mv prefocusing for 20 min, 500 mv for 30 min, 1,500 mv for 90 min, and 2,000 mv for 30 min. The IEF gels were silver stained with SilverQuest per the manufacturer's protocol (Invitrogen, Carlsbad, CA) to visualize IgG species. Fractions (0.5 ml) were extensively dialyzed against 4 liters PBS (three times for at least 24 h total) at 4°C prior to further use.
Solid-phase IEF of MAbs was carried out by applying 10 μg of MAb to a pH 6 to 11 gradient gel. Gels were silver stained as described above. Standard pI markers (GE Healthcare, Piscataway, NJ) were run in parallel lanes of each gel. Each marker was plotted versus its distance from the top of the gel to develop a pI-versus-distance standard curve. The distance of each MAb band from the gel top was measured and compared to the curve to assign a pI.
HIV-1 envelope and IgG subclass ELISA.
HIV-1 envelope capture ELISAs were performed as previously described (18) with various antigens (as indicated in the text) that were directly coated (HIV-1 Ba-L SOSIP trimer, 1 μg/ml; YU2 gp120 core construct and YU2 gp120 core plus V3, 2 μg/ml) or captured (Bal-gp120 or FLSC at a concentration of 0.15 μg/ml) by antibody D7324, which had been adsorbed to the solid phase at 2 μg/ml. For IEF-fractionated whole IgG, 0.5 μg from each Mini Rotofor fraction was tested in a total assay volume of 50 μl; 5.0 ng of IEF-fractionated, affinity-purified IgG was tested in the same volume. All IgG preparations were incubated with antigens for 1 h at 37°C. Bound Abs were then detected with 1:1,000-diluted alkaline phosphatase (AP)-goat antihuman IgG (Southern Biotech; Birmingham, AL) and detected using the Blue Phos microwell phosphatase substrate system (KPL, Gaithersburg, MD). All assays were performed in duplicate or repeated several times. Negative-control assays were carried out with secondary antibody; background values were subtracted from all test absorbance readings.
ELISAs that detect human IgG subclasses were used according to the manufacturer's instructions (Invitrogen, Carlsbad, CA) to evaluate Mini Rotofor fractions from selected subjects. All assays were performed in duplicate. This assay has a limit of detection of 0.43 μg/ml for IgG1, 0.17 μg/ml for IgG2, and 0.024 μg/ml for IgG4.
Neutralization assays.
HIV-1 neutralization testing was performed using a luciferase-based assay with TZM.bl cells as previously described (26, 38). This assay measures the reduction in luciferase expression following a single round of virus infection. Stocks of Env-pseudotyped viruses were prepared by transfection of 293T/17 cells as previously described (26). These included pseudoviruses expressing a tier 1 clade B envelope (SF162.LS) and those expressing tier 2 clade B envelopes 6535.3, QH0692.42, SC422661.8, PVO.4, TRO.11, AC10.0.29, RHPA4259.7, THRO4156.18, REJO4541.67, TRJO4551.58, WITO4160.33, and CAAN5342.A2. All whole IgG samples were tested against a murine leukemia virus (MuLV) control, one tier 1 clade B pseudovirus (SF162.LS), and three tier 2 clade B pseudoviruses which the plasma of all patients with broad neutralizing activity demonstrated activity against (SC422661.8, RHPA4259.7, and REJO4541.67). Affinity-purified samples underwent testing against tier 1 clade B pseudoviruses (SF162.LS) and tier 2 tier 2 clade B pseudoviruses, as determined by the reported (38) plasma neutralization profiles of the subjects and sample quantities. Threefold serial dilutions of IgG were tested in duplicate (96-well flat-bottom plate) in 10% Dulbecco's modified Eagle medium (DMEM) growth medium (100 μl/well). Pseudovirus (200 50% tissue culture infective doses [TCID50]) was added to each well in a volume of 50 μl, and the plates were incubated for 1 h at 37°C. TZM.bl cells were then added (1 × 104/well in a 100-μl volume) in 10% DMEM growth medium containing DEAE-dextran (Sigma, St. Louis, MO) at a final concentration of 11 μg/ml. The final volume for each well was 250 μl. Assay controls included replicate wells of TZM.bl cells alone (cell control), TZM.bl cells with virus (virus control), and the MuLV control. Following a 48-h incubation at 37°C, 150 μl of assay medium was removed from each well, and 100 μl of Bright-Glo luciferase reagent (Promega, Madison, WI) was added. The cells were allowed to lyse for 2 min, and then 150 μl of the cell lysate was transferred to a 96-well black solid plate and luminescence was measured using a Victor 3 luminometer (Perkin Elmer, Waltham, MA). The 50% inhibitory dose (IC50) titer was calculated as the immunoglobulin concentration that caused a 50% reduction in relative luminescence units (RLU) compared to results for the virus control wells after subtraction of cell control RLUs (26).
RESULTS
Selection of HIV-1-positive subjects with broad neutralizing activity in plasma.
For the current study, we utilized a cohort of individuals who demonstrated broad HIV-1 neutralization activity in a previous study (38). Specifically, both plasma and purified IgG from five selected individuals (subjects 1, 2, 6, 8, and 9) neutralized at least 75% of pseudoviruses representing 12 tier 2 subtype B isolates tested, as well as isolates of the subtypes A, C, and CRF02_AG (38). These individuals had between 104 and 3,610 HIV-1 RNA copies/ml. Most samples used in this study were contemporaneous with respect to those used in the published evaluations (in cases where noncontemporaneous samples were used, plasma neutralization assays were repeated to ensure a matching neutralization profile). To provide a basis for comparison, our analyses included five randomly chosen HIV-1-positive subjects (NVS 7, NVS 14, NVS 203, J5, and LT 8) who exhibited limited HIV-1 neutralizing activity (38). Four of these 5 subjects had neutralizing activity against SF162.LS (ID80 range, 1:1,432 to 1:6,826), while subject LT 8 had minor activity (ID80, 1:27). These 5 individuals had no significant tier 2 pseudovirus-neutralizing activity (4 individuals with plasma ID80 of <1:20 for the tier 2 panel and 1 individual, NVS 7, with an ID80 of 1:52 for REJO4541.67 only).
IEF of plasma immunoglobulin.
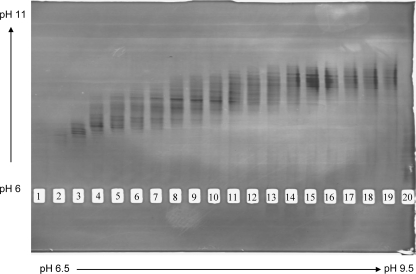
Protein A-purified whole immunoglobulin preparations from the selected individuals were subjected to preparative isoelectric focusing using a pH range of 7 to 9, which is sufficient to cover the isoelectric range of most human IgG (36). Separation of antibodies was verified by subjecting the separate isoelectric fractions to a solid-phase IEF gel spanning a pH range from 6 to 11. A representative IEF gel profile is shown in Fig. 1. These analyses verified that plasma antibodies were substantially separated according to pI. As expected, immunoglobulin species recovered from each preparative IEF fraction focused in the corresponding pH range of the solid-phase IEF gel.
Fig 1.
Preparative IEF fractionation of plasma IgG with broad HIV neutralizing activity. Representative data from subject 6 (see the text) are shown. Fractionation of 20 mg IgG was achieved by preparative IEF with a pH range of 6.5 to 9.5 (indicated at bottom), as described in Materials and Methods. An aliquot of each fraction (10 μl) was electrophoresed over an IEF gel with a pH range of 6 to 11 (indicated at left). Immunoglobulin bands were visualized with silver stain. Lanes 1 to 20 correspond to fractions 1 to 20, respectively.
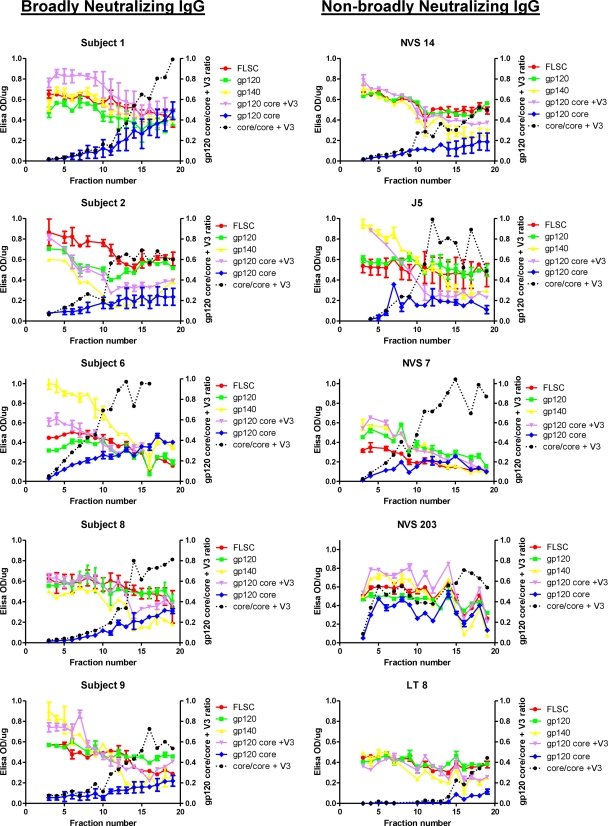
Variable specificities of IEF-fractionated IgG species.
Immunoglobulin fractions from each subject were screened for reactivity with a series of antigens representing various domains and structures (Fig. 2). For these experiments, an equal amount of IgG (0.5 μg) from each fraction was tested. Relative reactivities were determined as a function of absorbance values (see Materials and Methods) per 0.5 μg of immunoglobulin. Test antigens included monomeric HIV-1 Ba-L gp120 (12), YU2 core (60), the YU2 gp120 core containing the V3 loop (YU2 gp120 core +V3) (60), a single-chain gp120-CD4 complex (FLSC) presenting a full-length CD4-induced gp120 structure in which the CD4 binding site is occupied (12), and a stabilized, cleaved HIV-1 Ba-L SOSIP trimer (8). In all cases, reactivity with monomeric gp120, FLSC, gp140, and gp120 core +V3 was highest in IgG fractions with a more neutral pI (Fig. 2, lower fraction numbers). Such reactivity diminished concurrently as fractions progressed to more basic isolectric points (i.e., higher fraction numbers). An inverse trend was seen with reactivity to the YU2 gp120 core. Reactivity with this antigen was negligible in IgG fractions representing more neutral pIs and increased as fractions proceeded to more-basic pIs. Similar patterns were seen with IEF-fractionated gp120 affinity-purified antibody for the 5 subjects with broad HIV-1 neutralizing activity (data not shown).
Fig 2.
ELISA reactivity patterns of fractionated IgG from subjects with broad or nonbroad plasma HIV neutralizing activity. Whole IgG from each subject was fractionated by preparative IEF (see Materials and Methods). Aliquots (0.5 μg each) of IgG from each fraction were tested by ELISA for reactivity against the indicated HIV antigens. The x axis represents the IEF fractions (spanning a pH gradient of 6.5 to 9.5 from left to right). The left y axis represents ELISA signals expressed as background-corrected optical density at 450 nm (OD450) readings/μg IgG. Assays were repeated at least twice. Error bars indicate the high and low values observed. The gp120 core/gp120 core +V3 ratio (right y axis) is calculated from mean absorbance values for each antigen.
In all subjects, the ratio of reactivity with YU2 gp120 core/gp120 core +V3 increased as fractions progressed to more basic isolectric points. These ratios in the most basic fractions tended to be higher in broadly neutralizing subjects than in non-broadly neutralizing subjects, although the overall differences between groups was not significant (P > 0.05; t test).
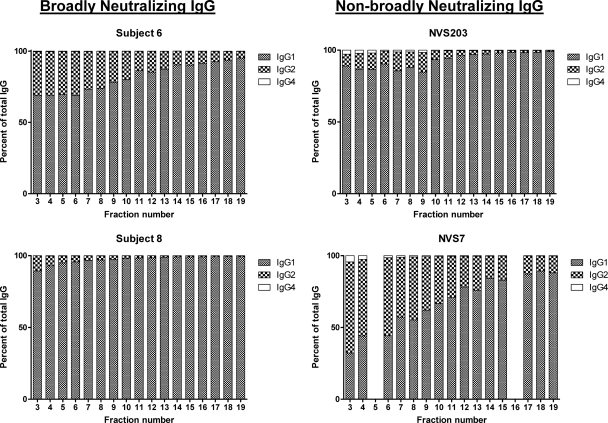
Subclass distribution of protein A-purified, preparative IEF fractions.
IEF fractions from two representative broadly neutralizing subjects (subject 6 and subject 8) and two non-broadly neutralizing subjects (NVS 7 and NVS 203) were evaluated for IgG subclass content using a commercial detection assay. As shown in Fig. 3, IgG1 was the major subclass detected in the vast majority of fractions regardless of subject. Lower levels of IgG2 and IgG4 were also detected. IgG1 concentrations tended to be higher in the fractions with more basic pIs. Conversely, IgG2 and IgG4 concentrations were higher in fractions with more neutral pIs. For the two patients with broad neutralization, there was a 10 to 20% maximal difference in IgG1 percentages between fractions with basic versus neutral pIs.
Fig 3.
IgG Subclass distribution among IEF-fractioned IgG from subjects with broad or nonbroad plasma HIV neutralizing activity. IEF fraction numbers are indicated on the x axis. All fractions were assayed in duplicate by ELISA for IgG1, IgG2, and IgG4 concentrations as described in Materials and Methods. Total IgG was determined by the sum of all IgG subclasses tested. The quantities of IgG subclasses in each fraction were extrapolated from the ELISAs and used to calculate percentages of total IgG (y axis).
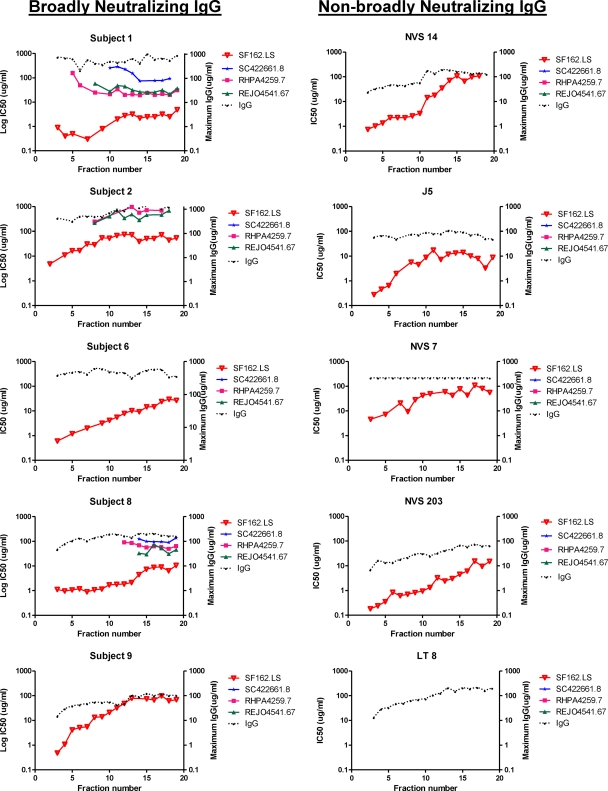
Neutralizing activity of IEF-fractionated IgG.
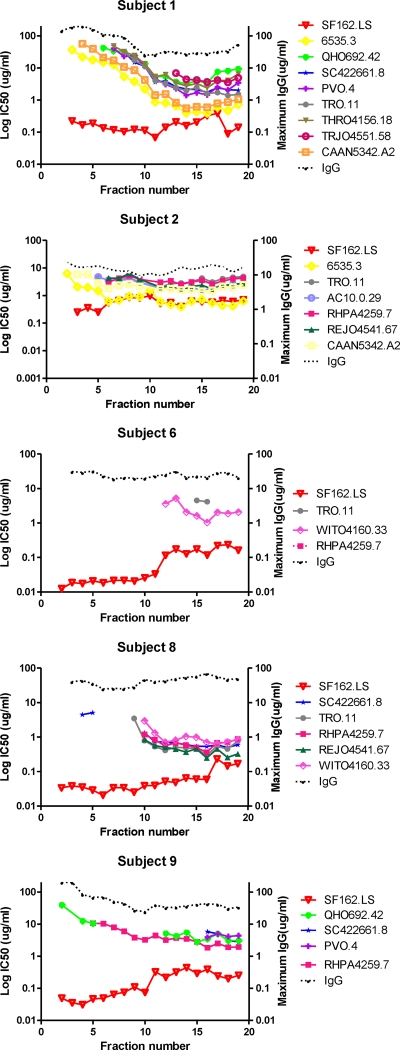
Fractionated IgG from all subjects was further tested for neutralizing activities against a panel of pseudoviruses, including a tier 1 reference isolate (SF162.LS) as well as three difficult-to-neutralize (26) tier 2 isolates (SC422661.8, RHPA4259.7, and REJO4541.67) selected based on established sensitivity to plasma neutralizing activity (38). Virus pseudotyped with MuLV envelope served as negative controls. Each fraction was tested in a 3-fold dilution series starting at the highest IgG concentration recovered from each chamber of the Mini Rotofor preparative IEF instrument (see Materials and Methods).
Regardless of subject, the tier 1 pseudovirus (SF162.LS) was most potently neutralized by IgG fractions representing more-neutral pIs. The exception was control subject LT 8, whose plasma lacked neutralizing activity against SF162.LS; in accordance, fractionated IgG also demonstrated no neutralizing activity (Fig. 3). In all cases, potency against the SF162.LS pseudovirus declined (i.e., the IC50 increased) as fractions proceeded toward more basic pIs. Fractionated whole IgG from the control subjects lacking tier 2 neutralizing activity in plasma did not neutralize the tier 2 pseudoviruses (Fig. 4). However, neutralization of two or more tier 2 pseudoviruses was observed with fractioned IgG from subjects 1, 2, and 8. Remarkably, neutralization potency repeatedly increased across the more basic fractions, with maximum potency or lowest IC50s at fractions 13 to 17 (pIs of approximately 8.5 to 9) (Fig. 4). Since equivalent fractions from the control subjects did not neutralize these viruses, this pattern was not a general artifact arising from the IEF fractionation procedure. In comparison, more neutral pI fractions from subjects 1, 2, and 8 exhibited no detectable neutralization of the tier 2 pseudoviruses (indicated by the absence of symbols for these fractions) even though the overall IgG concentrations of all fractions were similar. None of the IEF fractions from any subject neutralized the MuLV control virus (data not shown). Notably, IEF-fractionated IgG from two subjects (subjects 6 and 9) failed to neutralize tier 2 viruses even though unfractionated IgG from these subjects was active against the pseudoviruses tested here (38).
Fig 4.
Neutralization patterns of IEF-fractioned IgG from subjects with broad or nonbroad plasma HIV neutralizing activity. IEF fractions are indicated on the x axis; the pH gradient of 6.5 to 9.5 spans the fractions from left to right. IgG from each fraction was tested for neutralizing activity against the indicated pseudoviruses as described in Materials and Methods. SF162.LS is a tier 1 pseudovirus; the rest fall within the tier 2 category. Assays were run in duplicate. Mean infectivity values were used to calculate IC50 titers (left y axis). The maximum IgG concentrations tested per fraction are shown on the right y axis. The absence of a symbol for any pseudovirus-fraction pair indicates that an IC50 was not achieved at the highest testable IgG concentration in the fraction.
Protein A chromatography does not efficiently capture all IgG subclasses, particularly IgG3. This introduced the possibility that the neutralizing patterns seen among the IEF fractions (Fig. 4) might be affected by the absence of other neutralizing subclasses not captured by protein A. This was addressed by subjecting the protein A-unbound material derived from representative subjects 1 and 8 to protein G chromatography, which captures all human IgG subclasses. However, because of the purification sequence, the protein G column in this case was expected to bind primarily IgG3. Protein G-purified material was fractionated by preparative IEF using the Mini Rotofor instrument as before. The resulting fractions were then tested for neutralizing activity against SF162.LS, SC422661.8, RHPA4259.7, and REJO4541.76. None of the resulting fractions exhibited detectable neutralizing activity against any pseudovirus (data not shown). This strongly indicated that broadly neutralizing activity was contained within the immunoglobulin subclasses that bind protein A.
Neutralizing activity of affinity-purified, IEF-fractionated IgG.
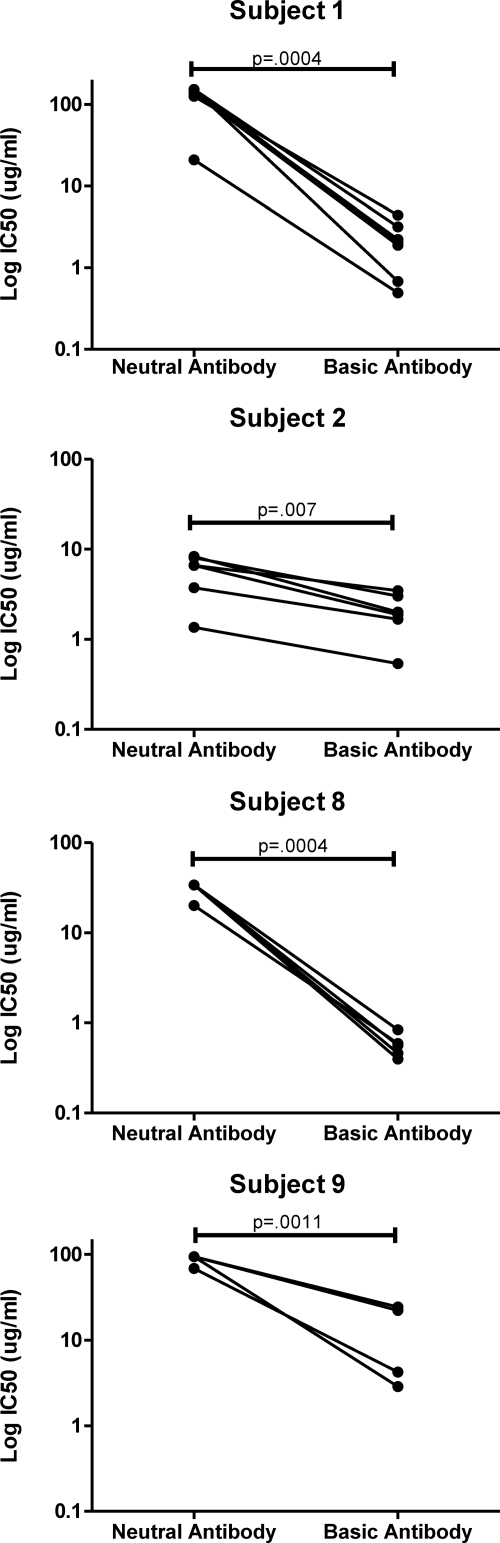
It was possible that the concurrence of antigen binding specificities and broad neutralizing activity in certain preparative IEF fractions was due to a fortuitous combination of nonneutralizing, ELISA-reactive antibodies and neutralizing antibodies with separate specificities. Alternatively, the immunoreactivity patterns might have indicated that the broadly neutralizing antibodies also recognize the antigens reactive in the ELISAs. To distinguish these possibilities, whole IgG from the subjects with broad HIV-1 neutralizing activity was subjected to affinity chromatography using gp120 to purify specific immunoglobulin, which was then fractionated by IEF as before. The fractions were then tested for neutralizing activity against a range of tier 2 pseudoviruses matching the source subjects' plasma neutralization profiles, along with the tier 1 SF162.LS pseudovirus (the total number of pseudoviruses tested varied depending on the amount of IgG recovered, which varied among subjects). Figure 5 shows the neutralization patterns of IEF fractions generated from the gp120-specific IgG. In general, neutralizing potencies were roughly 10-fold higher with the gp120-specific IgG versus whole-plasma IgG. In particular, IEF fractions of gp120-specific IgG from subject 9 exhibited detectable broadly neutralizing activity, whereas the corresponding fractions of whole IgG did not (Fig. 4 and 5). For subjects 1, 2, 8, and 9, neutralizing potency was greatest in basic-pI fractions (13 to 17) compared to results for the more-neutral fractions (3 to 8) for all pseudoviruses tested (P ≤ 0.007; paired t test) (Fig. 6). This followed the patterns seen with IEF fractions of whole IgG (Fig. 4). Notably, the most potent IEF fraction (fraction 16) from subject 1 exhibited the lowest IC50 values for all viruses tested and represented less than 2% of the entire anti-gp120 population in the Mini Rotofor fractions by ELISA. In comparison, IEF fractions from subject 2 also displayed relatively broad, albeit less potent, activity in the more-neutral fractions. The IEF fractions of gp120-specific IgG from subject 6 demonstrated relatively narrow neutralization breadth, in accordance with the results obtained for whole IgG (Fig. 4). In this case, no individual fraction or group of fractions could recapitulate the capacity of the source plasma (or the gp120 affinity-purified antibody) to potently neutralize the viruses tested here (38).
Fig 5.
Neutralization patterns of gp120-affinity-purified, IEF-fractioned IgG from subjects with broad HIV neutralizing activity. IEF fractions are shown on the x axis. A pH gradient of 6.5 to 9.5 spans the fractions from left to right. IgG from each fraction was tested for neutralizing activity against the indicated pseudoviruses as described in Materials and Methods. Assays were run in duplicate. Mean infectivity values were used to calculate IC50 titers (left y axis). The maximum IgG concentrations tested per fraction are shown on the right y axis. The absence of a symbol for any pseudovirus-fraction pair indicates that an IC50 was not achieved at the highest testable IgG concentration in that fraction.
Fig 6.
Neutralization activity of basic versus neutral anti-gp120 IgG. Data from Fig. 5 were reanalyzed to calculate the mean IC50 values for the tier 2 virus SC422661.8, RHPA4259.7, or REJO4541.76 among pools of the basic fractions (13 to 17) versus the more neutral fractions (3 to 7). Mean IC50 values for the two pools were compared by paired t test (shown). A P value < 0.05 was taken as significant. Subject 6 was not evaluated due to restricted neutralization breadth among IEF fractions.
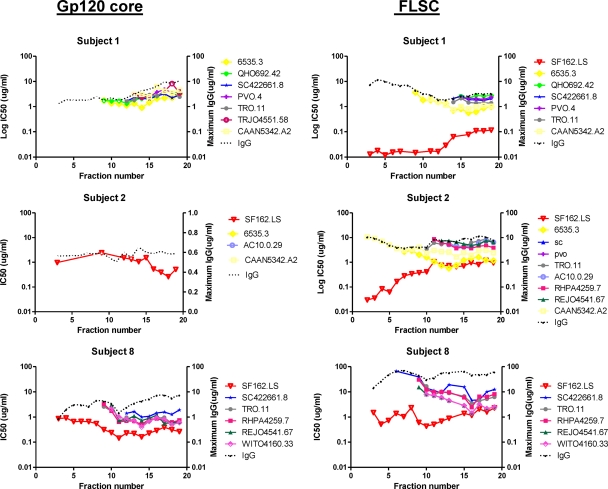
Neutralizing specificities were narrowed by affinity purification using either FLSC or YU2 gp120 core followed by preparative IEF fractionation in the Mini Rotofor instrument. IgGs from the three subjects (subjects 1, 2, and 8) with the greatest neutralization breadth and potency were selected for analyses (Fig. 7). The resulting IEF fractions were again tested against tier 1 and/or tier 2 pseudoviruses that were sensitive to the plasma neutralizing activity of the source subject (38). Subject 1 IgG demonstrated similar neutralization patterns regardless of the affinity handle used for purification (compare Fig. 4, 5, and 7). Neutralizing activity with the greatest breadth and potency was consistently observed in comparable fractions corresponding to more-basic pIs. Notably, the weaker neutralizing activity recovered in more-neutral fractions of gp120-purified IgG was not recovered in YU2 gp120 core-purified IgG of subject 1. This was consistent with the ELISA results (Fig. 2), which showed low binding of neutral-pI fractions to the YU2 gp120 core antigen. Subject 8 also exhibited similar tier 2 neutralization patterns among IEF fractions regardless of whether material was recovered from gp120, FLSC, or YU2 gp120 core affinity matrices.
Fig 7.
Neutralization patterns of HIV envelope-affinity purified, IEF-fractioned IgG from subjects with broad HIV neutralizing activity. The affinity handles used to capture IgG (see Materials and Methods) are shown at top. IEF fractions are shown on the x axis. A pH gradient of 6.5 to 9.5 spans the fractions from left to right. IgG from each fraction was tested for neutralizing activity against the indicated pseudoviruses as described in Materials and Methods. Assays were run in duplicate (except for gp120 core-purified material from subject 1). Mean infectivity values were used to calculate IC50 titers (left y axis). The maximum IgG concentrations tested per fraction are shown on the right y axis. Fractions derived from subject 1 were tested as singletons due to the paucity of sample.
Subject 2 exhibited a distinct pattern of specificity in which only low quantities of IgG were recovered from the YU2 gp120 core affinity matrix, again consistent with the low YU2 gp120 core binding titers in ELISA (Fig. 2). The quantity of YU2 gp120 core-purified IgG was insufficient to achieve IC50 values for any tier 2 pseudovirus. However, the larger quantity of material recovered from the FLSC affinity matrix produced a neutralization pattern similar to that observed with the gp120 affinity-purified immunoglobulin (compare Fig. 5 and 7), with the greatest breadth and potency in the more-basic-pI fractions 13 to 17.
Isoelectric points of various HIV-1 MAbs.
It was of interest to compare the pI values estimated for neutralizing plasma IgGs to those of human anti-gp120 MAbs. Five broadly neutralizing MAbs (PG9, PG16, VRC01, b12, and 2G12), two narrowly neutralizing MAbs (17b and E51), and three nonneutralizing MAbs (A32, C11, and 19e) were selected for these analyses. The pI of each MAb was calculated as described in Materials and Methods and is shown in Table 2. Predicted positions among Mini Rotofor fractions based on pI values are also shown. The broadly neutralizing MAbs VRC01, 2G12, and b12 exhibited basic pIs corresponding to fractions 10 to 20 in the Mini Rotofor IEF gradient. The broadly neutralizing MAbs PG9 and PG16 exhibited more-neutral pIs (around 7.8), corresponding to fractions 7 and 8 in the Mini Rotofor IEF gradient. This position matches the more-neutral end of the plasma-derived fraction series, showing broadly neutralizing activity (compare with results in Fig. 5), but is outside the basic pI area of the most potent activity. In comparison, MAbs 17b and E51, with restricted neutralizing activity, exhibited pIs from 7.0 to 7.85, corresponding to Mini Rotofor IEF fractions 4 to 8. Plasma-derived, anti-gp120 IgG fractions in this range also exhibited narrow neutralization breadth (Fig. 5). The nonneutralizing MAbs exhibited a range of pIs (7.4 to 8.8), theoretically distributing them throughout the Mini Rotofor IEF gradient (i.e., fractions 5 to 16).
Table 2.
Isoelectric points of human anti-gp120 MAbsa
| Antibody | Neutralization breadth | Calculated PI | Predicted IEF fraction |
|---|---|---|---|
| E51 | Restricted | 7.0 | 4 |
| 19E | None | 7.4 | 5 |
| PG9 | Broad | 7.75 | 7 |
| 17b | Restricted | 7.85 | 8 |
| C11 | Broad | 7.85 | 8 |
| PG16 | Broad | 7.85 | 8 |
| 2G12 | Broad | 8.1 | 10 |
| A32 | None | 8.8 | 16 |
| VRC01 | Broad | 8.8 | 16 |
| b12 | Broad | >9.0 | 20 |
The pI values were used to predict where the MAbs would focus in a typical preparative IEF procedure using the Rotofor instrument.
DISCUSSION
IEF has long been used as a means to characterize and differentiate antibody responses during infection and disease. This technique was classically used in studies of multiple myeloma (4) and is currently used for analyzing the degradation of monoclonal antibodies (14, 24, 61). IEF has also been used in the study of the humoral response to a number of pathogens (10, 11, 15, 17, 20–22, 30, 37, 39, 45, 46, 50, 51, 55). In contrast, IEF has seen limited application in HIV-1 infection and immunity (33, 56), with most early efforts focused on diagnostic applications (1–5, 7, 47, 48, 58). One complication for using IEF as a serological tool in HIV-1 infection is that titers of anti-envelope antibodies tend to rapidly decline once viremia is brought under control. However, a subset of chronically infected, asymptomatic, non-HAART individuals with low viremia maintain sustained levels of anti-envelope antibodies and broad plasma neutralizing activities over time (38, 41). Such subjects provide a means to study this subject using comprehensive biochemical methods, including IEF.
Our analyses indicate that among HIV-1-infected individuals, gross anti-gp120 antibody responses exhibit electrophoretic characteristics that commonly track with broad specificity (Fig. 2). In the HIV-1-infected individuals we examined, antibodies that preferentially recognize monomeric and CD4-constrained gp120 structures, trimeric gp140, and a gp120 core containing the V3 loop tend to have more neutral pIs, whereas antibodies that are able to recognize the minimal gp120 core structure appear to have more basic pIs. Given that the same quantity of IgG was tested for all IEF fractions, the ELISAs (Fig. 2) indicate that more-basic antibodies have roughly equivalent reactivities with all of the antigens we tested whereas more neutral antibodies fail to recognize the core gp120 structure. Notably, the same basic profiles occurred regardless of whether or not the source subject harbored broadly neutralizing antibodies, although differences in antigen binding trends seemed more pronounced for subjects with broadly neutralizing antibodies (Fig. 2, left panels versus right panels).
The broadly neutralizing antibodies in our subjects seemed to primarily track with the IgG1 subclass. Any significant involvement of IgG3 in plasma neutralizing activity was precluded by findings that neutralizing activity was consistently recovered with protein A. Furthermore, protein G-purified material that did not bind protein A had no detectable neutralizing activity in the preparative IEF fractions. IgG2 and IgG4 were detected in the preparative IEF fractions of protein A-purified antibody but at lower concentrations than IgG1. IEF fractions containing the largest amounts of IgG2 and IgG4 subclasses corresponded to more neutral pIs and restricted neutralization breadth (compare Fig. 2 and 3). Overall, it is important to note that the association of broadly neutralizing antibodies with more basic pIs cannot be explained simply by the electrophoretic properties of IgG1. This subclass was distributed and dominant throughout the preparative IEF gradients regardless of pI and neutralizing activity (Fig. 3). Similarly, the anti-gp120 MAbs we evaluated by IEF (Table 2) exhibited a range of pIs even though they were all expressed as IgG1.
Our data show that neutralizing anti-gp120 antibodies in plasma repeatedly fall into at least 2 groups that are distinguished by consistent neutralization profiles, epitope specificities, and electrophoretic properties. One group is marked by more neutral pIs and falls within the pool of antibodies that react with gp120, FLSC, and/or the gp120 core containing a V3 loop but have low or no reactivity to the gp120 core (compare corresponding panels in Fig. 2, 4, and 5). This group has narrow breadth and low or no capacity to neutralize tier 2 pseudoviruses (Fig. 4 and 5), although it potently neutralizes the sensitive tier 1 SF162.LS pseudovirus. The second group is marked by more basic pIs and falls within the population of antibodies that are capable of binding to a gp120 core structure. This group seems routinely capable of potent and broad neutralization of tier 2 pseudoviruses (Fig. 4 and 5).
A longstanding and extensive body of literature shows that antibody pI mainly reflects paratope biochemistry and epitope specificity (13, 16, 19, 35, 44, 49, 57). This fact is evident within a collection of anti-gp120 MAbs that have identical IgG1 Fc portions (Table 2). Accordingly, a straightforward interpretation of our findings is that the broadly neutralizing plasma antibodies with basic pIs recognize gp120 targets containing key acidic residues. Notably, the antibodies falling in the more neutral pI range recognized gp120 core +V3 but not gp120 core. This is consistent with hypothetical recognition of cross-reactive (albeit poorly neutralizing) V3 epitopes, which hold a basic charge in a number of different HIV-1 viruses (31).
Affinity purification allowed a general picture of cognate targets for broadly neutralizing antibodies with basic pIs. The gp120 affinity purification methods recovered neutralizing antibodies from five broadly neutralizing subjects. In four cases, the affinity fractionated antibodies retained broadly neutralizing activity (Fig. 5). This trend indicates that the cognate epitopes for these antibodies are efficiently presented on monomeric envelope in agreement with previous findings (9, 28). However, it contrasts with the recently reported broadly neutralizing human monoclonal antibodies PG9 and PG16 (53), which preferentially bind “quaternary epitopes” of trimeric HIV-1 envelope (34). The gp120 core, lacking all variable loops, recovered basic, broadly neutralizing antibodies from subject 1 and 8 whole-plasma IgG. These findings are in accordance with a study by Scheid at al. that showed how broadly neutralizing MAbs recovered from memory B cell pools frequently recognize the gp120 core antigen (43). The MAbs VRC01 and b12, with basic pIs (Table 2), also recognize epitopes in and around the CD4 binding site preserved in the gp120 core (27, 40). However, affinity chromatography with both FLSC and gp120 core recovered basic, broadly neutralizing antibodies with equivalent breadth from subject 1 and subject 8 plasma. Only FLSC but not gp120 core successfully recovered broadly neutralizing antibodies from subject 2 plasma. Since the CD4 binding site is blocked in FLSC, subjects 1, 2, and 8 may express plasma neutralizing antibodies with novel specificities linked to basic pIs.
Two recent studies of HIV-infected patients by Walker et al. indicated that only one or two antibody specificities were responsible for the majority of broad neutralizing activities in plasma (52, 54). Our data for subjects 1, 8, and 9 appear to follow the same trend. The most potent gp120-affinity-purified fraction (fraction 16) recovered from subject 1 that exhibited very broad neutralizing activity (Fig. 5) represented less than 2% of the entire anti-gp120 antibody pool. This is consistent with linkage between broadly neutralizing activity and single-target specificity. In comparison, gp120-purified antibodies from subject 2 exhibited broadly neutralizing activity across the entire preparative IEF gradient (Fig. 5). FLSC apparently captured only the subset of these antibodies that fell within the more basic-pI fractions (Fig. 7). Thus, subject 2 may represent a case where at least two antibody specificities (i.e., one neutral and one basic) collaborate to create the full spectrum of broad plasma neutralizing activity.
Subject 6 is an interesting case where whole-plasma IgG and gp120-affinity-purified IgG were broadly neutralizing but fractionated antibodies were not. It is difficult to attribute the absence of activity to low immunoglobulin concentrations, since the preparative IEF fractions for subject 6 contained amounts of IgG and exhibited ELISA immunoreactivity (Fig. 2, 4, and 5) equivalent to those of the other subjects' plasma treated in an identical manner. One likely explanation for these results is that the plasma neutralizing activity for subject 6 demands a certain balance of polyclonal anti-envelope antibodies that is disrupted by fractionation. An alternative explanation is that the broadly neutralizing plasma antibody in subject 6 is unexpectedly diluted away or otherwise lost during the fractionation procedures. Such an effect cannot be attributed entirely to the preparative IEF procedure, since fractionated gp120-affinity-purified material from subject 6 also exhibited relatively poor neutralizing activity (Fig. 5).
Overall, this study shows that preparative IEF provides an informative means for fractionating and characterizing HIV-1 neutralizing antibodies without the predetermined bias for epitope specificity introduced by methods relying exclusively on affinity purification and/or antigen depletion. Our findings that broadly neutralizing antibodies commonly possess basic pIs and recognize potentially unique epitopes outside the CD4 binding site, outside variable loops, and within monomeric gp120 structures could inform future immunogen design efforts to elicit similar antiviral responses. Further, the combination of IEF with affinity fractionation should facilitate detailed comparisons of plasma antibodies versus MAbs derived from B cell pools in order to recapitulate circulating broadly neutralizing responses. Finally, it should be noted that the analyses described here involved only subtype B-infected subjects. Additional studies will be needed to extend our findings into infections by other subtypes.
ACKNOWLEDGMENTS
We thank members of the NVS study and Becky Boyce, study coordinator. We also thank Brian Taylor and the IHV Core for help in purifying IgG.
This research was supported by grants NIH 1K23AI084580-01A1 to M.M.S., NIH R01AI087181-01 to G.K.L., and the Bill and Melinda Gates Foundation, grant no. 38619 to M.S.S.
Footnotes
Published ahead of print 29 February 2012
REFERENCES
- 1. Amadori A, et al. 1990. IgG oligoclonal bands in sera of HIV-1 infected patients are mainly directed against HIV-1 determinants. AIDS Res. Hum. Retroviruses 6:581–586 [DOI] [PubMed] [Google Scholar]
- 2. Biselli R, et al. 1996. Anti-V3 loop spectrotype in HIV-infected individuals during zidovudine therapy. Infection 24:227–233 [DOI] [PubMed] [Google Scholar]
- 3. Bukasa KS, et al. 1988. Anti-HIV antibodies in the CSF of AIDS patients: a serological and immunoblotting study. J. Neurol. Neurosurg. Psych. 51:1063–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dale G, Latner AL, Muckle TJ. 1970. The investigation of myelomatosis by gel isoelectric focusing followed by electrophoresis. J. Clin. Pathol. 23:35–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. D'Amelio R, et al. 1992. Spectrotype of anti-gp120 antibodies remains stable during the course of HIV disease. J. Acquir. Immune Defic. Syndr. 5:930–935 [PubMed] [Google Scholar]
- 6. Decker JM, et al. 2005. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J. Exp. Med. 201:1407–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. del Bono V, et al. 1998. Isoelectric focusing and reverse blotting as a diagnostic tool in pediatric HIV infection. J. Clin. Virol. 11:203–210 [DOI] [PubMed] [Google Scholar]
- 8. Dey AK, David KB, Klasse PJ, Moore JP. 2007. Specific amino acids in the N-terminus of the gp41 ectodomain contribute to the stabilization of a soluble, cleaved gp140 envelope glycoprotein from human immunodeficiency virus type 1. Virology 360:199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dhillon AK, et al. 2007. Dissecting the neutralizing antibody specificities of broadly neutralizing sera from human immunodeficiency virus type 1-infected donors. J. Virol. 81:6548–6562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drouet E, et al. 1999. Oligo-monoclonal immunoglobulins frequently develop during concurrent cytomegalovirus (CMV) and Epstein-Barr virus (EBV) infections in patients after renal transplantation. Clin. Exp. Immunol. 118:465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feutren G, de The G, Bach JF. 1992. Epstein-Barr virus serology and isoelectrofocusing pattern of serum immunoglobulins in cyclosporin or placebo-treated type I diabetics. J. Autoimmun. 5:161–172 [DOI] [PubMed] [Google Scholar]
- 12. Fouts TR, et al. 2000. Expression and characterization of a single-chain polypeptide analogue of the human immunodeficiency virus type 1 gp120-CD4 receptor complex. J. Virol. 74:11427–11436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freedman MH. 1972. The use of preparative liquid isoelectric focusing for the further purification of rabbit antihapten antibodies. J. Immunol. Methods 1:177–198 [DOI] [PubMed] [Google Scholar]
- 14. Gandhi S, et al. 2012. Elucidation of degradants in acidic peak of cation exchange chromatography in an IgG1 monoclonal antibody formed on long-term storage in a liquid formulation. Pharm. Res. 29:209–224 [DOI] [PubMed] [Google Scholar]
- 15. Gea S, et al. 1993. Chagas' disease cardioneuropathy: association of anti-Trypanosoma cruzi and anti-sciatic nerve antibodies. Am. J. Trop. Med. Hyg. 49:581–588 [DOI] [PubMed] [Google Scholar]
- 16. Gibas CJ, Subramaniam S, McCammon JA, Braden BC, Poljak RJ. 1997. pH dependence of antibody/lysozyme complexation. Biochemistry 36:15599–15614 [DOI] [PubMed] [Google Scholar]
- 17. Grimaldi LM, et al. 1988. HTLV-I-associated myelopathy: oligoclonal immunoglobulin G bands contain anti-HTLV-I p24 antibody. Ann. Neurol. 24:727–731 [DOI] [PubMed] [Google Scholar]
- 18. Guan Y, et al. 2009. Discordant memory B cell and circulating anti-Env antibody responses in HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 106:3952–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Imanishi T, Hurme M, Sarvas H, Makela O. 1975. Expression of a mouse Ig V gene in antibodies of three immunoglobulin classes. Eur. J. Immunol. 5:198–202 [DOI] [PubMed] [Google Scholar]
- 20. Insel RA, Adderson EE, Carroll WL. 1992. The repertoire of human antibody to the Haemophilus influenzae type b capsular polysaccharide. Int. Rev. Immunol. 9:25–43 [DOI] [PubMed] [Google Scholar]
- 21. Johnson GC, Adams DS, McGuire TC. 1983. Pronounced production of polyclonal immunoglobulin G1 in the synovial fluid of goats with caprine arthritis-encephalitis virus infection. Infect. Immun. 41:805–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaiser R, et al. 1989. Intrathecal synthesis of virus-specific oligoclonal antibodies in patients with enterovirus infection of the central nervous system. J. Neurol. 236:395–399 [DOI] [PubMed] [Google Scholar]
- 23. Lagenaur LA, Villarroel VA, Bundoc V, Dey B, Berger EA. 2010. sCD4-17b bifunctional protein: extremely broad and potent neutralization of HIV-1 Env pseudotyped viruses from genetically diverse primary isolates. Retrovirology 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lehermayr C, Mahler HC, Mader K, Fischer S. 2011. Assessment of net charge and protein-protein interactions of different monoclonal antibodies. J. Pharm. Sci. 100:2551–2562 [DOI] [PubMed] [Google Scholar]
- 25. Lewis GK, et al. 2011. Identification and characterization of an immunogenic hybrid epitope formed by both HIV gp120 and human CD4 proteins. J. Virol. 85:13097–13104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li M, et al. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108–10125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Y, et al. 2011. Mechanism of neutralization by the broadly neutralizing HIV-1 monoclonal antibody VRC01. J. Virol. 85:8954–8967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Y, et al. 2009. Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. J. Virol. 83:1045–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moore JP, Sodroski J. 1996. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J. Virol. 70:1863–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mortimer GE, Widdowson JP. 1979. Predominance of immunoglobulin G sub-class 3 among the complement-fixing antibodies to streptococcal M-associated protein. Clin. Exp. Immunol. 37:247–258 [PMC free article] [PubMed] [Google Scholar]
- 31. Moulard M, et al. 2000. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120. J. Virol. 74:1948–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mouquet H, et al. 2011. Memory B cell antibodies to HIV-1 gp140 cloned from individuals infected with clade A and B viruses. PLoS One 6:e24078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Muller S, et al. 1993. B-cell abnormalities in AIDS: stable and clonally-restricted antibody response in HIV-1 infection. Scand. J. Immunol. 38:327–334 [DOI] [PubMed] [Google Scholar]
- 34. Pancera M, et al. 2010. Crystal structure of PG16 and chimeric dissection with somatically related PG9: structure-function analysis of two quaternary-specific antibodies that effectively neutralize HIV-1. J. Virol. 84:8098–8110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perlmutter RM, Davie JM. 1977. Characterization of molecular heterogeneity and multispecificity in homologous idiotypic antisera. J. Immunol. 118:769–774 [PubMed] [Google Scholar]
- 36. Prin C, Bene MC, Gobert B, Montagne P, Faure GC. 1995. Isoelectric restriction of human immunoglobulin isotypes. Biochim. Biophys. Acta 1243:287–289 [DOI] [PubMed] [Google Scholar]
- 37. Roos RP, Nalefski EA, Nitayaphan S, Variakojis R, Singh KK. 1987. An isoelectric focusing overlay study of the humoral immune response in Theiler's virus demyelinating disease. J. Neuroimmunol. 13:305–314 [DOI] [PubMed] [Google Scholar]
- 38. Sajadi MM, et al. 2011. Correlation between circulating HIV-1 RNA and broad HIV-1 neutralizing antibody activity. J. Acquir. Immune Defic. Syndr. 57:9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Salazar-Grueso EF, et al. 1989. Isoelectric focusing studies of serum and cerebrospinal fluid in patients with antecedent poliomyelitis. Ann. Neurol. 26:709–713 [DOI] [PubMed] [Google Scholar]
- 40. Saphire EO, et al. 2001. Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science 293:1155–1159 [DOI] [PubMed] [Google Scholar]
- 41. Sather DN, et al. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J. Virol. 83:757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sather DN, Stamatatos L. 2010. Epitope specificities of broadly neutralizing plasmas from HIV-1 infected subjects. Vaccine 28(Suppl 2):B8–B12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scheid JF, et al. 2009. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458:636–640 [DOI] [PubMed] [Google Scholar]
- 44. Sela M, Mozes E. 1966. Dependence of the chemical nature of antibodies on the net electrical charge of antigens. Proc. Natl. Acad. Sci. U. S. A. 55:445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seppala IJ, Larinkari U, Rasanen T, Valtonen VV. 1981. Occurrence and specificity of antibodies against group-specific polysaccharides in beta-hemolytic streptococcal infections. Acta Pathol. Microbiol. Scand. B 89:323–334 [DOI] [PubMed] [Google Scholar]
- 46. Shackelford PG, et al. 1987. Subclass distribution of human antibodies to Haemophilus influenzae type b capsular polysaccharide. J. Immunol. 138:587–592 [PubMed] [Google Scholar]
- 47. Sinclair D, Galloway E, McKenzie S, Follett EA, Wallace L. 1989. Oligoclonal immunoglobulins in HIV infection. Clin. Chem. 35:1669–1671 [PubMed] [Google Scholar]
- 48. Slade HB, Pica RV, Pahwa SG. 1989. Detection of HIV-specific antibodies in infancy by isoelectric focusing and affinity immunoblotting. J. Infect. Dis. 160:126–130 [DOI] [PubMed] [Google Scholar]
- 49. Teplyakov A, et al. 2009. Epitope mapping of anti-interleukin-13 neutralizing antibody CNTO607. J. Mol. Biol. 389:115–123 [DOI] [PubMed] [Google Scholar]
- 50. Ulanova M, Hahn-Zoric M, Lau YL, Lucas A, Hanson LA. 1996. Expression of Haemophilus influenzae type b idiotype 1 on naturally acquired antibodies. Clin. Exp. Immunol. 105:422–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ulanova M, et al. 1997. The clonal antibody response to Pseudomonas aeruginosa heat shock protein is highly diverse in cystic fibrosis patients. APMIS 105:449–456 [PubMed] [Google Scholar]
- 52. Walker LM, et al. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Walker LM, et al. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Walker LM, et al. 2010. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS Pathog. 6:e1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wassall DA, Sinclair IJ. 1985. The serum antibody response of infected rats, rabbits, lambs and calves to Fasciola hepatica adult antigen fractions separated by preparative flatbed iso-electrofocusing. Parasite Immunol. 7:359–366 [DOI] [PubMed] [Google Scholar]
- 56. Wenisch E, et al. 1989. Isolation of human monoclonal antibody isoproteins by preparative isoelectric focusing in immobilized pH gradients. J. Biochem. Biophys. Methods 18:309–322 [DOI] [PubMed] [Google Scholar]
- 57. Williamson AR. 1971. Antibody isoelectric spectra. Analysis of the heterogeneity of antibody molecules in serum by isoelectric focusing in gel and specific detection with hapten. Eur. J. Immunol. 1:390–394 [DOI] [PubMed] [Google Scholar]
- 58. Wisnewski A, Cavacini L, Posner M. 1996. Human antibody variable region gene usage in HIV-1 infection. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 11:31–38 [DOI] [PubMed] [Google Scholar]
- 59. Wrammert J, et al. 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453:667–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wu L, et al. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384:179–183 [DOI] [PubMed] [Google Scholar]
- 61. Wu SJ, et al. 2010. Structure-based engineering of a monoclonal antibody for improved solubility. Protein Eng. Des. Sel. 23:643–651 [DOI] [PubMed] [Google Scholar]
- 62. Wu X, et al. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wyatt R, et al. 1995. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J. Virol. 69:5723–5733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xiang SH, et al. 2003. Epitope mapping and characterization of a novel CD4-induced human monoclonal antibody capable of neutralizing primary HIV-1 strains. Virology 315:124–134 [DOI] [PubMed] [Google Scholar]