Abstract
The concept of the cardiovascular continuum, introduced during the early 1990s, created a holistic view of the chain of events connecting cardiovascular-related risk factors with the progressive development of pathological-related tissue remodelling and ultimately, heart failure and death. Understanding of the tissue-specific changes, and new technologies developed over the last 25–30 years, enabled tissue remodelling events to be monitored in vivo and cardiovascular disease to be diagnosed more reliably than before. The tangible product of this evolution was the introduction of a number of biochemical markers such as troponin I and T, which are now commonly used in clinics to measure myocardial damage. However, biomarkers that can detect specific earlier stages of the cardiovascular continuum have yet to be generated and utilised. The majority of the existing markers are useful only in the end stages of the disease where few successful intervention options exist. Since a large number of patients experience a transient underlying developing pathology long before the signs or symptoms of cardiovascular disease become apparent, the requirement for new markers that can describe the early tissue-specific, matrix remodelling process which ultimately leads to disease is evident. This review highlights the importance of relating cardiac biochemical markers with specific time points along the cardiovascular continuum, especially during the early transient phase of pathology progression where none of the existing markers aid diagnosis.
Keywords: biomarkers, cardiovascular disease, extracellular matrix remodeling, ECMr, diagnostic markers, cardiovascular continuum, biomarker continuum, cardiac matrikine
Cardiovascular Continuum (CVC) and Biomarkers
The evidence-based concept of a cardiovascular continuum (CVC) introduced in 1991 by Dzau and Braunwald ingeniously described the vast number of different and diverse tissue remodelling processes that gradually lead to cardiovascular-related pathology, heart failure and death. Further validation and expansion of the CVC model was performed through pathophysiology and clinical trial evidence1,2 which highlighted the impact of risk factors related to cardiovascular disease (CVD), such as cigarette smoking and diabetes, in the initiation of the CVC vicious circle which was also extended to include other affected organs such as the brain and the kidney. A key remark by Dzau et al in recent CVC validation work2 stresses the value of biomarkers for risk assessment, early diagnosis and prognosis while emphasising that markers that may also act as mediators of disease. Undoubtedly, a biomarker or a panel of markers which could facilitate stratification of patients in the appropriate CVC segment could prove invaluable in a clinical setting, as it would allow early intervention at the beginning of the CVC where prevention may be possible.3–5 However, existing biomarkers cannot fully realize this goal as only a handful of these, mainly troponin, have been found to be cardiac specific and even then, are only useful in detecting myocardial damage in the late stages of CVD, in what has recently been described as the vascular aging continuum (VAC).6 The vast majority of other biomarkers seem to be up-or down-regulated in non CVD-related pathologies. At this time, the selection of biomarkers that can reliably facilitate prognosis during the early stages of CVC is limited (Fig. 1). An overview of frequently used CVD biomarkers is presented in this paper to underline some of the strengths and weaknesses that these have, and propose an approach for future, novel biomarker development.
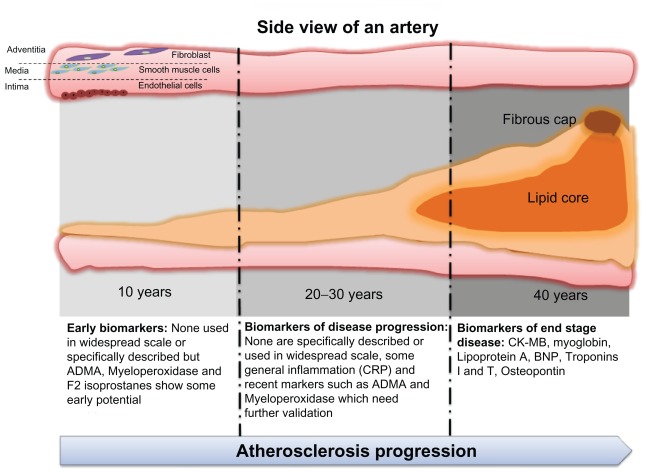
Figure 1.
Existing biomarkers are valuable diagnostic and monitoring tools mainly for the end stages of cardiovascular disease.
Notes: There is currently a lack of biomarkers that can reliably describe the transient, underlying abnormal extracellular matrix remodelling (ECMR) which ultimately leads to cardiovascular-related pathology. The illustration of atherosclerosis progression and the lack of early biomarkers of atheromatic formation is indicative of this unmet need. The timeline of atheromatic formation is suggestive of the large extent of matrix remodelling which takes place over decades and remains unmonitored for the large part. Accurate monitoring of early cardiac ECMR could prompt early intervention and prevention of disease progression.
Clinically Relevant Cardiac Markers
Creatinine kinase (CK) and CK-MB
Creatinine kinase MB is an enzyme present primarily in cardiac muscle. The MB is one of the three CK isoenzymes the other being the MM and BB. CK-MB is released rapidly after myocardial injury.7 During an onset of acute myocardial infarction (AMI), CK-MB rises to twice the normal levels within 6 hours and peaks within 12–24 hours.8–10 Serial CK-MB mass measurements have a nearly 90% sensitivity of AMI three hours after a patient is first assessed in a hospital emergency department, which equates to approximately 6 hours after symptom onset, but these measurements are only 36%–48% sensitive when used at, or shortly after, presentation.9,11 CK-MB plays an important role in defining the infarct size, expansion and risk of re-infarction. If a cTn is not available, the CK-MB is considered the best alternative marker of AMI. Decades ago, elevated serum levels of CK-MB, the cardiac-specific isoform of CK, were also used as biomarkers for the diagnosis of myocardial necrosis. This measure satisfied one component of the diagnostic criteria for MI, as proposed by the World Health Organization, and its use was later extended to monitor trends and determinants in a cardiovascular disease study.12 Even though the CK-MB has been proven a relatively sensitive measure of myocardial necrosis and AMI, this enzyme is not exclusively specific to myocardial damage, as elevated levels in several conditions following acute or chronic muscle injury and in patients undergoing surgical procedures, have been found.13 Furthermore, CK is present in the intestine, diaphragm, uterus and prostate, and injury to these organs would result in release of CK-MB and thus impair the specificity of CK-MB serum measurements. In order increase the specificity of CK-MB measurements and thereby distinguish the “true positive” serum elevations secondary to myocardial injury from the “false positive” elevations due to other tissue injury, the measurement of CK-MB as a percentage of total CK has been used. There is no clear consensus on whether absolute CK-MB or the CK-MB relative index is the preferred test for patients with potential acute coronary syndromes, but the World Health Organization international diagnostic criteria, and several others, recommend use of absolute CK-MB.14
Myoglobin
Myoglobin is a relatively small, 17.8 kDa, heme protein that is abundant in the cytoplasm of cardiac and skeletal muscle cells. The main function of myoglobin is to transport oxygen within muscle cells, and it constitutes approximately 2% of muscle protein in both skeletal and cardiac muscles.
The tissue/plasma ratio of myoglobin is very high, and combined with its small size, myoglobin is rapidly released into the circulation upon tissue necrosis and injury. Of the biomarkers routinely collected from patients suspected or diagnosed with CVD, myoglobin is generally accepted as one of the earliest to appear during the development of the disease. Elevated levels following an AMI appear in the circulation after 0.5–2 hours. Since myoglobin is only released as a result of tissue necrosis, it is a poor biomarker of acute cardiac ischemia. Furthermore, myocardial and skeletal muscle myoglobins share 100% homology, thus making this marker tissue unspecific. Myoglobin is cleared by kidneys, and it has been reported that patients suffering from renal insufficiency have increased plasma levels of myoglobin, and thus readings may be falsely high. There is difference of opinion as to whether myoglobin is a useful biomarker in the evaluation of patients with suspected acute coronary syndromes. As assays for measurements of cardiac-specific biomarkers such as cTnI and cTnT have become available, the value of myoglobin as a cardiac biomarker has decreased.15 Current guidelines recommend myoglobin measurements only in patients presenting within 6 hours of chest-pain onset.16 Recent studies have demonstrated that, among patients with ST-elevation myocardial infarction (MI), those with raised myoglobin levels before the initiation of fibrinolytic therapy are at high risk for death and heart failure.17 For patients presenting to the emergency room with chest pain in the absence of ST-elevation, the addition of myoglobin to biomarker panels that include CK-MB and cTnI or cTnT improves sensitivity for the detection of MI, particularly in patients presenting early after symptom onset.8,9,11,15,18,19 Beyond the diagnosis of MI, there are discrepancies as to whether myoglobin is useful for risk-stratification in patients with non–ST-elevation acute coronary syndromes (ACS). One recent study has suggested that myoglobin provides incremental prognostic information to CK-MB and troponin,9 but several others have not reached the same conclusion.8,18,19
Lipoprotein A
Lipoprotein (A) is a low-density lipoprotein (LDL) particle with an apolipoptotein A (apoA) attached. Apo(A) is linked to LDL by a disulfide bond.20 This structure has significant homology to plasminogen, and the enhanced coronary heart disease (CHD) risk associated with Lp(a) is reportedly due to inhibiting the effects of this lipoprotein particle on plasminogen activation, enhancing the risk of thrombosis. Lp(a) may also increase atherogenicity of LDL.21,22 Many observational trials support the association of Lp(a) with enhanced cardiovascular risk.23 In general, it has been postulated that every 30 mg/dL increase in Lp(a) doubles the risk of CHD. Therapeutic modification of Lp(a) is controversial. Only estrogen and niacin have been showed to moderately lower Lp(a).24
Brain natriuretic peptide (BNP)
Measurement of plasma brain natriuretic peptide (BNP) concentration is a very efficient and cost-effective mass screening technique for identifying patients with various cardiac abnormalities, regardless of aetiology.25 BNP is a 32-amino acid polypeptide cardiac neuro-hormone secreted from membrane granules in the cardiac ventricles, particularly the left ventricle, as a response to ventricular volume expansion and pressure overload.25,26 Atrial natriuretic peptide (ANP) and B-type natriuretic peptide (B-NP) are of myocardial cell origin, while C-type natriuretic peptide (CNP) is of endothelial origin.25 BNP was originally named brain natriuretic peptide, and it was first detected in porcine brain.27,28 BNP levels have been found elevated in patients with various clinical conditions such as heart failure, MI, left ventricular hypertrophy, cardiac inflammation, primary pulmonary hypertension, renal failure, ascetic cirrhosis and is associated with advanced age.29 The levels correlate with severity of symptoms and with prognosis, and so it helps to detect the presence of heart failure, determine its severity, and estimate prognosis. BNP has the potential to considerably improve the management of patients with congestive heart failure (CHF) and may become a routinely assessed serum parameter in clinical medicine. BNP is considerably less costly than other tests for CHD, and due to its cost-effectiveness is highly desirable in developing countries. Originally, the US Food and Drug Administration (FDA) approved the use of BNP or NT-proBNP (amino terminal pro-brain natriuretic peptide) to assist in differentiating a cardiac cause (such as congestive heart failure) from a non-cardiac origin (such as chronic obstructive pulmonary disease) for dyspnea. Recently, NT-proBNP was approved by the FDA for use in assessing the prognosis of patients with congestive heart failure and acute coronary syndrome, while the BNP assay is also approved for risk stratification in acute coronary syndrome.30
Troponins I and T
The troponin protein complex consists of 3 subunits, the C (TnC) subunit which is the calcium binding component, the I (TnI) which maintains the structural position of the troponin-tropomyosin complex, and the T (TnT) which is the tropomyosin binding subunit. All are located on the thin filament of both skeletal and myocardial myocytes, the latter playing an integral role in the Frank-Starling mechanism of the heart.31,32 Interestingly, both TnT and TnI sub-units have distinct isoforms for each muscle type, hence there is a specific cardiac isoform.33 Cardiac troponins T and I (cTnT and cTnI) are now recognized as the most tissue-specific biomarkers related to cardiac damage and have been included as a diagnostic criterion for several cardiac-related pathologies.34–39 This success is closely related to the troponins’ unique position and function in the cardiomyocyte and the ability to generate specific monoclonal antibodies against both cTnT and cTnI which are precise tissue-specific biomarkers of myocardial injury that are not detected in healthy individuals.31 Due to the integral role of troponins in myocardial contraction and their success as cardiac-specific markers, the question has risen as to whether troponin-related proteolysis is somehow also implicated in the development of cardiac damage that leads to heart failure, through a gradual procedure which eventually leads to decreased diastolic and systolic function.34 The notion that proteolysis is present early in cardiac disease and can facilitate progress to cardiac damage through troponin degradation has not been yet broadly utilised for the development of proteolytic fragments as cardiac-specific markers.
Osteopontin
Osteopontin (OPN) is a matricellular glycoprotein/cytokine that has been recently found to be a promising prognostic biomarker for patients with heart failure, ischemic heart disease and cardiac remodelling in both clinical and pre-clinical settings.40–44 Even though its expression by macrophages during myocardial necrosis has been reported since 199445 its precise function is not fully understood. OPN has previously been described as a regulator of inflammation and bio-mineralisation via macrophage interaction while also associated with bone remodelling.46–48 OPN has been characterised as an independent predictor of death within 4 years for patients with heart failure and was found highly elevated in patients with left ventricular dysfunction.40 However, OPN is expressed in many tissues and has also been described as a marker in non-CVD related pathologies which include cancer, myeloma, multiple sclerosis, bone destruction, angiogenesis, Graves’ disease and pulmonary hypertension.48–54 This inherently impedes the direct association of OPN up-regulation with the early phase of any of its related pathologies, particularly early CVC, prior to the development of other clinical symptoms that can facilitate reliable diagnosis.
C-reactive protein (CRP)
C-reative protein (CRP) is a non-specific acute-phase reactant protein produced in the liver. It is associated with a variety of diverse functions related to immune reactivity including complement activation, innate immunity and phagocyte stimulation.55 Even though its usefulness was initially greeted with scepticism due to the fact that it has been previously used as an non-specific inflammatory marker,56 it has since been widely used as an acute inflammation marker. It has been found to be a reliable marker for a variety of CVD-related pathologies which include atheromatic plaque vulnerability, atherosclerosis, coronary artery disease, coronary vasospasm, left ventricular dysfunction, angina pectoris and myocardial infarction.57–61 CRP levels have been found to be related to levels of cardiac enzymes and troponin I,61 while in some cases it was found to be a better marker of CVD than troponin T.62 CRP has been found to have a role in myocardial and cerebral infarct growth and has been consequently targeted by inhibitors to induce a cardio- protective effect.63 However this application has yet to be fully realised.64 Its reliability has several limitations as human CRP levels greatly vary, depending on ethnicity, gender, and genetics, and it has also been associated with obesity and weight loss.64,65 In addition, it has been described as an indicator/ marker for non-cardiac related pathologies such as anastomotic leakage, systemic lupus erythematosus (SLE), and dementia.66–68
Recent advances in identifying clinical markers with promising early prognostic and diagnostic capacity
A number of additional novel clinical markers have also been studied recently. Some of these show promising results as early prognostic and diagnostic markers, as outlined below, although their ultimate utility remains to be tested in large clinical settings.
During the last 10 years, asymmetric dimethylarginine (ADMA) has received much attention as a promising cardiovascular biomarker. ADMA is an endogenous competitive inhibitor of nitric oxide synthase although it can also cause vasoconstriction.69 It has been found to be increased in a number of cardiovascular events that include atherosclerosis, hypertension, coronary artery disease and chronic heart failure, and even on its own, is believed to be a novel cardiovascular risk factor.69–72 ADMA has also been associated with inflammation and increased risk of death in cardiovascular related events.73
Myeloperoxidase (MPO) is another recently described biomarker that has been found to be relevant for heart failure, acute coronary syndrome and, recently, atherosclerosis.74,75 MPO is an enzyme which among other molecules can produce hypochlorite and has been shown to be released early in the inflammatory process, while it has been linked to both inflammation and oxidative stress.75,76 MPO has been found to be related to CVD due to its involvement in LDL and HDL oxidation which is closely related to plaque formation in arterial walls through increased cholesterol aggregation. 75 MPO has shown some promising early results in clinical settings, being able to demonstrate a diagnostic value of CVD even in individuals showing negative results for troponin T. However, a key characteristic of MPO utilisation is that its elevation may not be directly related to cardiac or vascular tissue remodelling and may be attributed to underlying inflammatory processes which ultimately lead to organ failure.77
F2 isoprostanes are a family of prostaglandin compounds derived from arachidonic acid peroxidation which have recently shown promising potential as in vivo markers of oxidant injury in cardiovascular pathologies such as atherosclerosis, hypertension and, recently, ACS.78–80 Even though increased levels of this marker have been found in non-cardiovascular related pathologies such as Alzheimer’s disease, pulmonary disorders and renal failure, its presence has been strongly linked with well-known cardiovascular risk factors.78,81,82 However, and despite these promising results, use of F2 isoprostanes have not been used on a large scale. Relevant literature in large cohorts is limited, which restricts evaluation of its potential.
Cardiac Extracellular Matrix Components and Opportunities for Biomarker Development
Cardiac extracellular matrix
The cardiac extracellular matrix (CECM) is a vibrant three-dimensional entity which offers structural support to which cells adhere and migrate. It consists primarily of collagen, mainly type I but also III, IV, V, VI, glycoproteins, proteoglycans as well as diverse cell types such as fibroblasts and endothelial cells.83 Recognition of the constantly active dynamics of the CECM attracted additional attention to the possibility that its close monitoring could enhance our understanding of the underlying mechanisms occurring in the transition from physiology to pathology.84
CECM modifications during the natural ECM remodelling process increase with age and are part of a physiologic process, particularly because the CECM activates cardiac fibroblasts.85,86 However, unbalanced cardiac extracellular matrix remodelling (CECMR) can result in cardiovascular-related pathology through a mast cell-driven process and ultimately in heart failure.87,88
The collagenous cardiac CECM has been shown to have higher turnover rates than other tissues, possibly due to its contribution to diastolic stiffness.89,90 The practical outcome of this observation was the introduction and utilisation of CECM-related biomarkers such as procollagen types I and III (PINP, PICP, and PIIINP) that could monitor CECMR91 and its effects on diastolic dysfunction, pumping capacity and ventricular volume. An additional key property of cardiac CECM constituents is their ability to participate in inflammatory pathways that ultimately affect cardiac repair and pathogenesis.92 Due to these exceptional properties as active participants of cardiac and vascular remodelling, the use of CECM constituents as promising non-invasive early indicators of underlying developing pathology and inadequate tissue adaptation has been proposed.91,93–96
Cardiac matrix metalloproteinases (MMPs)
MMPs are a family of proteases that together with other proteases, such as cathepsins and elastases, play a key regulatory role in tissue remodelling in both physiology and in a number of pathologies which include cancer, fibrosis and CVD.97,98 Under normal physiologic conditions, there is a balance between MMPs that degrade CECM components and tissue inhibitors of metalloproteinases (TIMPs).99,100 However, during developing pathology, events such as decreased TIMP expression and activation of mast cells through chronic stimuli or increased stress, can induce increased MMP activation which in turn drives abnormal CECMR, eventually leading to cardiac and vascular disease and ultimately death.88,101 MMP activity has been implicated in a large number of diverse cardiac and vascular pathologies which include cardiomyopathies, atherosclerosis, aneurism, myocarditis, hypertension and viral heart disease.88,97,100,102–105 MMP effects and activity are closely related to the availability of substrates, some of which have been found to be specific for some MMPs.86 An example of such specificity is MMP-8, -3 and -13 which have been described as specific for fibrillar collagens; MMP-7 against collagens I, III and proteoglycans; while MMP-2 and -9 were found to preferentially cleave proteoglycans in myocardial tissue.97,102 Analogous substrate-MMP specificities and interactions have been reported in the ECM of both cardiac and vascular components, further highlighting the importance of these interactions in ECMR during physiology and pathology.106–111 The tangible product of this recognition was MMP utilisation as CECMR specific markers with promising results.105,112 MMPs are also actively employed in non-cardiovascular related tissues and organs which include liver, skin, and lung. This presents the practical problem of how to pin-point the precise tissue source of the substrate-MMP biomarker. Biomarkers that rely on MMPs and their action on specific substrates to form tissue-specific neoepitopes have been successfully employed for ECMR- related pathologies other than cardiovascular. The utilisation of such technologies should be further investigated for cardiovascular pathologies.98,113–119
MMPs and ECM remodelling in atherosclerosis
In most cases the underlying cause of CVD is atherosclerosis. Vulnerable atherosclerotic plaques are characterized by their propensity to rupture, exposing thrombogenic material to the circulation and consequently initiating the formation of luminal thrombi and ischemia. One of the key determinants of lesion stability is the composition and integrity of the plaque ECM. The quantity and quality of the ECM is of paramount importance in defining plaque stability. For instance, stable ECM-rich plaques which remain generally asymptomatic are characterized by their dense ECM, composed primarily of fibrillar and non-fibrillar collagens and proteoglycans. On the other hand, vulnerable or unstable plaques have a thin and disrupted fibrous cap and the collagen content in the ECM is clearly reduced.108 The proteolytic activity of MMPs is a key regulator of ECM integrity in atherosclerosis. 120 Several studies have shown increased expression and activity of MMPs, including MMP-1, -2, -3 and -9, in vulnerable areas of atherosclerotic plaques.121,122 While MMPs could collectively target many different ECM components, most studies have focused on the collagenolysis, especially of type I collagen, taking place in lesions. In one of the key studies, the proteolytic activity was attributed to MMP-1 and -13.123 Another proteinase which has received substantial attention is MMP-9. Synthesis of active MMP-9 by macrophages and smooth muscle cells was demonstrated in human coronary atherectomy specimens taken from patients with unstable angina but was not found in stable patients.124 MMP-9 is one of the few extracellular proteins with biomarker potential in CVD.125 While collagen degradation has been studied in detail, little is known of the proteolytic processing of other ECM components, especially glycoproteins and proteoglycans. One of the main non-collagen components of vessels with important structural and regulatory functions is the large aggregating proteoglycan versican. Halpert et al showed that versican could be a substrate for MMP-7 and -12 at sites of plaque rupture.126 Although MMP-12 is a protease with general substrate specificity and is highly expressed by activated macrophages, its role in atherosclerosis is not well understood.127 However, it is known to play a key role in the pathological development of abdominal aortic aneurysms, not only because it cleaves elastin and type I collagen but because it targets various ECM glycoproteins, including collagen XII, tenascin, fibronectin, thrombospondins and periostin.106 Proteolysis of the ECM not only compromises its structural properties, but its degradation products have been recently shown to activate pro-inflammatory signalling via toll-like receptors (TLRs). Hyaluronic acid degradation products (that is, tetra- and hexa-saccharides) have the ability to act as endogenous TLR-2 and -4 ligands in a variety of cell types, including macrophages and endothelial cells.128 Similarly, the fibronectin splice variant extra-type III domain A (EDA), has been shown to activate T-cells and induce MMP-9 expression in human monocytes by activating TLR-4.129 The role of EDA fragments in CVD was recently highlighted by Arslan and colleagues.130 Moreover, Kim et al recently showed that versican fragments activate TLR-2131 and Babelova et al described a proinflammatory effect of biglycan via TLR-2 and -4.132 Given the well-documented importance of TLR-2 and -4 in human atherosclerosis133 and the extensive remodelling of the ECM in CVD, the connection between MMP activity and the generation of bioactive, pro-inflammatory fragments from ECM proteins needs to be further investigated.
CECM matrikines
As seen in the previous sections, CECM and its constant interaction with proteases constitutes a complex and active entity in both physiology and pathology. The accumulated information on the continuous interaction between proteases and ECM gave birth to the conception of matrikines. The term was used to describe peptides formed by protease-driven ECM proteolysis that were able to regulate cell activities via interaction cell surface receptors-.134 The finding that matrikines can regulate both ECM synthesis and remodelling as well as MMP production and activation is indicative of the strong influence they may have in physiology and in transition processes that ultimately lead to pathology. 135 A number of proteins including elastin, various collagen types, glycoproteins and laminin134,136 have been recently described as ECM matrikine sources and mainly indicate the presence of tumours. However, the fact that most of these proteins exist in a large variety of tissues increases the possibility that these may be implicated in other pathologies. Angiogenesis is a prime example of such an occurrence, since it takes place in a variety of diverse processes such as arthritis, wound healing, tumour growth, and cardiovascular- related events such as atherosclerosis and post-ischemic vascularisation of the myocardium.137 Even though cardiovascular specific matrikines have not yet been described in detail, cardiovascular-related proteins such as elastin and their remodelling has been previously associated with angiogenesis, related inflammatory infiltrate and severity of atherosclerosis and aneurism progression.137–139 The possibility of cardiac-specific matrikines being implicated in cardiovascular pathology-related events, whether directly or indirectly should be further studied. The large number of potential combinations of peptide cleavages between cardiac-specific ECM protein constituents and proteases could result in a great number of matrikines that even though locally generated could have an effect on both adjacent and distant cells and tissues.
The above is a significant task., The example of successful utilisation of neoepitopes as biomarkers for a number of different pathologies is indicative of potential benefits of such approach98,113–116,119 in this case. The question arises whether certain neoepitopes could also act as matrikines. Since both neoepitopes and matrikines are generated by proteolytic action of proteases on ECM, this possibility may exist and should be further assessed.
Discussion-Cardiovascular Continuum and Novel Cardiac Biomarkers
The discovery of novel and tissue-specific cardiac biomarkers that can reliably assess pathologic conditions is important for early detection and prevention of cardiac damage. The existing panel of markers with the important addition of troponin markers constitutes a reliable set of tools. However, other markers are needed to describe underlying and developing pathology in the early, transient stages of CVC which elude existing late-stage diagnostic and prognostic means. The technology of combining protease-driven ECMR and the resulting peptide fragment “fingerprints” as markers of ECM monitoring has provided a number of reliable markers for ECMR pathologies98,113,115,116,119 which recently included cardiac-specific events such as arterial remodelling.140 The long timeline that separates risk factors which transiently develop to CVD-related pathology and ultimately cardiovascular death in the CVC include, as already described above, a large number of protease and protein interactions as key participants in ECMR. These protease and protein interactions could provide excellent markers of tissue remodelling closely reflecting different stages in the disease continuum (Figs. 2 and 3).
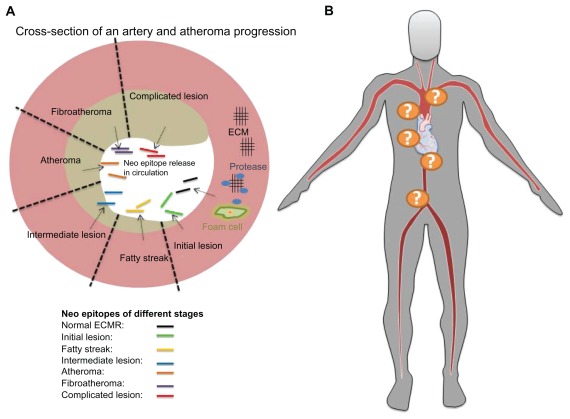
Figure 2.
Proteolytic activity by proteases such as matrix metalloproteinases (MMPs) is an important regulator of extracellular matrix (ECM) integrity in atherosclerosis, which is the central pathological feature of CVD. Interaction of different proteases combined with an altered ECM phenotype during atheroma formation and disease progression could result in a distinct neo epitope formation which could be used to monitor abnormal cardiac ECM remodelling and stage the disease. These neoepitopes are informative of protease activity, potential post- translational modifications of proteins, and tissue remodelling (A). Combining such biomarkers with a specific relationship to the location of atheroma formation could enable close monitoring of atherosclerosis progression (B).
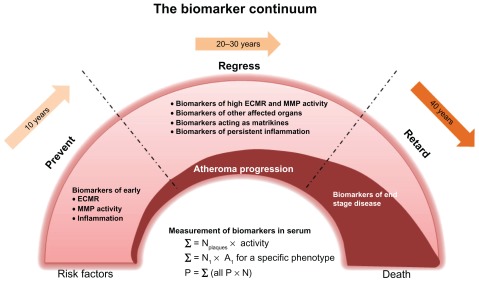
Figure 3.
A proposed illustration of a biomarker continuum, which could facilitate disease staging by utilisation of specific biomarkers that correspond to precise extracellular matrix remodelling (ECMR) events.
Notes: A serum measurement of biomarkers would provide information on all atherosclerotic plaques and the underlying activity of both proteases and ECMR. For a specific clinical phenotype, the marker or combination of markers may provide more information on the specific number of plaques and degree of ECMR and protease activity, and thus of disease-staging.
The possibility that such CECMR fragments may also act as matrikines which affect cell activities via cell surface receptors further adds to the importance of accurate measurements of such fragments. Measuring these fragments could potentially reflect protease production, cell apoptosis, proliferation and migration. Cardiac-specific proteins and a detailed description of the precise proteolytic activity of proteases on these proteins in vivo could provide a prime resource of such biomarker development. The successful use of troponins suggests that other cardiac-specific proteins such as titin, which also have cardiac specific regions, could be identified as useful matrikines. During the identification of biomarkers, it would be helpful to investigate their tissue and disease stage-specific post-translational modifications (PTMs), which may add supplementary information for detailed disease-staging. We previously discussed that since PTMs are modifications that take place following protein translation and are not DNA-coded, their presence may be related to tissue physiology or pathology either as a cause or a consequence and could therefore be included in the design of tissue-specific biomarkers.141 Identification of specific PTMs and their association with specific time points of disease progression could add important information to the proposed biomarker continuum, thus creating a detailed disease staging network of biomarkers that are informative of underlying and developing pathology. Introduction and utilisation of well-described biomarkers that are closely related to CVD-staging could facilitate their classification to specific pathology-linked time points in a similar way as the BIPED criteria did for biomarkers in osteoarthritis142.
We believe that the main challenge of future biomarker translational research lies in the detailed mapping of tissue-specific relevant protein substrates, identification of tissue-specific acting proteases, and tissue-specific PTMs. These could be combined in biomarker development and concurrently increase our understanding of CVD initiation and progression. Biomarkers relating to the early transient stages of CVD could enable early intervention and modification of the path of such a commonplace, but often fatal, disease.
Footnotes
Author Contributions
EV has conceived, designed and wrote the first draft of the manuscript. NB has contributed in adding and writing sections of the existing clinical cardiac markers. AD has contributed in adding and writing sections of the extracellular matrix remodeling processes. MK has contributed by critical revisions and approval of the final versions. All authors reviewed and approved of the final manuscript.
Competing Interests
Authors disclose that Efstathios Vassiliadis, Natasha Barascuk and Morten Karsdal are full-time employees at Nordic Bioscience A/S.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Dzau VJ, Antman EM, Black HR, et al. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease) Circulation. 2006 Dec 19;114(25):2850–70. doi: 10.1161/CIRCULATIONAHA.106.655688. [DOI] [PubMed] [Google Scholar]
- 2.Dzau VJ, Antman EM, Black HR, et al. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part II: Clinical trial evidence (acute coronary syndromes through renal disease) and future directions. Circulation. 2006 Dec 19;114(25):2871–91. doi: 10.1161/CIRCULATIONAHA.106.655761. [DOI] [PubMed] [Google Scholar]
- 3.Dzau V. The cardiovascular continuum and renin-angiotensin-aldosterone system blockade. J Hypertens Suppl. 2005 Apr;23(1):S9–17. [PubMed] [Google Scholar]
- 4.Barrios V, Escobar C. Rosuvastatin and cardiovascular continuum when time is important. J Am Coll Cardiol. 2010 Apr 13;55(15):1645–6. doi: 10.1016/j.jacc.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 5.Barrios V, Escobar C. Rosuvastatin along the cardiovascular continuum: from JUPITER to AURORA. Expert Rev Cardiovasc Ther. 2009 Nov;7(11):1317–27. doi: 10.1586/erc.09.119. [DOI] [PubMed] [Google Scholar]
- 6.O’Rourke MF, Safar ME, Dzau V. The Cardiovascular Continuum extended: aging effects on the aorta and microvasculature. Vasc Med. 2010 Dec;15(6):461–8. doi: 10.1177/1358863X10382946. [DOI] [PubMed] [Google Scholar]
- 7.Young GP, Gibler WB, Hedges JR, et al. Serial creatine kinase-MB results are a sensitive indicator of acute myocardial infarction in chest pain patients with nondiagnostic electrocardiograms: the second Emergency Medicine Cardiac Research Group Study. Acad Emerg Med. 1997 Sep;4(9):869–77. doi: 10.1111/j.1553-2712.1997.tb03812.x. [DOI] [PubMed] [Google Scholar]
- 8.Jurlander B, Clemmensen P, Wagner GS, Grande P. Very early diagnosis and risk stratification of patients admitted with suspected acute myocardial infarction by the combined evaluation of a single serum value of cardiac troponin-T, myoglobin, and creatine kinase MB(mass) Eur Heart J. 2000 Mar;21(5):382–9. doi: 10.1053/euhj.1999.1760. [DOI] [PubMed] [Google Scholar]
- 9.Newby LK, Storrow AB, Gibler WB, et al. Bedside multimarker testing for risk stratification in chest pain units: The chest pain evaluation by creatine kinase-MB, myoglobin, and troponin I (CHECKMATE) study. Circulation. 2001 Apr 10;103(14):1832–7. doi: 10.1161/01.cir.103.14.1832. [DOI] [PubMed] [Google Scholar]
- 10.Hedges JR, Young GP, Henkel GF, Gibler WB, Green TR, Swanson JR. Early CK-MB elevations predict ischemic events in stable chest pain patients. Acad Emerg Med. 1994 Jan;1(1):9–16. doi: 10.1111/j.1553-2712.1994.tb02794.x. [DOI] [PubMed] [Google Scholar]
- 11.de Winter RJ, Koster RW, Sturk A, Sanders GT. Value of myoglobin, troponin T, and CK-MBmass in ruling out an acute myocardial infarction in the emergency room. Circulation. 1995 Dec 15;92(12):3401–7. doi: 10.1161/01.cir.92.12.3401. [DOI] [PubMed] [Google Scholar]
- 12.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000 Sep;36(3):959–69. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 13.Moe KT, Wong P. Current trends in diagnostic biomarkers of acute coronary syndrome. Ann Acad Med Singapore. 2010 Mar;39(3):210–5. [PubMed] [Google Scholar]
- 14.Capellan O, Hollander JE, Pollack C, Jr, et al. Prospective evaluation of emergency department patients with potential coronary syndromes using initial absolute CK-MB vs. CK-MB relative index. J Emerg Med. 2003 May;24(4):361–7. doi: 10.1016/s0736-4679(03)00030-1. [DOI] [PubMed] [Google Scholar]
- 15.Jernberg T, Lindahl B, James S, Ronquist G, Wallentin L. Comparison between strategies using creatine kinase-MB(mass), myoglobin, and troponin T in the early detection or exclusion of acute myocardial infarction in patients with chest pain and a nondiagnostic electrocardiogram. Am J Cardiol. 2000 Dec 15;86(12):1367–71. A5. doi: 10.1016/s0002-9149(00)01245-5. [DOI] [PubMed] [Google Scholar]
- 16.Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction—summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina) J Am Coll Cardiol. 2002 Oct 2;40(7):1366–74. doi: 10.1016/s0735-1097(02)02336-7. [DOI] [PubMed] [Google Scholar]
- 17.de Lemos JA, Antman EM, Giugliano RP, et al. Very early risk stratification after thrombolytic therapy with a bedside myoglobin assay and the 12-lead electrocardiogram. Am Heart J. 2000 Sep;140(3):373–8. doi: 10.1067/mhj.2000.109216. [DOI] [PubMed] [Google Scholar]
- 18.Stork TV, Wu AH, Muller-Bardorff M, et al. Diagnostic and prognostic role of myoglobin in patients with suspected acute coronary syndrome. North-Wurttemberg Infarction Study (NOWIS) Group. Am J Cardiol. 2000 Dec 15;86(12):1371–4. A5. doi: 10.1016/s0002-9149(00)01246-7. [DOI] [PubMed] [Google Scholar]
- 19.Polanczyk CA, Lee TH, Cook EF, Walls R, Wybenga D, Johnson PA. Value of additional two-hour myoglobin for the diagnosis of myocardial infarction in the emergency department. Am J Cardiol. 1999 Feb 15;83(4):525–9. doi: 10.1016/s0002-9149(98)00907-2. [DOI] [PubMed] [Google Scholar]
- 20.Seed M, Doherty E, Stubbs P. Lipoprotein (a): a prothrombotic risk factor for coronary artery disease. J Cardiovasc Risk. 1995 Jun;2(3):206–15. [PubMed] [Google Scholar]
- 21.Morrisett JD. The role of lipoprotein[a] in atherosclerosis. Curr Atheroscler Rep. 2000 May;2(3):243–50. doi: 10.1007/s11883-000-0026-z. [DOI] [PubMed] [Google Scholar]
- 22.Stubbs P, Seed M, Lane D, Collinson P, Kendall F, Noble M. Lipoprotein(a) as a risk predictor for cardiac mortality in patients with acute coronary syndromes. Eur Heart J. 1998 Sep;19(9):1355–64. doi: 10.1053/euhj.1998.1043. [DOI] [PubMed] [Google Scholar]
- 23.Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. 1990 Aug;82(2):495–506. doi: 10.1161/01.cir.82.2.495. [DOI] [PubMed] [Google Scholar]
- 24.Angelin B. Therapy for lowering lipoprotein (a) levels 34. Curr Opin Lipidol. 1997 Dec;8(6):337–41. doi: 10.1097/00041433-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Kambayashi Y, Nakao K, Kimura H, et al. Biological characterization of human brain natriuretic peptide (BNP) and rat BNP: species-specific actions of BNP. Biochem Biophys Res Commun. 1990 Dec 14;173(2):599–605. doi: 10.1016/s0006-291x(05)80077-4. [DOI] [PubMed] [Google Scholar]
- 26.Kambayashi Y, Nakao K, Mukoyama M, et al. Isolation and sequence determination of human brain natriuretic peptide in human atrium 37. FEBS Lett. 1990 Jan 1;259(2):341–5. doi: 10.1016/0014-5793(90)80043-i. [DOI] [PubMed] [Google Scholar]
- 27.Sudoh T, Minamino N, Kangawa K, Matsuo H. Brain natriuretic peptide-32: N-terminal six amino acid extended form of brain natriuretic peptide identified in porcine brain. Biochem Biophys Res Commun. 1988 Sep 15;155(2):726–32. doi: 10.1016/s0006-291x(88)80555-2. [DOI] [PubMed] [Google Scholar]
- 28.Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988 Mar 3;332(6159):78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- 29.Raizada A, Bhandari S, Khan MA, et al. Brain type natriuretic peptide (BNP)- a marker of new millennium in diagnosis of congestive heart failure. Indian Journal of Clinical Biochemistry. 2007;22:4–9. doi: 10.1007/BF02912873. Ref Type: Generic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shapiro BP, Chen HH, Burnett JC, Jr, Redfield MM. Use of plasma brain natriuretic peptide concentration to aid in the diagnosis of heart failure. Mayo Clin Proc. 2003 Apr;78(4):481–6. doi: 10.4065/78.4.481. [DOI] [PubMed] [Google Scholar]
- 31.Lewandrowski K, Chen A, Januzzi J. Cardiac markers for myocardial infarction. A brief review. Am J Clin Pathol. 2002 Dec;118(Suppl):S93–9. doi: 10.1309/3EK7-YVV9-228C-E1XT. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda N, Terui T, Ohtsuki I, Ishiwata S, Kurihara S. Titin and troponin: central players in the frank-starling mechanism of the heart. Curr Cardiol Rev. 2009 May;5(2):119–24. doi: 10.2174/157340309788166714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkinson JM, Grand RJ. Comparison of amino acid sequence of troponin I from different striated muscles. Nature. 1978 Jan 5;271(5640):31–5. doi: 10.1038/271031a0. [DOI] [PubMed] [Google Scholar]
- 34.Adamcova M, Sterba M, Simunek T, Potacova A, Popelova O, Gersl V. Myocardial regulatory proteins and heart failure. Eur J Heart Fail. 2006 Jun;8(4):333–42. doi: 10.1016/j.ejheart.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Adams JE, III, Bodor GS, vila-Roman VG, et al. Cardiac troponin I. A marker with high specificity for cardiac injury. Circulation. 1993 Jul;88(1):101–6. doi: 10.1161/01.cir.88.1.101. [DOI] [PubMed] [Google Scholar]
- 36.Kim LJ, Martinez EA, Faraday N, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation. 2002 Oct 29;106(18):2366–71. doi: 10.1161/01.cir.0000036016.52396.bb. [DOI] [PubMed] [Google Scholar]
- 37.Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation. 2003 Aug 19;108(7):833–8. doi: 10.1161/01.CIR.0000084543.79097.34. [DOI] [PubMed] [Google Scholar]
- 38.Muehlschlegel JD, Perry TE, Liu KY, et al. Troponin is superior to electrocardiogram and creatinine kinase MB for predicting clinically significant myocardial injury after coronary artery bypass grafting. Eur Heart J. 2009 Jul;30(13):1574–83. doi: 10.1093/eurheartj/ehp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007 Nov 27;116(22):2634–53. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg M, Zugck C, Nelles M, et al. Osteopontin, a new prognostic biomarker in patients with chronic heart failure. Circ Heart Fail. 2008 May;1(1):43–9. doi: 10.1161/CIRCHEARTFAILURE.107.746172. [DOI] [PubMed] [Google Scholar]
- 41.Francia P, Balla C, Ricotta A, et al. Plasma osteopontin reveals left ventricular reverse remodelling following cardiac resynchronization therapy in heart failure. Int J Cardiol. 2010 Sep 20; doi: 10.1016/j.ijcard.2010.08.048. [DOI] [PubMed] [Google Scholar]
- 42.Georgiadou P, Iliodromitis EK, Kolokathis F, et al. Osteopontin as a novel prognostic marker in stable ischaemic heart disease: a 3-year follow-up study. Eur J Clin Invest. 2010 Apr;40(4):288–93. doi: 10.1111/j.1365-2362.2010.02257.x. [DOI] [PubMed] [Google Scholar]
- 43.Schoensiegel F, Bekeredjian R, Schrewe A, et al. Atrial natriuretic peptide and osteopontin are useful markers of cardiac disorders in mice. Comp Med. 2007 Dec;57(6):546–53. [PubMed] [Google Scholar]
- 44.Kossmehl P, Schonberger J, Shakibaei M, et al. Increase of fibronectin and osteopontin in porcine hearts following ischemia and reperfusion. J Mol Med (Berl) 2005 Aug;83(8):626–37. doi: 10.1007/s00109-005-0642-8. [DOI] [PubMed] [Google Scholar]
- 45.Murry CE, Giachelli CM, Schwartz SM, Vracko R. Macrophages express osteopontin during repair of myocardial necrosis. Am J Pathol. 1994 Dec;145(6):1450–62. [PMC free article] [PubMed] [Google Scholar]
- 46.Rittling SR, Denhardt DT. Osteopontin function in pathology: lessons from osteopontin-deficient mice. Exp Nephrol. 1999 Mar;7(2):103–13. doi: 10.1159/000020591. [DOI] [PubMed] [Google Scholar]
- 47.Giachelli CM, Steitz S. Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol. 2000 Dec;19(7):615–22. doi: 10.1016/s0945-053x(00)00108-6. [DOI] [PubMed] [Google Scholar]
- 48.Anborgh PH, Mutrie JC, Tuck AB, Chambers AF. Pre- and post-translational regulation of osteopontin in cancer. J Cell Commun Signal. 2011 Jun;5(2):111–22. doi: 10.1007/s12079-011-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bornsen L, Khademi M, Olsson T, Sorensen PS, Sellebjerg F. Osteopontin concentrations are increased in cerebrospinal fluid during attacks of multiple sclerosis. Mult Scler. 2011 Jan;17(1):32–42. doi: 10.1177/1352458510382247. [DOI] [PubMed] [Google Scholar]
- 50.Sfiridaki A, Miyakis S, Pappa C, et al. Circulating osteopontin: a dual marker of bone destruction and angiogenesis in patients with multiple myeloma. J Hematol Oncol. 2011;4:22. doi: 10.1186/1756-8722-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maier T, Laubender RP, Sturm RA, et al. Osteopontin expression in plasma of melanoma patients and in melanocytic tumours. J Eur Acad Dermatol Venereol. 2011 Aug 12; doi: 10.1111/j.1468-3083.2011.04210.x. [DOI] [PubMed] [Google Scholar]
- 52.Xu L, Ma X, Wang Y, et al. The Expression and Pathophysiological Role of Osteopontin in Graves’ Disease. J Clin Endocrinol Metab. 2011 Sep 7; doi: 10.1210/jc.2011-1339. [DOI] [PubMed] [Google Scholar]
- 53.Weber GF, Lett GS, Haubein NC. Osteopontin is a marker for cancer aggressiveness and patient survival. Br J Cancer. 2010 Sep 7;103(6):861–9. doi: 10.1038/sj.bjc.6605834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lorenzen JM, Nickel N, Kramer R, et al. Osteopontin in patients with idiopathic pulmonary hypertension. Chest. 2011 May;139(5):1010–7. doi: 10.1378/chest.10-1146. [DOI] [PubMed] [Google Scholar]
- 55.Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004 Nov 19;279(47):48487–90. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 56.Sepulveda JL, Mehta JL. C-reactive protein and cardiovascular disease: a critical appraisal. Curr Opin Cardiol. 2005 Sep;20(5):407–16. doi: 10.1097/01.hco.0000175518.57804.94. [DOI] [PubMed] [Google Scholar]
- 57.rroyo-Espliguero R, Avanzas P, Cosin-Sales J, Aldama G, Pizzi C, Kaski JC. C-reactive protein elevation and disease activity in patients with coronary artery disease. Eur Heart J. 2004 Mar;25(5):401–8. doi: 10.1016/j.ehj.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 58.Hung MJ, Cherng WJ, Yang NI, Cheng CW, Li LF. Relation of high-sensitivity C-reactive protein level with coronary vasospastic angina pectoris in patients without hemodynamically significant coronary artery disease. Am J Cardiol. 2005 Dec 1;96(11):1484–90. doi: 10.1016/j.amjcard.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 59.Shah SJ, Marcus GM, Gerber IL, et al. High-sensitivity C-reactive protein and parameters of left ventricular dysfunction. J Card Fail. 2006 Feb;12(1):61–5. doi: 10.1016/j.cardfail.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Tanaka H, Tsurumi Y, Kasanuki H. Prognostic value of C-reactive protein and troponin T level in patients with unstable angina pectoris. J Cardiol. 2006 Apr;47(4):173–9. [PubMed] [Google Scholar]
- 61.Tsakiris AK, Marnelos PG, Nearchou NS, Papadakis JE, Karatzis EN, Skoufas PD. The influence of thrombolytic therapy on C-reactive protein in ST-segment elevation acute myocardial infarction. Hellenic J Cardiol. 2006 Jul;47(4):218–22. [PubMed] [Google Scholar]
- 62.Blum A, Safori G, Hous N, Lupovitch S. The prognostic value of high-sensitive C-reactive protein and cardiac troponin T in young and middle-aged patients with chest pain without ECG changes. Eur J Intern Med. 2003 Aug;14(5):310–4. doi: 10.1016/s0953-6205(03)00099-2. [DOI] [PubMed] [Google Scholar]
- 63.Pepys MB, Hirschfield GM, Tennent GA, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006 Apr 27;440(7088):1217–21. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- 64.Kraus VB, Jordan JM. Serum C-Reactive Protein (CRP), Target for Therapy or Trouble? Biomark Insights. 2007;1:77–80. [PMC free article] [PubMed] [Google Scholar]
- 65.Dietrich M, Jialal I. The effect of weight loss on a stable biomarker of inflammation, C-reactive protein. Nutr Rev. 2005 Jan;63(1):22–8. doi: 10.1111/j.1753-4887.2005.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 66.Deitmar S, Anthoni C, Palmes D, Haier J, Senninger N, Bruwer M. Are leukocytes and CRP early indicators for anastomotic leakage after esophageal resection? Zentralbl Chir. 2009 Feb;134(1):83–9. doi: 10.1055/s-0028-1098768. [DOI] [PubMed] [Google Scholar]
- 67.Lee SS, Singh S, Link K, Petri M. High-sensitivity C-reactive protein as an associate of clinical subsets and organ damage in systemic lupus erythematosus. Semin Arthritis Rheum. 2008 Aug;38(1):41–54. doi: 10.1016/j.semarthrit.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davis G, Baboolal N, Nayak S, McRae A. Sialic acid, homocysteine and CRP: potential markers for dementia. Neurosci Lett. 2009 Nov 20;465(3):282–4. doi: 10.1016/j.neulet.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 69.Boger RH. Asymmetric dimethylarginine (ADMA) and cardiovascular disease: insights from prospective clinical trials. Vasc Med. 2005 Jul;10(Suppl 1):S19–25. doi: 10.1177/1358836X0501000104. [DOI] [PubMed] [Google Scholar]
- 70.Boger RH. Association of asymmetric dimethylarginine and endothelial dysfunction. Clin Chem Lab Med. 2003 Nov;41(11):1467–72. doi: 10.1515/CCLM.2003.225. [DOI] [PubMed] [Google Scholar]
- 71.Tousoulis D, Siasos G, Oikonomou E, et al. Asymmetric dimethylarginine (ADMA): is really a biomarker for cardiovascular prognosis? Int J Cardiol. 2011 Dec 1;153(2):123–5. doi: 10.1016/j.ijcard.2011.08.070. [DOI] [PubMed] [Google Scholar]
- 72.Wolf C, Lorenzen JM, Stein S, et al. Urinary asymmetric dimethylarginine (ADMA) is a predictor of mortality risk in patients with coronary artery disease. Int J Cardiol. 2010 Dec 13; doi: 10.1016/j.ijcard.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 73.Tripepi G, Mattace RF, Sijbrands E, et al. Inflammation and asymmetric dimethylarginine for predicting death and cardiovascular events in ESRD patients. Clin J Am Soc Nephrol. 2011 Jul;6(7):1714–21. doi: 10.2215/CJN.11291210. [DOI] [PubMed] [Google Scholar]
- 74.Sinning C, Schnabel R, Peacock WF, Blankenberg S. Up-and-coming markers: myeloperoxidase, a novel biomarker test for heart failure and acute coronary syndrome application? Congest Heart Fail. 2008 Jul;14(4 Suppl 1):46–8. doi: 10.1111/j.1751-7133.2008.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 75.Schindhelm RK, van der Zwan LP, Teerlink T, Scheffer PG. Myeloperoxidase: a useful biomarker for cardiovascular disease risk stratification? Clin Chem. 2009 Aug;55(8):1462–70. doi: 10.1373/clinchem.2009.126029. [DOI] [PubMed] [Google Scholar]
- 76.Loria V, Dato I, Graziani F, Biasucci LM. Myeloperoxidase: a new biomarker of inflammation in ischemic heart disease and acute coronary syndromes. Mediators Inflamm. 2008;135625 doi: 10.1155/2008/135625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu AH. Novel biomarkers of cardiovascular disease: myeloperoxidase for acute and/or chronic heart failure? Clin Chem. 2009 Jan;55(1):12–4. doi: 10.1373/clinchem.2008.118208. [DOI] [PubMed] [Google Scholar]
- 78.Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers. 2005 Nov;10(Suppl 1):S10–23. doi: 10.1080/13547500500216546. [DOI] [PubMed] [Google Scholar]
- 79.Nourooz-Zadeh J. Key issues in F2-isoprostane analysis. Biochem Soc Trans. 2008 Oct;36(Pt 5):1060–5. doi: 10.1042/BST0361060. [DOI] [PubMed] [Google Scholar]
- 80.LeLeiko RM, Vaccari CS, Sola S, et al. Usefulness of elevations in serum choline and free F2-isoprostane to predict 30-day cardiovascular outcomes in patients with acute coronary syndrome. Am J Cardiol. 2009 Sep 1;104(5):638–43. doi: 10.1016/j.amjcard.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 81.Wiswedel I, Hirsch D, Carluccio F, Hampl H, Siems W. F2-isoprostanes as biomarkers of lipid peroxidation in patients with chronic renal failure. Biofactors. 2005;24(1–4):201–8. doi: 10.1002/biof.5520240124. [DOI] [PubMed] [Google Scholar]
- 82.Young IS. Oxidative stress and vascular disease: insights from isoprostane measurement. Clin Chem. 2005 Jan;51(1):14–5. doi: 10.1373/clinchem.2004.039768. [DOI] [PubMed] [Google Scholar]
- 83.Pelouch V, Dixon IM, Golfman L, Beamish RE, Dhalla NS. Role of extra-cellular matrix proteins in heart function. Mol Cell Biochem. 1993 Dec 22;129(2):101–20. doi: 10.1007/BF00926359. [DOI] [PubMed] [Google Scholar]
- 84.Brown L. Cardiac extracellular matrix: a dynamic entity. Am J Physiol Heart Circ Physiol. 2005 Sep;289(3):H973–4. doi: 10.1152/ajpheart.00443.2005. [DOI] [PubMed] [Google Scholar]
- 85.Burgess ML, McCrea JC, Hedrick HL. Age-associated changes in cardiac matrix and integrins. Mech Ageing Dev. 2001 Oct;122(15):1739–56. doi: 10.1016/s0047-6374(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 86.Jourdan-Lesaux C, Zhang J, Lindsey ML. Extracellular matrix roles during cardiac repair. Life Sci. 2010 Sep 25;87(13–'14):391–400. doi: 10.1016/j.lfs.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wesley RB, Meng X, Godin D, Galis ZS. Extracellular matrix modulates macrophage functions characteristic to atheroma: collagen type I enhances acquisition of resident macrophage traits by human peripheral blood monocytes in vitro. Arterioscler Thromb Vasc Biol. 1998 Mar;18(3):432–40. doi: 10.1161/01.atv.18.3.432. [DOI] [PubMed] [Google Scholar]
- 88.Levick SP, Melendez GC, Plante E, McLarty JL, Brower GL, Janicki JS. Cardiac mast cells: the centrepiece in adverse myocardial remodelling. Cardiovasc Res. 2011 Jan 1;89(1):12–9. doi: 10.1093/cvr/cvq272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bishop JE, Laurent GJ. Collagen turnover and its regulation in the normal and hypertrophying heart. Eur Heart J. 1995 May;16(Suppl C):38–44. doi: 10.1093/eurheartj/16.suppl_c.38. [DOI] [PubMed] [Google Scholar]
- 90.Bishop JE, Rhodes S, Laurent GJ, Low RB, Stirewalt WS. The regulation of collagen deposition in the hypertrophying heart. Ann N Y Acad Sci. 1995 Mar 27;752:236–9. doi: 10.1111/j.1749-6632.1995.tb17432.x. [DOI] [PubMed] [Google Scholar]
- 91.de JS, van Veen TA, de Bakker JM, Vos MA, van Rijen HV. Biomarkers of myocardial fibrosis. J Cardiovasc Pharmacol. 2011 May;57(5):522–35. doi: 10.1097/FJC.0b013e31821823d9. [DOI] [PubMed] [Google Scholar]
- 92.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extra-cellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010 Mar;48(3):504–11. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Espira L, Czubryt MP. Emerging concepts in cardiac matrix biology. Can J Physiol Pharmacol. 2009 Dec;87(12):996–1008. doi: 10.1139/Y09-105. [DOI] [PubMed] [Google Scholar]
- 94.Piper C, Schultheiss HP, Akdemir D, Rudolf J, Horstkotte D, Pauschinger M. Remodeling of the cardiac extracellular matrix differs between volume-and pressure-overloaded ventricles and is specific for each heart valve lesion. J Heart Valve Dis. 2003 Sep;12(5):592–600. [PubMed] [Google Scholar]
- 95.Zannad F, Pitt B. Biomarkers of extracellular matrix turnover. Heart Fail Clin. 2009 Oct;5(4):589–99. doi: 10.1016/j.hfc.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 96.Iraqi W, Rossignol P, Angioi M, et al. Extracellular cardiac matrix biomarkers in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) study. Circulation. 2009 May 12;119(18):2471–9. doi: 10.1161/CIRCULATIONAHA.108.809194. [DOI] [PubMed] [Google Scholar]
- 97.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007 Oct;87(4):1285–342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 98.Vassiliadis E, Veidal SS, Simonsen H, et al. Immunological detection of the type V collagen propeptide fragment, PVCP-1230, in connective tissue remodeling associated with liver fibrosis. Biomarkers. 2011 Aug;16(5):426–33. doi: 10.3109/1354750X.2011.584131. [DOI] [PubMed] [Google Scholar]
- 99.Lopez B, Gonzalez A, Diez J. Role of matrix metalloproteinases in hypertension-associated cardiac fibrosis. Curr Opin Nephrol Hypertens. 2004 Mar;13(2):197–204. doi: 10.1097/00041552-200403000-00008. [DOI] [PubMed] [Google Scholar]
- 100.Hutchinson KR, Stewart JA, Jr, Lucchesi PA. Extracellular matrix remodeling during the progression of volume overload-induced heart failure. J Mol Cell Cardiol. 2010 Mar;48(3):564–9. doi: 10.1016/j.yjmcc.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baghelai K, Marktanner R, Dattilo JB, et al. Decreased expression of tissue inhibitor of metalloproteinase 1 in stunned myocardium. J Surg Res. 1998 Jun;77(1):35–9. doi: 10.1006/jsre.1998.5330. [DOI] [PubMed] [Google Scholar]
- 102.Kandasamy AD, Chow AK, Ali MA, Schulz R. Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res. 2010 Feb 1;85(3):413–23. doi: 10.1093/cvr/cvp268. [DOI] [PubMed] [Google Scholar]
- 103.Marchant D, McManus BM. Matrix metalloproteinases in the pathogenesis of viral heart disease. Trends Cardiovasc Med. 2009 Jan;19(1):21–6. doi: 10.1016/j.tcm.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 104.Loftus IM, Naylor AR, Bell PR, Thompson MM. Matrix metalloproteinases and atherosclerotic plaque instability. Br J Surg. 2002 Jun;89(6):680–94. doi: 10.1046/j.1365-2168.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- 105.Sundstrom J, Evans JC, Benjamin EJ, et al. Relations of plasma matrix metalloproteinase-9 to clinical cardiovascular risk factors and echocardiographic left ventricular measures: the Framingham Heart Study. Circulation. 2004 Jun 15;109(23):2850–6. doi: 10.1161/01.CIR.0000129318.79570.84. [DOI] [PubMed] [Google Scholar]
- 106.Didangelos A, Yin X, Mandal K, et al. Extracellular matrix composition and remodeling in human abdominal aortic aneurysms: a proteomics approach. Mol Cell Proteomics. 2011 Aug;10(8):M111. doi: 10.1074/mcp.M111.008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Didangelos A, Yin X, Mandal K, Baumert M, Jahangiri M, Mayr M. Proteomics characterization of extracellular space components in the human aorta. Mol Cell Proteomics. 2010 Sep;9(9):2048–62. doi: 10.1074/mcp.M110.001693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Didangelos A, Simper D, Monaco C, Mayr M. Proteomics of acute coronary syndromes. Curr Atheroscler Rep. 2009 May;11(3):188–95. doi: 10.1007/s11883-009-0030-x. [DOI] [PubMed] [Google Scholar]
- 109.Lombardi F, Belletti S, Battezzati PM, Pacciolla R, Biondi ML. MMP-1 and MMP-3 polymorphism and arrhythmia recurrence after electrical cardioversion in patients with persistent atrial fibrillation. J Cardiovasc Med (Hagerstown ) 2011 Jan;12(1):37–42. doi: 10.2459/JCM.0b013e3283403366. [DOI] [PubMed] [Google Scholar]
- 110.Lombard C, Saulnier J, Wallach J. Assays of matrix metalloproteinases (MMPs) activities: a review. Biochimie. 2005 Mar;87(3–4):265–72. doi: 10.1016/j.biochi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 111.Naito Y, Tsujino T, Lee-Kawabata M, et al. Matrix metalloproteinase-1 and -2 levels are differently regulated in acute exacerbation of heart failure in patients with and without left ventricular systolic dysfunction. Heart Vessels. 2009 May;24(3):181–6. doi: 10.1007/s00380-008-1100-7. [DOI] [PubMed] [Google Scholar]
- 112.Ramos-Fernandez M, Bellolio MF, Stead LG. Matrix metalloproteinase-9 as a marker for acute ischemic stroke: a systematic review. J Stroke Cerebrovasc Dis. 2011 Jan;20(1):47–54. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 113.Veidal SS, Karsdal MA, Vassiliadis E, et al. MMP Mediated Degradation of Type VI Collagen Is Highly Associated with Liver Fibrosis—Identification and Validation of a Novel Biochemical Marker Assay. PLoS One. 2011;6(9):e24753. doi: 10.1371/journal.pone.0024753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vassiliadis E, Larsen DV, Clausen RE, et al. Measurement of CO3-610, a Potential Liver Biomarker Derived from Matrix Metalloproteinase-9 Degradation of Collagen Type III, in a Rat Model of Reversible Carbon- Tetrachloride-Induced Fibrosis. Biomark Insights. 2011;6:49–58. doi: 10.4137/BMI.S6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vassiliadis E, Veidal SS, Barascuk N, et al. Measurement of matrix metalloproteinase 9-mediated collagen type III degradation fragment as a marker of skin fibrosis. BMC Dermatol. 2011;11:6. doi: 10.1186/1471-5945-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leeming D, He Y, Veidal S, et al. A novel marker for assessment of liver matrix remodeling: An enzyme-linked immunosorbent assay (ELISA) detecting a MMP generated type I collagen neo-epitope (C1M) Biomarkers. 2011 Oct 11; doi: 10.3109/1354750X.2011.620628. [DOI] [PubMed] [Google Scholar]
- 117.Veidal SS, Karsdal MA, Nawrocki A, et al. Assessment of proteolytic degradation of the basement membrane: A fragment of type IV collagen as a biochemical marker for liver fibrosis. Fibrogenesis Tissue Repair. 2011 Oct 5;4(1):22. doi: 10.1186/1755-1536-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karsdal MA, Delvin E, Christiansen C. Protein fingerprints—Relying on and understanding the information of serological protein measurements. Clin Biochem. 2011 Nov;44(16):1278–9. doi: 10.1016/j.clinbiochem.2011.08.1135. [DOI] [PubMed] [Google Scholar]
- 119.Veidal SS, Vassiliadis E, Barascuk N, et al. Matrix metalloproteinase-9- mediated type III collagen degradation as a novel serological biochemical marker for liver fibrogenesis. Liver Int. 2010 Oct;30(9):1293–304. doi: 10.1111/j.1478-3231.2010.02309.x. [DOI] [PubMed] [Google Scholar]
- 120.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003 May 2;92(8):827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 121.Dollery CM, Libby P. Atherosclerosis and proteinase activation. Cardiovasc Res. 2006 Feb 15;69(3):625–35. doi: 10.1016/j.cardiores.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 122.Monaco C, Andreakos E, Kiriakidis S, et al. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci U S A. 2004 Apr 13;101(15):5634–9. doi: 10.1073/pnas.0401060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sukhova GK, Schonbeck U, Rabkin E, et al. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation. 1999 May 18;99(19):2503–9. doi: 10.1161/01.cir.99.19.2503. [DOI] [PubMed] [Google Scholar]
- 124.Brown DL, Hibbs MS, Kearney M, Loushin C, Isner JM. Identification of 92-kD gelatinase in human coronary atherosclerotic lesions. Association of active enzyme synthesis with unstable angina. Circulation. 1995 Apr 15;91(8):2125–31. doi: 10.1161/01.cir.91.8.2125. [DOI] [PubMed] [Google Scholar]
- 125.Didangelos A, Stegemann C, Mayr M. The -omics era: Proteomics and lipidomics in vascular research. Atherosclerosis. 2011 Oct 2; doi: 10.1016/j.atherosclerosis.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 126.Halpert I, Sires UI, Roby JD, et al. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci U S A. 1996 Sep 3;93(18):9748–53. doi: 10.1073/pnas.93.18.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Johnson JL, George SJ, Newby AC, Jackson CL. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc Natl Acad Sci U S A. 2005 Oct 25;102(43):15575–80. doi: 10.1073/pnas.0506201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006 Jul 15;177(2):1272–81. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 129.Okamura Y, Watari M, Jerud ES, et al. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001 Mar 30;276(13):10229–33. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 130.Arslan F, Smeets MB, Riem Vis PW, et al. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res. 2011 Mar 4;108(5):582–92. doi: 10.1161/CIRCRESAHA.110.224428. [DOI] [PubMed] [Google Scholar]
- 131.Kim S, Takahashi H, Lin WW, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009 Jan 1;457(7225):102–6. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Babelova A, Moreth K, Tsalastra-Greul W, et al. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem. 2009 Sep 4;284(36):24035–48. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Monaco C, Gregan SM, Navin TJ, Foxwell BM, Davies AH, Feldmann M. Toll-like receptor-2 mediates inflammation and matrix degradation in human atherosclerosis. Circulation. 2009 Dec 15;120(24):2462–9. doi: 10.1161/CIRCULATIONAHA.109.851881. [DOI] [PubMed] [Google Scholar]
- 134.Maquart FX, Pasco S, Ramont L, Hornebeck W, Monboisse JC. An introduction to matrikines: extracellular matrix-derived peptides which regulate cell activity. Implication in tumor invasion. Crit Rev Oncol Hematol. 2004 Mar;49(3):199–202. doi: 10.1016/j.critrevonc.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 135.Maquart FX, Bellon G, Pasco S, Monboisse JC. Matrikines in the regulation of extracellular matrix degradation. Biochimie. 2005 Mar;87(3–4):353–60. doi: 10.1016/j.biochi.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 136.Tran KT, Lamb P, Deng JS. Matrikines and matricryptins: Implications for cutaneous cancers and skin repair. J Dermatol Sci. 2005 Oct;40(1):11–20. doi: 10.1016/j.jdermsci.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 137.Bellon G, Martiny L, Robinet A. Matrix metalloproteinases and matrikines in angiogenesis. Crit Rev Oncol Hematol. 2004 Mar;49(3):203–20. doi: 10.1016/j.critrevonc.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 138.Thompson MM, Jones L, Nasim A, Sayers RD, Bell PR. Angiogenesis in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 1996 May;11(4):464–9. doi: 10.1016/s1078-5884(96)80183-3. [DOI] [PubMed] [Google Scholar]
- 139.Herron GS, Unemori E, Wong M, Rapp JH, Hibbs MH, Stoney RJ. Connective tissue proteinases and inhibitors in abdominal aortic aneurysms. Involvement of the vasa vasorum in the pathogenesis of aortic aneurysms. Arterioscler Thromb. 1991 Nov;11(6):1667–77. doi: 10.1161/01.atv.11.6.1667. [DOI] [PubMed] [Google Scholar]
- 140.Barascuk N, Vassiliadis E, Zheng Q, et al. Levels of Circulating MMCN-151, a Degradation Product of Mimecan, Reflect Pathological Extracellular Matrix Remodeling in Apolipoprotein E Knockout Mice. Biomark Insights. 2011;6:97–106. doi: 10.4137/BMI.S7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Karsdal MA, Henriksen K, Leeming DJ, Woodworth T, Vassiliadis E, Bay-Jensen AC. Novel combinations of Post-Translational Modification (PTM) neo-epitopes provide tissue-specific biochemical markers—are they the cause or the consequence of the disease? Clin Biochem. 2010 Jul;43(10–11):793–804. doi: 10.1016/j.clinbiochem.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 142.Bauer DC, Hunter DJ, Abramson SB, et al. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage. 2006 Aug;14(8):723–7. doi: 10.1016/j.joca.2006.04.001. [DOI] [PubMed] [Google Scholar]