Abstract
Background
How tandem autologous-allogeneic stem cell transplantation should be integrated in the treatment of multiple myeloma remains controversial. We examined the long-term outcome of patients with multiple myeloma managed with tandem autologous-allogeneic stem cell transplantation and present a prognostic factor analysis based on the experience of the Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC).
Design and Methods
This French, retrospective, registry-based study included 146 patients who had undergone tandem autologous-allogeneic transplantation for multiple myeloma at 20 SFGM-TC centers between 1998 and 2010. The patients included in the study had fully completed the two steps of a planned tandem autologous-allogeneic transplantation. No treatment had to be administered between the autologous and allogeneic parts of the tandem procedure.
Results
Seventy-seven patients (53%) underwent tandem autologous-allogeneic transplantation as part of upfront treatment, i.e. after a single line of treatment not including autologous transplantation. The median follow-up from the allogeneic transplant was 47.5 months (range, 1.2–132 months). At 4 years, the overall survival and event-free survival rates were 48% (95% CI 39–57 %) and 27% (95% CI 19–36), respectively. Eighteen patients (12%) experienced grade III–IV acute graft-versus-host disease and 43 patients (30%) had chronic graft-versus-host disease. The transplant-related mortality rate at 1 year was 15% (95% CI 10–22). Patients receiving tandem transplantation as upfront treatment had significantly improved event-free survival (36% versus 11%; P=0.005) and overall survival (56% versus 34%; P=0.02). Donor’s age ≤50 years was associated with improved event-free survival (35% versus 16%; P=0.005) and overall survival (54% versus 41%; P=0.02). In the multivariable analysis, upfront tandem transplantation, donor’s age ≤50 years and full chimerism were independent prognostic factors for better outcome.
Conclusions
We confirmed the feasibility of tandem autologour-allogeneic transplantation in heavily treated patients with multiple myeloma. We identified younger donor’s age and upfront tandem transplantation as two independent prognostic factors for survival which could be further explored in prospective studies.
Keywords: multiple myeloma, tandem autologous-allogeneic transplantation
Introduction
The so-called tandem autologous-allogeneic stem cell transplantation (SCT) is a sequential two-step procedure which combines tumor burden reduction by high-dose chemotherapy followed by autologous SCT and the graft-versus-myeloma effect mediated by immunocompetent lymphocytes following allogeneic SCT.1,2 The feasibility of tandem autologous-allogeneic SCT, initially described in resistant lymphomas3 has been demonstrated by several studies in multiple myeloma (MM).4–7 However, given the relatively limited number of patients, various issues related to tandem SCT remain unsolved. In the largest series reported so far8,9 including 102 MM patients, the median overall survival was more than 6 years and median event-free survival was 3 years. Another retrospective study on 17 MM patients undergoing tandem autologous-allogeneic SCT reported 2-year overall and event-free survival rates of 74% and 56%, respectively.10 Bruno published the results of a series of 100 newly diagnosed MM patients who underwent tandem autologous-allogeneic SCT.11 With a median follow-up of more than 5 years, the median event-free survival was 37 months. Collectively these results indicate that tandem autologous-allogeneic SCT is a potential treatment for MM patients, having a beneficial impact on survival.
To further characterize the benefit of tandem autologous-allogeneic SCT in MM, studies comparing its efficacy to double autologous-SCT have been conducted in newly diagnosed MM patients. In the French Intergroupe Francophone du Myélome (IFM) study, high-risk patients [with β2 microglobulin >3.5 mg/L and chromosome 13 deletion (del13)] were randomized to either double autologous SCT or tandem autologous-allogeneic SCT.12,13 The comparison of the two treatment arms suggested a trend for a better overall survival in the double autologous SCT arm (P=0.07). Event-free survival was not statistically different between the two arms (P=0.58). In an Italian study, both overall and event-free survival were significantly better in the tandem autologous-allogeneic SCT arm (P=0.01 and 0.02, respectively).14 In the Spanish PETHEMA study, MM patients not in complete response or near complete response after a first autologous SCT were randomized to either a second autologous SCT or a non-myeloablative allogeneic SCT.15 This study did not find any significant difference in either overall survival or event-free survival between the two arms. The conflicting conclusions of these three studies might be attributed to differences in the patients’ prognostic factors (high versus standard risk), the randomization criteria for tandem transplantation (HLA-matched donor available versus quality of response after autologous SCT) and the allogeneic conditioning regimen (total body irradiation 2 Gy versus fludarabine-melphalan).
In order to identify the best candidates for tandem autologous-allogeneic SCT, recent prospective studies included cytogenetic data. MM patients were randomized to double autologous SCT or tandem autologous-allogeneic-SCT when an HLA-matched sibling donor was available. In the US BMT CTN102 phase III trial, there was no significant benefit in survival between the two treatment arms, in either high-risk (β2 microglobulin ≥4 mg/L and del13) or standard-risk patients, whereas the 3-year overall survival rates in the tandem autologous-allogeneic SCT arm were 59% and 77%, respectively.16,17 In the recently published EBMT study, the 5-year overall survival rates were 65% and 58% in the tandem autologous-allogeneic SCT and autologous SCT arms, respectively (P=0.006).18 For patients with del13, the overall survival rate was 69% in the autologous-allogeneic SCT arm versus 55% in the autologous SCT arm (P=0.003). For patients with no del13, overall survival was not statistically different between the two arms. Thus, an improvement or trend to an improvement was observed in both standard (no del13) and high-risk (presence of del13) prognostic groups receiving tandem autologous-allogeneic SCT compared to autologous SCT. In the German DSMMM group, the 3-year overall survival rate for newly diagnosed MM patients with del13 was 60% in the tandem autologous-allogeneic SCT arm, which was not significantly different from that in the double autologous SCT arm.19 In the upfront setting, the benefit, in terms of survival, of tandem autologous-allogeneic SCT and the best candidates for this procedure remain unclear.
In this context, we report the experience of the Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC) on tandem autologous-allogeneic SCT in MM. We present long-term follow-up data and an analysis of prognostic factors for the largest series published so far.
Design and Methods
Patients
This was a French, retrospective, registry-based study which included 146 patients who had undergone tandem autologous-allogeneic SCT for MM at 20 SFGM-TC centers. The patients included in the study had fully completed both steps of a planned tandem autologous-allogeneic transplantation. No treatment had to have been administered between the autologous and allogeneic parts of the tandem transplantation. The decision to perform tandem transplantation was taken by each participating center. Given the retrospective nature of the study, the criteria for performing tandem transplantation or not could not be further characterized and the number of patients withdrawn at any stage before autologous SCT or between the autologous and allogeneic transplants of the tandem could not be assessed. For each patient, the following data were recorded at diagnosis in the French SFGM-TC database: demographics (age, gender), biological factors (level and type of serum paraprotein and urinary light chains, serum level of creatinine and β2 microglobulin, and cytogenetics when available). Stage was assessed according to the Durie-Salmon and International Staging System classifications.20 Conventional cytogenetic metaphases and del13 were studied with fluorescent in situ hybridization (FISH) analysis for 13q14. Approval was obtained from the SGFM-TC Scientific Board and the Ethics Committee of the Institut Gustave Roussy in accordance with the Declaration of Helsinki. Results were analyzed as of June 30, 2010.
Tandem autologous-allogeneic procedure
Patients included in this study received an induction regimen before tandem autologous-allogeneic SCT according to institutional protocols or IFM guidelines. A treatment line before tandem autologous-allogeneic SCT was defined as an induction regimen which did not include an autologous transplantation. Autologous stem cells were mobilized using granulocyte colony-stimulating factor with or without intermediate-dose (3 g/m2) cyclophosphamide depending on the policy of the participating center. After peripheral blood stem cell collection, patients underwent autologous SCT prepared by high-dose chemotherapy (melphalan 200 or 140 mg/m2 according to renal function). A tandem SCT performed after a single line of treatment, i.e. with no preceding autologous SCT, was defined as an upfront tandem SCT. Patients were considered to have chemosensitive disease if they had a complete response, very good partial response, partial response or minimal response, whereas they were considered to have refractory disease if they had either stable disease or progressive disease. Supportive care for all transplants was performed according to each centers’ policy.
Assessment of response
Based on clinical and laboratory data recorded in the SFGM-TC database and collected from participating centers for the purpose of this analysis, disease response was evaluated according to IMWG criteria21 before the autologous SCT, between the autologous and allogeneic transplants of the tandem, and within 6 months after completion of the planned tandem procedure (best response after allogeneic SCT).
Study end-points
The primary end-points were the overall response rate, event-free survival, overall survival and transplant-related mortality following completion of the whole tandem procedure. We also assessed engraftment, chimerism, and acute and chronic GVHD. Peripheral blood stem cell or bone marrow donor-recipient chimerism was determined after the allogeneic SCT at the usual days and as clinically indicated, through analysis of DNA microsatellite polymorphisms by polymerase chain reaction in sex-matched cases and through conventional cytogenetic analysis by G-banding or FISH studies for the Y-chromosome in sex-mismatched cases. GVHS was diagnosed and clinically graded according to established criteria.22,23 Patients who had successful engraftment and survived for at least 4 weeks were evaluable for acute GVHD, whereas patients surviving for at least 100 days were evaluable for chronic GHVD. Transplant-related mortality was defined as deaths related to the allogeneic transplant of the tandem procedure.
Statistical analysis
Overall survival was calculated from the time of inclusion into the study (i.e. from the date of the allogeneic transplant of the tandem procedure) until the date of death from any cause. Data on patients who did not die were censored on the last date they were known to be alive. Event-free survival was calculated from the date of allogeneic transplantation until the date of disease progression, disease relapse, or death or the date of the last visit. The probabilities of overall and event-free survival were estimated using the Kaplan-Meier method. The occurrence of transplant-related mortality and GVHD was estimated by the cumulative incidence method. The impact of several variables on overall survival, event-free survival and transplant-related mortality was tested using log-rank tests. The factors found to be statistically significant (P≤0.05) in univariate analyses were entered into a stepwise Cox model to determine their independent contribution to survival (overall survival, EFS, transplant-related mortality).24
Results
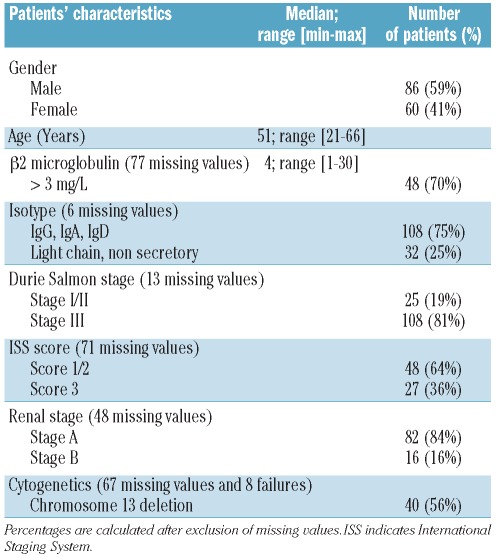
Patients’ characteristics at diagnosis (Table 1)
Table 1.
Patients’ characteristics at diagnosis.
Forty patients (56%) displayed del13. In 34 patients, this aberration was isolated, in five patients the del13 was accompanied by t(4;14) and one patient had both del13 and del17.
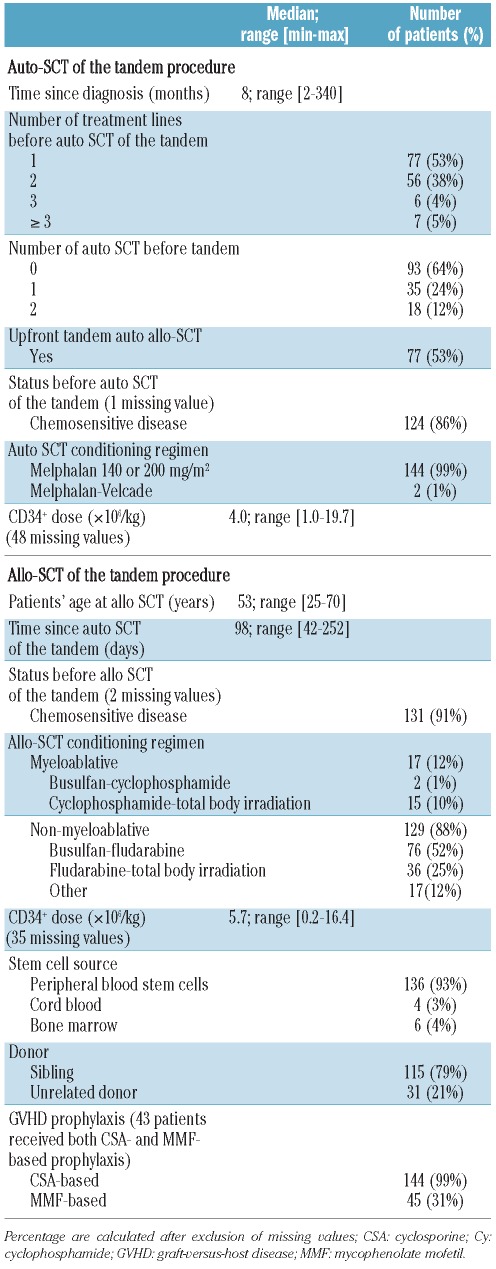
Characteristics of the tandem autologous-allogeneic stem cell transplantation (Table 2)
Table 2.
Characteristics of the tandem autologous (auto)-allogeneic (allo) SCT.
The median time between diagnosis and the autologous transplant of the tandem procedure was 8 months (range, 2–340 months); for two patients with Durie-Salmon stage I MM, the median was >300 months. After several years with no therapy, these two patients required late treatment because of evolving MM. Among the 93 patients (64%) who received the autologous SCT of the tandem as their first autologous-SCT, 77 (53%) received tandem autologous-allogeneic SCT as upfront therapy. One hundred and twenty-four patients (86%) had chemosensitive disease before the tandem SCT. Induction treatment consisted of the vincristine, doxorubicin and dexamethasone (the VAD regimen) in 124 patients (85%), whereas the remaining patients received bortezomib-based treatments. All except two patients received high-dose chemotherapy (melphalan 140 or 200 mg/m2) followed by autologous stem cell support. For the allogeneic transplant of the tandem procedure, 65 patients (45%) received a conditioning regimen including total body irradiation. T-cell depletion was performed in vivo (with antithymocyte globulin, ATG) for 84 patients (58%) and ex vivo for seven patients (5%). Among the 146 patients of the cohort, one patient received a haploidentical transplantation. HLA and sex mismatches were present in 16 (11%) and 67 (46%) of the patients, respectively. The median age of the donors at allogeneic SCT was 49 years (range, 22–70 years, excluding four patients transplanted with cord blood grafts). No primary or secondary graft failure was observed. One hundred and twenty-five of the patients (86%) achieved full donor chimerism in the first 3 months after allogeneic SCT.
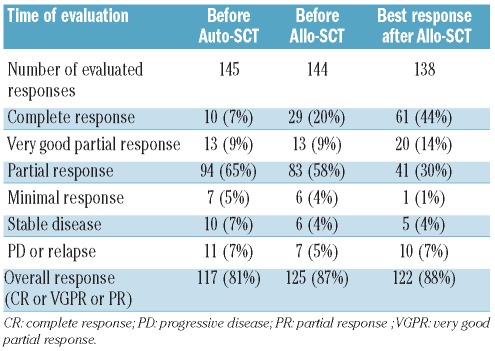
Disease response and relapse (Table 3)
Table 3.
Disease response (IMWG criteria) according to time of evaluation.
The overall response rate was 87% before allogeneic SCT, including 29 patients (20%) with a complete response, 13 patients (9%) with a very good partial response and 83 patients (58%) with a partial response. After allogeneic SCT, the overall response rate reached 88%, including 61 patients (44%) with a complete response, 20 patients (14%) with a very good partial response and 41 patients (30%) with a partial response. Due to the retrospective nature of the study, the time of achievement of the best response after allogeneic SCT could not be assessed precisely. Of the four patients who had cord blood transplants, all were alive at the time of analysis: one had a complete response, two had very good partial responses and one had a partial response. The haploidentical transplant recipient died 1 month after the tandem procedure from a viral infection. Sixty-six patients (46%) have relapsed after allogeneic SCT. Among them, 29 patients (20%) received donor lymphocyte infusions (DLI) as treatment for the relapse, with a median time from allogeneic SCT of 10 months (range, 3–76 months). In 25 cases the DLI were from a related donor and in four cases from an unrelated donor. Nine patients who received DLI are in complete response at the current follow-up. Treatment of relapse other than DLI was not recorded in the database. At the time of statistical analysis, 77 patients (53%) of the cohort are alive. Among them, 72 patients are alive without relapse: 44 patients have a complete response, three have a very good partial response and seven have a partial response.
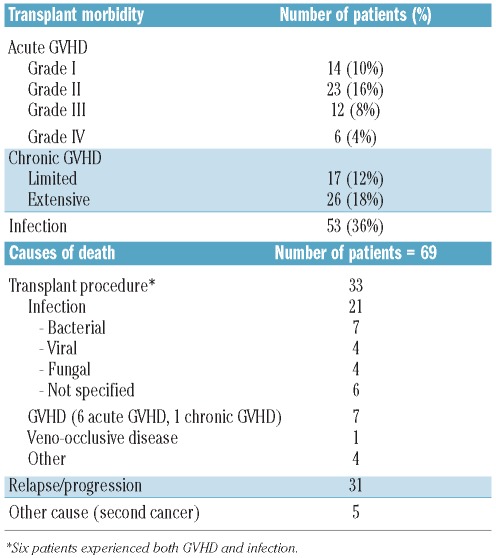
Morbidity and causes of death after tandem stem cell transplantation (Table 4)
Table 4.
Morbidity and causes of death after tandem autologous-allogeneic-SCT.
Acute GVHD developed in 55 patients (38%): 37 patients (26%) had grade I–II acute GVHD and 18 (12%) had grade III–IV GVHD which was the cause of death in six patients. Chronic GVHD was evaluable in 141 patients. Forty-three patients (30%) developed chronic GVHD: 17 patients had limited disease (12%) and 26 (18%) had extensive disease. Extensive chronic GVHD was fatal in one patient. Infection developed in 53 patients (36%). Transplant-related mortality rates at day 100 and 1 year after allogeneic SCT were 6.3% (95% CI 3.4–11.5) and 15% (95% CI 10–22), respectively. Among tandem-related complications, 21 patients died of infection, including seven bacterial, four viral and four fungal infections. Seven patients died of GVHD (6 acute GVHD and 1 chronic GVHD) and one patient died of veno-occlusive disease. Five patients died from a second hematologic disease or cancer, including one acute myeloid leukemia, one B-cell lymphoma and one pancreatic cancer. For two patients, the type of second cancer was not specified. The median follow-up after the tandem procedure was 47.5 months (range, 1.2–132 months). In univariate analysis, younger donor’s age was significantly associated with a reduced transplant-related mortality, with a median transplant-related mortality of 8% (95% CI 3–16) in patients ≤50 years versus 24% (95% CI 15–36) in patients >50 years (P=0.006). Online Supplementary Table S1A summarizes the results of univariate analysis for transplant-related mortality. Two independent factors were found to be predictive of transplant-related mortality after tandem autologous-allogeneic SCT in the multivariable Cox’s regression models. Donor’s age >50 years was associated with a higher risk of transplant-related mortality [hazard ratio (HR)=3.86, 95% CI 1.49–9.97; P=0.006]. The number of autologous transplants before the tandem procedure was also an independent predictor of higher risk of transplant-related mortality (HR=1.91, 95% CI 1.15–3.19; P=0.01). Online Supplementary Table S1B summarizes the results of multivariable analysis for transplant-related mortality.
Survival
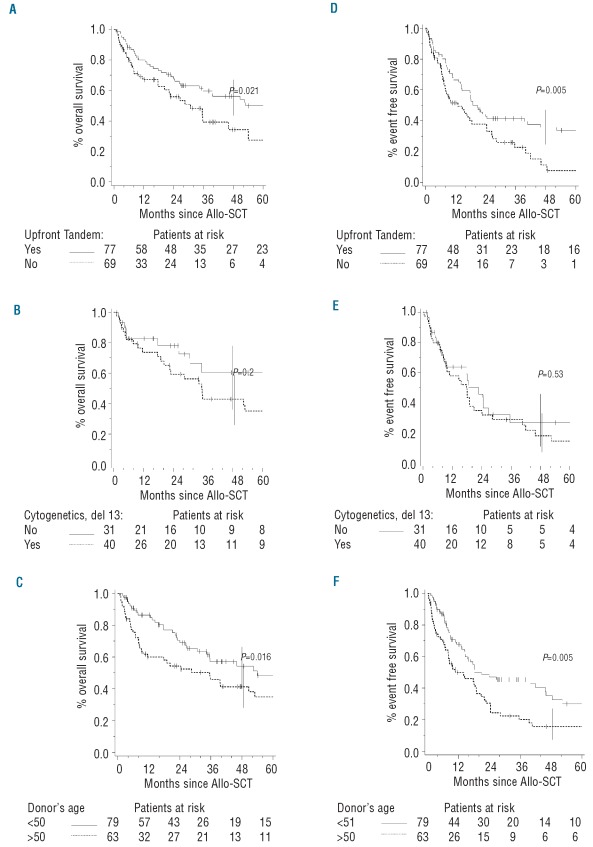
The post-tandem event-free survival rate reached 27% (95% CI 19–36). The event-free survival curve demonstrates a continuous risk of disease recurrence with no plateau. The post-tandem overall survival rate was 48% (95% CI 39–57) (Figure 1). Younger patient’s age at diagnosis (≤50 years) was associated with better survival (P=0.05 for overall survival and P=0.02 for event-free survival). The median overall survival of patients for whom the tandem transplants were performed as an upfront procedure was significantly better: 56% (95% CI 45–67) versus 34% (95% CI 21–50) (P=0.02) (Figure 2A). As for overall survival, the median event-free survival of patients transplanted upfront was significantly higher (36%; 95% CI 26–47) than that in patients in whom the procedure was not upfront (11%; 95% CI 4–26) (P=0.005) (Figure 2D). Univariate analyses for overall and event-free survival did not show any significant difference according to cytogenetics (presence or absence of del13) (Figure 2BE). Overall survival for patients without del13 was 60% (95% CI 40–78) versus 43% (95% CI 28–59) for those with del13 (P=0.2). Event-free survival for patients without del13 reached 26% (95% CI 13–46) versus 18% (95% CI 8–34) for those with del13 (P=0.53). No statistically significant difference in survival was observed according to the type of donor (related or unrelated): 51% (95% CI 41–60) versus 31% (95% CI 14–56) for overall survival and 26% (95% CI 18–36) versus 27% (95% CI 12–50) for event-free survival. As for the type of donor, no statistically significant difference in overall or event-free survival was observed depending on the type of allogeneic conditioning regimen (myeloablative or non-myeloablative) (Online Supplementary Table S1A). Younger donor’s age was significantly associated with a better overall survival: median 54% (95% CI 41–67) for patients ≤50 years versus 41% (95% CI 29–55) for patients >50 years (P=0.02) (Figure 2C). Younger donor’s age was also associated with an improved event-free survival: median 35% (95% CI 24–48) for patients ≤50 years versus 16% (95% CI 8–28) for patients >50 years (P=0.005) (Figure 2F). Online Supplementary Table S1A summarizes the results of the univariate analyses for overall and event-free survival. Two independent factors were found to be predictive for both overall and event-free survival after the tandem autologous-allogeneic SCT in the multivariable Cox’s regression models. Donor’s age >50 years was associated with a worse overall survival (HR=1.99, 95% CI 1.22–3.25; P=0.006). Higher donor’s age was also associated with a worse event-free survival (HR=2.13, 95% CI 1.40–3.25; P=0.0004). Achievement of full chimerism after the tandem SCT was associated with a better outcome (overall survival: HR=0.46, 95% CI 0.25–0.86; P=0.02; event-free survival: HR=0.35, 95% CI 0.2–0.61; P=0.0002). Online Supplementary Table S1B summarizes the results of multivariable analysis for overall and event-free survival.
Figure 1.
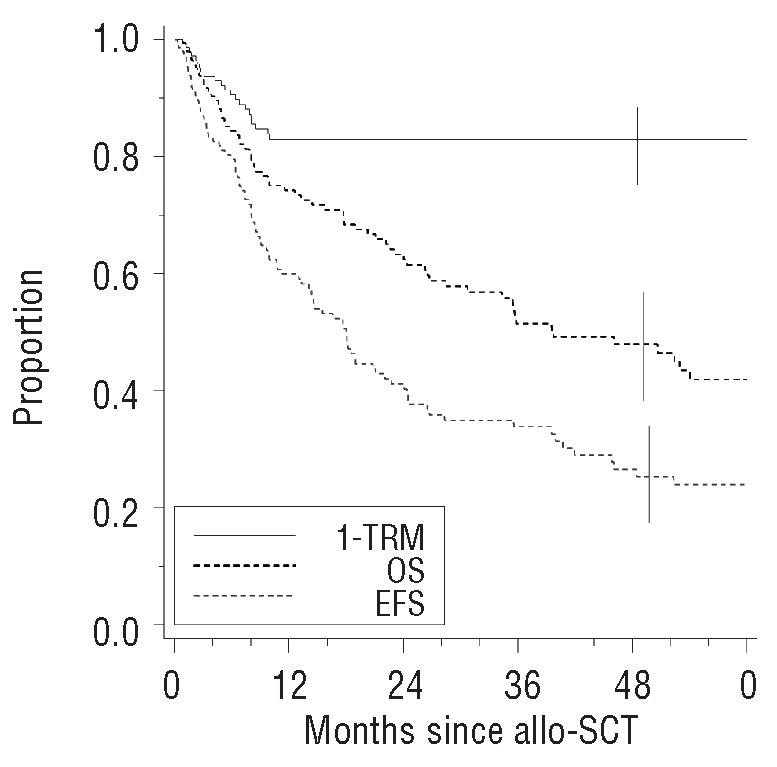
Kaplan-Meier estimates of overall survival (OS), event-free survival (EFS) and 1 minus the transplant-related mortality (1-TRM) following tandem autologous-allogeneic (allo)-SCT.
Figure 2.
Overall survival (OS) and event-free survival (EFS) according to the status of the tandem procedure, cytogenetics and donor’s age. (A) OS according to the status of tandem autologous (auto) - allogeneic (allo)-SCT. (B) OS according to the presence of del13. (C) OS according to donor’s age. (D) EFS according to the status of tandem auto-allo-SCT. (E) EFS according to the presence of del13. (F) EFS according to donor’s age.
Discussion
We identified an upfront procedure as a significant factor for improved survival after tandem autologous-allogeneic SCT. When tandem SCT was performed as an upfront procedure, the event-free survival was 36% (95% CI 26–49) versus 11% (95% CI 4–26) when it was not employed upfront (P=0.005). In our multivariable analysis, upfront tandem was an independent factor for better event-free survival (HR=0.56, 95% CI 0.37–0.85; P=0.006). In a prospective setting, for 100 newly diagnosed MM patients undergoing tandem autologous-allogeneic SCT, Bruno reported 5-year overall and event-free survival rates of 65% and 40%, respectively, with a median follow-up of 5 years.11 In a small retrospective series of 23 relapsed MM patients, Karlin showed the feasibility of tandem transplantation with a 2-year overall survival rate of 61%.25 However, the median follow-up was short (27.4 months) and the recently published EBMT study has emphasized the need for a prolonged follow-up to determine the benefit on survival of tandem autologous-allogeneic SCT.18 Finally, in the Seattle series of 102 MM patients, 5-year overall and progression-free survival rates were 64% and 36%, respectively, with a median follow-up of 6.3 years.8 In this series, only 20% of the patients had received more than one induction treatment for relapsed/refractory MM with no autologous transplantation prior to the tandem autologous-allogeneic SCT. In the multivariable analysis, a period of more than 10 months between starting treatment and the autologous transplant of the tandem procedure was correlated with shorter survival, but differences according to the number of treatment lines before the tandem procedure failed to reach statistical significance. In our series, 47% of the patients had received more than one line of treatment before the tandem SCT. Our results suggest that considering tandem autologous-allogeneic SCT early in the course of MM should further improve the benefit of tandem transplantation in the long-term control of the disease.
The incidences of acute and chronic GVHD were low in our cohort. In the Seattle series,8 among 102 patients transplanted after 2 Gy total body irradiation with or without fludarabine, 42% experienced grade II–IV acute GVHD and 74% extensive chronic GVHD. In the PETHEMA study,15 among 25 patients receiving allogeneic SCT from HLA sibling donors after fludarabine and melphalan, the incidence of grade II–IV acute GVHD was 32%, and the incidence of chronic GVHD was 66%. The low incidence of GVHD in our study was possibly correlated to the important number of patients (91, 62%) experiencing a T-cell depletion procedure, either ex vivo (7 patients) or in vivo with ATG (84 patients, 58%). The incidence of GVHD we report here is similar to that of the studies including ATG as part of the conditioning regimen.26 In the study published by Kröger,10 patients received a regimen based on fludarabine, melphalan and ATG (rabbit, Fresenius) (30 mg/kg). The incidence of grade II–III acute GVHD was 38% and that of chronic GVHD 40%. In the French IFM study,12,13 46 MM patients in the autologous-allogeneic SCT arm received busulfan, fludarabine and ATG. Patients received ATG (rabbit, Genzyme) at a dose of 2.5 mg/kg for 5 days. The incidence of grade II–IV acute GVHD was 23.9% and that of extensive chronic GVHD 35.7%. Compared to the IFM study, we used a reduced dose of ATG at 2.5 mg/kg for 2 or 3 days. However, we did not observe a higher rate of GVHD. Beside its low incidence, GVHD was rarely fatal in our study with seven deaths related to GVHD (6 acute GVHD and 1 chronic GVHD).
In our series the transplant-related mortality rate at 1 year was 15%. In the relapse setting, Karlin reported on a single-center experience of tandem autologous-allogeneic SCT in 23 MM patients:25 the 1-year transplant-related mortality rate was 17%. Our cohort included heavily treated patients with 53 (36%) having had a prior autologous SCT before the tandem procedure and 69 (47%) having been treated with more than one line of therapy before the tandem SCT. In the Seattle series,8,9 with a median follow-up of 6.3 years, transplant-related mortality was 18% but none of the patients had received an autologous SCT prior to the tandem SCT. As for the patients’ characteristics, the transplant characteristics in our study put patients at higher risk, with 17 patients (12%) having received myeloablative allogeneic conditioning and 31 patients (21%) transplanted from unrelated donors. In the studies mentioned above, MM patients received a non-myeloablative conditioning regimen based on 2 Gy total body irradiation. Furthermore, all patients, except 10 among 23 in the study by Karlin,25 were transplanted from HLA-identical sibling donors. In our univariate analysis, the number of autologous SCT before the tandem autologous-allogeneic SCT was a significant factor for transplant-related mortality (P=0.03). There was a trend for a lower transplant-related mortality when the tandem SCT was performed as an upfront procedure, with a transplant-related mortality of 10% (95% CI 5–20) versus 20% (95% CI 12–31) (P=0.08). The transplant-related mortality of patients who had not had a prior autologous SCT or had had one prior autologous SCT was not different (12 and 14%, respectively), whereas the transplant-related mortality rate was much higher (33%) for patients who had had two autologous SCT prior to the tandem procedure. These results could explain the lack of significance of upfront tandem for transplant-related mortality. Collectively, our results confirm the feasibility of tandem autologous-allogeneic SCT in a highly pretreated cohort.
In our cohort, 48 patients (70%) had a high β2 microglobulin (>3 mg/L). This parameter was not significantly associated with a poorer survival. In the Seattle series,8,9 only 44% of the patients had a high β2 microglobulin (>3.5 mg/L). In multivariable analysis, the authors identified β2 microglobulin >3.5 mg/L at diagnosis as a predictive factor of worse outcome (P=0.03 for overall survival; P=0.04 for progression-free survival). No firm conclusion could be drawn on the prognostic impact of del13, given the limited FISH data (18 patients). In our study, the presence of del13 was not predictive of a worse outcome with an overall survival of 43% (95% CI 28–59) when del13 was present versus 60% (95% CI 40–78) when it was not (P=0.2). Recently, Gahrton reported a better survival for patients with del13 who received tandem autologous-allogeneic SCT compared to those managed with autologous SCT.18 Our results are in accordance with those previously published by the IFM.12,13 In the IFM 99.03 study, no survival benefit was demonstrated in the tandem autologous-allogeneic SCT arm and the authors concluded that the tandem procedure might be best indicated for standard-risk patients. Furthermore, all patients in the two above studies and 90% in the Seattle series had received anthracycline-based induction (VAD) or similar treatment. Based on the results of the IFM 2005 study showing the superiority of bortezomib-dexamethasone (VD) with regards to response, regardless of adverse cytogenetics, VAD has been progressively replaced by VD.27,28 Therefore, 14 patients (10%) in our cohort received VD as induction, which could have contributed to the lack of significance of del13 on survival after tandem autologous-allogeneic-SCT.
We identified donor’s age as a significant factor for survival after tandem. When the donor’s age was ≤50 years, overall survival was 54% (95% CI 41–67) versus 41% (95% CI 29–55) (P=0.02). In multivariable analysis, donor’s age >50 years was an independent factor for worse overall survival (HR=1.99, 95% CI 1.22–3.25; P=0.006). When donor’s age was >50 years, the 1-year transplant-related mortality was 24% (95% CI 15–36) versus 8% (95% CI 3–16) for the older donors (P=0.006). No correlation was found between donor’s age and allogeneic conditioning regimen (myeloablative or not), donor type (sibling or unrelated) and HLA mismatches (data not shown). In our cohort, older donor’s age failed to be associated to with an increased incidence of GVHD or decreased cellularity of the CD34+ allogeneic stem cell source (data not shown). Donor’s age has been previously studied in both myeloablative and non-myeloablative allogeneic transplant conditioning settings.29,30 It has been hypothesized that as a donor’s age increases, both repopulation and homing abilities of the donor stem cells become impaired.31,32 Donor’s T lymphocytes are also affected by telomere shortening and decreased effector activity.33 Altogether, immune reconstitution in recipients of allogeneic transplants from younger donors is better. The reasons for improved survival with a younger donor and potential link with the graft-versus-myeloma effect could not be further clarified in our study. Our results emphasize that donor’s age should be a key criterion when selecting MM patients for tandem autologous-allogeneic SCT.
Here we have reported the French experience on the largest series published on tandem autologous-allogeneic SCT in MM. In multivariable analysis, an upfront tandem procedure and donor’s age ≤50 years were identified as two independent prognostic factors for improved survival. With the limits of a retrospective study, we underline that both patients’ pre-transplant characteristics (tandem as an upfront procedure) and intrinsic properties of the stem cell source (donor’s age) are important for the outcome of the tandem SCT. Finally, our study was initiated before the introduction of induction and maintenance regimens based on novel agents. The prognostic impact of these factors does, therefore, have to be further confirmed in prospective studies including the new MM induction treatments34 and post-transplant maintenance strategies.35
Supplementary Material
Acknowledgments
The authors would like to thank the Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC) data manager Nicole Raus for her helpful assistance. We thank Pr. Hervé Avet-Loiseau (Centre Hospitalier Universitaire Hôtel-Dieu, Laboratoire d’Hématologie Nantes, 44093, France) for providing missing cytogenetic data.
Appendix
The following additional centers and investigators from the Société Française de Greffe de Moelle et de Thérapie Cellulaire participated in this study; Pr. C. Berthou, Centre Hospitalier Universitaire Hôpital Morvan, Institut de Cancérologie et d’Hématologie, Brest, France; Pr. J-O. Bay, Centre Hospitalier Universitaire Estaing, Service de Thérapie Cellulaire et d’Hématologie Clinique, Clermont-Ferrand, France; Pr. C. Cordonnier, Hôpital Henri-Mondor, Assistance Publique-Hôpitaux de Paris, Service d’Hématologie Clinique, Créteil, France; Dr. L. Fouillard, Hôpital Saint-Antoine, Assistance Publique-Hôpitaux de Paris, Département d’Hématologie Clinique et Thérapie Cellulaire, Paris, France; Dr. B. Lioure, Centre Hospitalier Universitaire Hôpital de Hautepierre, Service d’Hématologie et Oncologie, Strasbourg, France.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B. Graft-versus-myeloma effect: proof of principle. Blood. 1996;87(3):1196–8. [PubMed] [Google Scholar]
- 2.Aschan J, Lonnqvist B, Ringden O, Kumlien G, Gahrton G. Graft-versus-myeloma effect. Lancet. 1996;348(9023):346. doi: 10.1016/s0140-6736(05)64525-4. [DOI] [PubMed] [Google Scholar]
- 3.Carella AM, Cavaliere M, Lerma E, Ferrara R, Tedeschi L, Romanelli A, et al. Autografting followed by nonmyeloablative immunosuppressive chemotherapy and allogeneic peripheral-blood hematopoietic stem-cell transplantation as treatment of resistant Hodgkin's disease and non-Hodgkin's lymphoma. J Clin Oncol. 2000;18(23):3918–24. doi: 10.1200/JCO.2000.18.23.3918. [DOI] [PubMed] [Google Scholar]
- 4.Carella AM, Beltrami G, Corsetti MT, Scalzulli P, Carella AM, Jr, Musto P. A reduced intensity conditioning regimen for allografting following autografting is feasible and has strong anti-myeloma activity. Haematologica. 2004;89(12):1534–6. [PubMed] [Google Scholar]
- 5.Bjorkstrand BB, Ljungman P, Svensson H, Hermans J, Alegre A, Apperley J, et al. Allogeneic bone marrow transplantation versus autologous stem cell transplantation in multiple myeloma: a retrospective case-matched study from the European Group for Blood and Marrow Transplantation. Blood. 1996;88(12):4711–8. [PubMed] [Google Scholar]
- 6.Vesole DH, Zhang L, Flomenberg N, Greipp PR, Lazarus HM, Huff CA. A phase II trial of autologous stem cell transplantation followed by mini-allogeneic stem cell transplantation for the treatment of multiple myeloma: an analysis of Eastern Cooperative Oncology Group ECOG E4A98 and E1A97. Biol Blood Marrow Transplant. 2009;15(1):83–91. doi: 10.1016/j.bbmt.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroger N. Autologous-allogeneic tandem stem cell transplantation in patients with multiple myeloma. Leuk Lymphoma. 2005;46(6):813–21. doi: 10.1080/10428190500080850. [DOI] [PubMed] [Google Scholar]
- 8.Rotta M, Storer BE, Sahebi F, Shizuru JA, Bruno B, Lange T, et al. Long-term outcome of patients with multiple myeloma after autologous hematopoietic cell transplantation and nonmyeloablative allografting. Blood. 2009;113(14):3383–91. doi: 10.1182/blood-2008-07-170746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maloney DG, Molina AJ, Sahebi F, Stockerl-Goldstein KE, Sandmaier BM, Bensinger W, et al. Allografting with non-myeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003;102(9):3447–54. doi: 10.1182/blood-2002-09-2955. [DOI] [PubMed] [Google Scholar]
- 10.Kroger N, Schwerdtfeger R, Kiehl M, Sayer HG, Renges H, Zabelina T, et al. Autologous stem cell transplantation followed by a dose-reduced allograft induces high complete remission rate in multiple myeloma. Blood. 2002;100(3):755–60. doi: 10.1182/blood-2002-01-0131. [DOI] [PubMed] [Google Scholar]
- 11.Bruno B, Rotta M, Patriarca F, Mattei D, Allione B, Carnevale-Schianca F, et al. Nonmyeloablative allografting for newly diagnosed multiple myeloma: the experience of the Gruppo Italiano Trapianti di Midollo. Blood. 2009;113(14):3375–82. doi: 10.1182/blood-2008-07-167379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garban F, Attal M, Michallet M, Hulin C, Bourhis JH, Yakoub-Agha I, et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood. 2006;107(9):3474–80. doi: 10.1182/blood-2005-09-3869. [DOI] [PubMed] [Google Scholar]
- 13.Moreau P, Garban F, Attal M, Michallet M, Marit G, Hulin C, et al. Long-term follow-up results of IFM99-03 and IFM99-04 trials comparing nonmyeloablative allotransplantation with autologous transplantation in high-risk de novo multiple myeloma. Blood. 2008;112(9):3914–5. doi: 10.1182/blood-2008-07-168823. [DOI] [PubMed] [Google Scholar]
- 14.Bruno B, Rotta M, Patriarca F, Mordini N, Allione B, Carnevale-Schianca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356(11):1110–20. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 15.Rosinol L, Perez-Simon JA, Sureda A, de la Rubia J, de Arriba F, Lahuerta JJ, et al. A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood. 2008;112(9):3591–3. doi: 10.1182/blood-2008-02-141598. [DOI] [PubMed] [Google Scholar]
- 16.Stadtmauer E, Krishnan A, Pasquini M, Ewell M, Alyea E, Antin J, et al. Tandem autologous stem cell transplants (auto-auto) with or without maintenance therapy versus single autologous transplant followed by HLA-Matched sibling non-myeloablative allogeneic stem cell transplant (auto-allo) for patients (pts) with high risk (HR) multiple myeloma (MM): results from the Blood and Marrow Transplant Clinical Trials Network (BMT-CTN) 0102 Trial. ASH Annual Meeting Abstracts. 2010;116:526. [Google Scholar]
- 17.Krishnan A, Pasquini M, Ewell M, Stadtmauer E, Alyea E, Antin J, et al. Tandem autologous hematopoietic stem cell transplants (AuHCT) with or without maintenance therapy (auto-auto) versus single AuHCT followed by HLA matched sibling non-myeloablative allogeneic HCT (auto-allo) for patients with standard risk (SR) multiple myeloma (MM): results from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0102 Trial. ASH Annual Meeting. Abstracts. 2010;116:41. [Google Scholar]
- 18.Bjorkstrand B, Iacobelli S, Hegenbart U, Gruber A, Greinix H, Volin L, et al. Tandem autologous/reduced-intensity conditioning allogeneic stem-cell transplantation versus autologous transplantation in myeloma: long-term follow-up. J Clin Oncol. 2011;29(22):3016–22. doi: 10.1200/JCO.2010.32.7312. [DOI] [PubMed] [Google Scholar]
- 19.Knop SLP, Hebart H, et al. Allogeneic stem cell transplant versus tandem high-dose melphalan for front-line treatment of deletion 13q14 myeloma: an interim analysis of the German DSMM V trial. Blood. 2009;114:Abstract 51. [Google Scholar]
- 20.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International Staging System for multiple myeloma. J Clin Oncol. 2005;23(15):3412–20. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 21.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 22.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–8. [PubMed] [Google Scholar]
- 23.Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28(3):250–9. [PubMed] [Google Scholar]
- 24.Lin DY. Cox regression analysis of multivariate failure time data: the marginal approach. Stat Med. 1994;13(21):2233–47. doi: 10.1002/sim.4780132105. [DOI] [PubMed] [Google Scholar]
- 25.Kroger N, Sayer HG, Schwerdtfeger R, Kiehl M, Nagler A, Renges H, et al. Unrelated stem cell transplantation in multiple myeloma after a reduced-intensity conditioning with pretransplantation antithymocyte globulin is highly effective with low transplantation-related mortality. Blood. 2002;100(12):3919–24. doi: 10.1182/blood-2002-04-1150. [DOI] [PubMed] [Google Scholar]
- 26.Karlin L, Arnulf B, Chevret S, Ades L, Robin M, De Latour RP, et al. Tandem autologous non-myeloablative allogeneic transplantation in patients with multiple myeloma relapsing after a first high dose therapy. Bone Marrow Transplant. 2011;46(2):250–6. doi: 10.1038/bmt.2010.90. [DOI] [PubMed] [Google Scholar]
- 27.Harousseau JL, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28(30):4621–9. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 28.Jagannath S, Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, et al. Bortezomib appears to overcome the poor prognosis conferred by chromosome 13 deletion in phase 2 and 3 trials. Leukemia. 2007;21(1):151–7. doi: 10.1038/sj.leu.2404442. [DOI] [PubMed] [Google Scholar]
- 29.Mehta J, Gordon LI, Tallman MS, Winter JN, Evens AM, Frankfurt O, et al. Does younger donor age affect the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies beneficially? Bone Marrow Transplant. 2006;38(2):95–100. doi: 10.1038/sj.bmt.1705388. [DOI] [PubMed] [Google Scholar]
- 30.Kollman C, Howe CW, Anasetti C, Antin JH, Davies SM, Filipovich AH, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043–51. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 31.Harrison DE, Astle CM. Loss of stem cell repopulating ability upon transplantation. Effects of donor age, cell number, and transplantation procedure. J Exp Med. 1982;156(6):1767–79. doi: 10.1084/jem.156.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106(4):1479–87. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamminga LM, van Os R, Ausema A, Noach EJ, Weersing E, Dontje B, et al. Impaired hematopoietic stem cell functioning after serial transplantation and during normal aging. Stem Cells. 2005;23(1):82–92. doi: 10.1634/stemcells.2004-0066. [DOI] [PubMed] [Google Scholar]
- 34.Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116(5):679–86. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van de Donk NW, Kroger N, Hegenbart U, Corradini P, San Miguel JF, Goldschmidt H, et al. Remarkable activity of novel agents bortezomib and thalidomide in patients not responding to donor lymphocyte infusions following nonmyeloablative allogeneic stem cell transplantation in multiple myeloma. Blood. 2006;107(8):3415–6. doi: 10.1182/blood-2005-11-4449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.