Abstract
Oncolytic herpes simplex virus type 1 (HSV-1) vectors are promising therapeutic agents for cancer. Their efficacy depends on the extent of both intratumoral viral replication and induction of a host antitumor immune response. To enhance these properties while employing ample safeguards, two conditionally replicating HSV-1 vectors, termed G47Δ and R47Δ, have been constructed by deleting the α47 gene and the promoter region of US11 from γ34.5-deficient HSV-1 vectors, G207 and R3616, respectively. Because the α47 gene product is responsible for inhibiting the transporter associated with antigen presentation (TAP), its absence led to increased MHC class I expression in infected human cells. Moreover, some G47Δ-infected human melanoma cells exhibited enhanced stimulation of matched antitumor T cell activity. The deletion also places the late US11 gene under control of the immediate-early α47 promoter, which suppresses the reduced growth properties of γ34.5-deficient mutants. G47Δ and R47Δ showed enhanced viral growth in a variety of cell lines, leading to higher virus yields and enhanced cytopathic effect in tumor cells. G47Δ was significantly more efficacious in vivo than its parent G207 at inhibiting tumor growth in both immune-competent and immune-deficient animal models. Yet, when inoculated into the brains of HSV-1-sensitive A/J mice at 2 × 106 plaque forming units, G47Δ was as safe as G207. These results suggest that G47Δ may have enhanced antitumor activity in humans.
The use of replication-competent viral vectors [in particular, herpes simplex virus type 1 (HSV-1) vectors] is an attractive strategy for tumor therapy because the virus can replicate and spread in situ, exhibiting oncolytic activity through direct cytopathic effect (1). A number of oncolytic HSV-1 vectors have been developed in recent years that have mutations in genes associated with neurovirulence and/or viral DNA synthesis to restrict viral replication to transformed cells and not cause disease (2).
In designing viral vectors for clinical use, it is essential that ample safeguards be employed. G207 is an oncolytic HSV-1 vector derived from wild-type HSV-1 strain F (3). It has deletions in both copies of the γ34.5 gene and an inactivating insertion of the Escherichia coli lacZ gene in UL39, encoding the infected cell protein 6 (ICP6; ref. 3). ICP6 is the large subunit of ribonucleotide reductase, a key enzyme for nucleotide metabolism and viral DNA synthesis in nondividing cells but not in dividing cells (4). γ34.5 is the major determinant of HSV neurovirulence (5). A second function of γ34.5 is to block host-cell-induced shutoff of protein synthesis in response to viral infection (6). Lack of this function is likely responsible for the less efficient growth of γ34.5− mutants compared with wild-type HSV, observed in many tumor cell types (7–9). This double mutation confers important advantages—minimal chance of reverting to wild type, preferential replication in tumor cells, attenuated neurovirulence, and ganciclovir/acyclovir hypersensitivity. G207 effectively kills multiple types of tumor cells in culture and in mice harboring tumors s.c. or intracranially (2). In several syngeneic tumor models in immunocompetent mice, oncolysis caused by intraneoplastic inoculation of G207 elicited a systemic immune response and tumor-specific cytotoxic T lymphocytes (10–12).
G207 has minimal toxicity when injected into the brains of HSV-1-susceptible mice or nonhuman primates (13–15). Recently, G207 has been examined in patients with recurrent malignant glioma (16), and the results from this phase I clinical trial indicate that intracerebral inoculation of G207 is safe at doses up to 3 × 109 plaque forming units (pfu), the highest dose tested. Whereas the use of oncolytic viruses is a promising approach for cancer therapy, the therapeutic benefits will likely depend on the dose and route of administration, the extent of intratumoral viral replication, and the host immune response.
HSV-1 infection causes down-regulation of major histocompatibility complex (MHC) class I expression on the surface of infected host cells (17, 18). The binding of ICP47 to the transporter associated with antigen presentation (TAP) blocks the antigenic peptide transport in the endoplasmic reticulum and the loading of MHC class I molecules (19–21). The binding of ICP47 is species-specific for TAPs from large mammals (22), with the affinity for murine TAP about 100-fold less than for human (23). ICP47 expression, therefore, could attenuate an antitumor immune response in humans that would be induced in the mouse tumor models.
In this paper, we describe a multimutated, replication-competent HSV-1 vector, termed G47Δ, derived from G207 by a deletion within the nonessential α47 gene (24). Because of the overlapping transcripts encoding ICP47 and US11 (Fig. 1b), the deletion in α47 also places the late US11 gene under control of the immediate-early α47 promoter. This alteration of US11 expression enhances the growth of γ34.5− mutants by precluding the shutoff of protein synthesis (25–27). Nevertheless, G47Δ was safe when inoculated into the brains of A/J mice. We show that human melanoma cells infected with G47Δ were more effective at stimulating their matched tumor-infiltrating lymphocytes (TILs) than those infected with G207, that G47Δ replicated better in the cell lines tested, and that G47Δ was more efficacious than G207 at inhibiting tumor growth in both the human U87MG xenograft and mouse Neuro2a syngeneic tumor models. These properties make G47Δ a promising vector for tumor therapy.
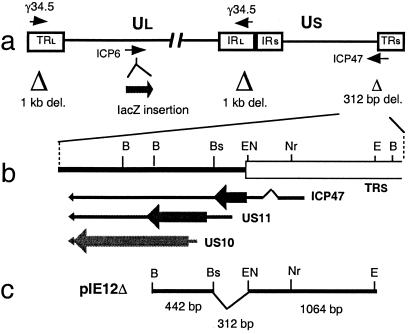
Figure 1.
Structure of G47Δ. (a) Schematic of the HSV-1 genome showing the regions modified in G47Δ. The HSV-1 genome consists of long and short unique regions (Ul and Us) each bounded by terminal (T) and internal (I) repeat regions (Rl and Rs). The parental virus G207 was engineered from wild-type HSV-1 strain F by deleting 1 kb within both copies of the γ34.5 gene and inserting the E. coli lacZ gene into the ICP6 coding region. G47Δ was derived from G207 by deleting 312 bp from the ICP47 locus, as indicated. (b) Map of the ICP47 locus showing locations of the overlapping 3′ coterminal transcripts (US10, US11, and ICP47), ORFs (thick arrow), and ICP47 splice junctions (∧). (c) Map of plasmid pIE12Δ used to generate deletion by homologous recombination with the indicated flanking sequences. Whereas US11 is regulated as a true late gene in wild-type HSV-1, deletion between the indicated BstEII and EcoNI sites places US11 under control of the ICP47 immediate-early promoter. Restriction site abbreviations: B, BamHI; Bs, BstEII; EN, EcoNI; Nr, NruI; E, EcoRI.
Materials and Methods
Cells.
Vero (African green monkey kidney), SK-N-SH (human neuroblastoma), U87MG (human glioma), U373MG (human glioma), Neuro2a (murine neuroblastoma), and Detroit 551 (diploid human fibroblast) cell lines were purchased from the American Type Culture Collection. SQ20B (head and neck squamous cell carcinoma) cells were provided by R. Weichselbaum (University of Chicago, Chicago). N18 murine neuroblastoma cells were provided by K. Ikeda (Tokyo Institute of Psychiatry, Tokyo). Human melanoma cell lines 624, 888, 938, 1102, and 1383 and human T cell lines TIL888 and TIL1413 were provided by J. Wunderlich (National Institutes of Health, Bethesda, MD). All tumor cells were maintained in DMEM supplemented with 10% FCS/2 mM glutamine/100 units/ml penicillin/100 μg/ml streptomycin/2.5 μg/ml Fungizone. Human T cells were maintained in AIM-V medium (GIBCO/BRL) supplemented with 10% human serum (type AB, Rh+; Valley Biomedical Products, Winchester, VA), 600 units/ml interleukin 2 (Chiron), 50 units/ml penicillin, and 1.25 μg/ml Fungizone.
Generation of G47Δ.
Plasmid pIE12 contains an 1,818-bp BamHI–EcoRI fragment from the HSV-1 BamHI x fragment, which encompasses the ICP47 coding region (28). A 312-bp fragment containing the ICP47 coding region between the BstEII and EcoNI sites was deleted from pIE12 to create pIE12Δ (Fig. 1c). Vero cells were seeded on 6-well dishes at 1–2 × 105 cells per well. Transfections were performed by using 1–3 μg of DNA, composing a 1:1:1 mixture of G207 (3) DNA, pIE12 (intact), and pIE12 cleaved with BamHI and XhoI, with 8 μl of Lipofectamine (Life Technologies, Rockville, MD), according to the manufacturer's instructions. The viral progeny from the transfection was then passaged twice in SK-N-SH cells to enrich for recombinants that contained the deletion in ICP47 as follows: SK-N-SH cells were seeded at 5 × 106 cells per 10-cm dish, infected the following day at a multiplicity of infection (moi) of 0.01–1, and harvested at 48 h after infection. This process was then repeated. The deletion was designed to generate a second-site suppresser mutation of γ34.5− that permits γ34.5− mutants to grow in SK-N-SH cells (25). Individual plaques from SK-N-SH-enriched stocks were plaque-purified on Vero cells under agarose overlays, and screened for the presence of the deletion in ICP47 by Southern blotting. A stock was prepared from one individual plaque that was homogeneous for the ICP47 deletion and designated as G47Δ. R47Δ was constructed similarly, except that R3616 (5) DNA was used in place of G207 DNA (R3616 was provided by B. Roizman, University of Chicago). Virus titration was performed as described (29).
Virus Yield Studies.
Cells were seeded on 6-well plates at 5 × 105, 8 × 105, or 1.6 × 106 cells per well. Triplicate or duplicate wells were infected with the viruses 6–8 h after seeding at an moi of 0.01. At 24 or 48 h after infection, the cells were scraped into the medium and lysed by three cycles of freeze thawing. The progeny virus was titered as described with a modification (29); briefly, Vero cells were plated in 6-well plates at 8 × 105 cells per well. After a 4- to 8-h incubation at 37°C, cells were infected in 1 ml of growth medium at 37°C overnight, after which 1 ml of medium containing 0.4% human IgG (ICN) was added. Wells were incubated at 37°C for another 2 days, and the number of plaques was counted after staining with methylene blue (0.5% wt/vol in 70% methanol).
In Vitro Cytotoxicity Studies.
In vitro cytotoxicity studies were performed as described (10), with a modification for human melanoma cells, which were grown in medium containing 10% (vol/vol) FCS. The number of surviving cells was counted daily with a Coulter Counter (Beckman Coulter) and expressed as a percentage of mock-infected controls.
Flow Cytometric Analyses.
Cells were plated in 6-well plates at 1 × 106 cells per well, and infected with virus (moi = 3) 24 h after seeding. Cells were incubated in the presence of ganciclovir (200 ng/ml) at 39.5°C for 6, 24, or 48 h, harvested by trypsinization, and washed once with 2 ml of PBS. G207 and G47Δ contain temperature-sensitive mutations in ICP4, so they can replicate at 37°C, but not at 39.5°C (3). Approximately 5 × 105 cells then were used for flow cytometric analyses using FITC-conjugated anti-human HLA class I antigen (clone W6/32, Sigma) and performed as described (10).
Human T Cell Stimulation Assays.
Human melanoma cells (888, 938, or 1102) were plated in 6-well plates at 5 × 105 cells per well, and infected with G207 or G47Δ (moi = 3) or without virus (mock) 24 h after seeding. Cells were incubated in growth medium containing 10% FCS and ganciclovir (200 ng/ml) at 39.5°C for 3 h (888) or 6 h (938 and 1102). Cells were then harvested by scraping, and a portion was used for cell counting. Infected melanoma cells (1 × 105) were then cocultured with an equal number of responding human T cells in 200 μl of AIM-V medium containing ganciclovir (200 ng/ml) in a flat-bottom 96-well plate. Melanoma 888 and 1102 were cocultured with TIL888 cells and melanoma 938 was cocultured with TIL1413 cells. TIL lines 888 and 1413 both recognize tyrosinase, a melanoma antigen, in an HLA-A24 restricted fashion (30, 31). After an 18 h-incubation at 37°C, the plate was centrifuged at 800 × g for 10 min, and conditioned medium was collected. IFN-γ concentrations were measured by ELISA with a human IFN-γ ELISA kit (Endogen, Cambridge, MA). The IFN-γ measurements in TIL cells without stimulator cells were considered the base release levels and were used to calculate the increase of IFN-γ secretion in stimulated TIL cells.
Animal Studies.
Six-week-old female A/J mice and athymic nude mice (BALB/c nu/nu) were purchased from the National Cancer Institute (Frederick, MD) and caged in groups of four or fewer. All animal procedures were approved by the Institutional Animal Care and Use Committee. Subcutaneous tumor therapy was performed as described (10, 32).
Intracerebral Inoculation Toxicity Studies.
Mock (PBS containing 10% glycerol), strain F (2 × 103 pfu), G207 (2 × 106 pfu), or G47Δ (2 × 106 pfu) in a volume of 5 μl was injected over a 5-min period into the right hemispheres of the brains of 6-week-old female A/J mice (n = 8, 10, 8, and 10, respectively) with a Kopf stereotactic frame (Kopf Instruments, Tujunga, CA). Cages were then blinded and mice were monitored daily for clinical manifestations for 3 weeks.
Results
Construction and Replication of G47Δ.
G47Δ was constructed by deleting 312 bp from G207 in the Us region adjacent to TRs (Fig. 1). Southern blot analyses of G47Δ DNA confirmed the presence of a 0.3-kb deletion in the α47 gene and a 1-kb deletion in the γ34.5 gene (data not shown). R47Δ (with the same deletion in the α47 locus) was generated from R3616, the parental virus of G207 that has an active ribonucleotide reductase (5).
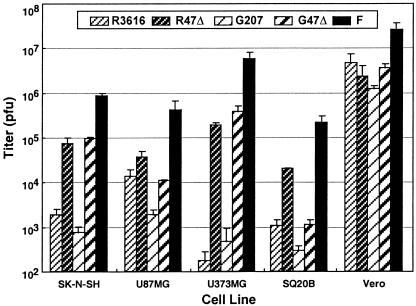
To investigate the effects of the α47 deletion on the growth properties of γ34.5-deficient mutants (G207 and R3616), we determined the yield of progeny virus after infection of human tumor cell lines SK-N-SH (neuroblastoma), U87MG (glioma), U373MG (glioma), and SQ20B (head and neck squamous cell carcinoma). By 24 h after infection at a low moi, G47Δ produced higher yields than G207, resulting in an ≈4- to 1,000-fold increase in titer (Fig. 2). In a single-step growth experiment in U87MG cells (moi = 2), the virus yield of G47Δ was 12 times greater than with G207 (Y. Ino and T.T., unpublished data). Similarly, R47Δ yielded higher titers than its parent R3616 in all tumor cell lines tested. However, neither G47Δ nor R47Δ grew as well as wild-type parental strain F. To determine whether virus yields were affected by cell density, Vero and SK-H-SH cells were seeded at a normal or higher density (8 × 105 or 1.6 × 106 cells per well), infected with strain F, G207, or G47Δ at an moi of 0.01, and harvested 48 h after infection. G47Δ produced a higher yield in the higher-density cultures, as opposed to G207, which had a reduced yield (data not shown).
Figure 2.
Virus yields of replication-competent HSV-1 mutants in various cell lines. Cells were seeded on 6-well plates at 5 × 105 cells per well. Triplicate wells were infected with R3616, R47Δ, G207, G47Δ, or strain F at an moi of 0.01. At 24 h after infection, cells were scraped into the medium and progeny virus was titered on Vero cells. In all cell lines tested, G47Δ showed a significantly higher replication capability than G207. Results represent the mean of triplicates ± SD.
Cytopathic Effect of G47Δ in Vitro.
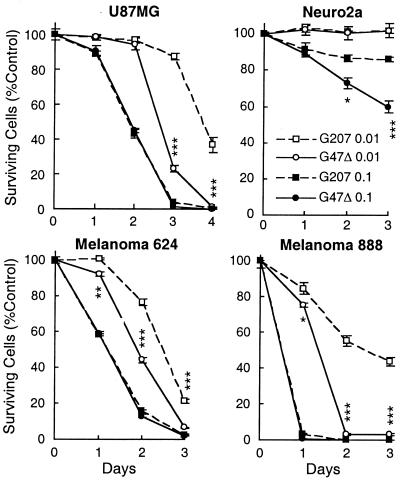
The cytolytic activity of G47Δ in vitro was compared with G207 in various neural crest-derived tumor cell lines. In human cell lines U87MG, melanoma 624, and melanoma 888, G47Δ killed tumor cells more rapidly than did G207 at a low moi of 0.01 (Fig. 3). At an moi of 0.1, both G207 and G47Δ killed all of the cells within 1–3 days of infection with no significant difference. Neuro2a, a murine neuroblastoma cell line, was resistant to killing by both G207 and G47Δ at an moi of 0.01. At an moi of 0.1, G47Δ was significantly more efficient at destroying Neuro2a cells than was G207 (Fig. 3), an effect also seen with N18 mouse neuroblastoma cells (data not shown). We have found that mouse tumor cells are generally more resistant to G207 replication than are human tumor cells (10, 11, 32).
Figure 3.
Cytopathic effect of G47Δ in vitro. Cells were plated into 6-well plates at 2 × 105 cells per well. After a 24-h incubation, cells were infected with G207 or G47Δ at an moi of 0.01 or 0.1, or without virus (Control). The number of surviving cells was counted daily and expressed as a percentage of mock-infected controls. G47Δ exhibited a significantly stronger cytopathic effect than did G207 in all three human tumor cell lines (U87MG, melanoma 624, and melanoma 888) at an moi of 0.01, and also in Neuro2a murine neuroblastoma cells at an moi of 0.1. The results are the mean of triplicates ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001; G207 versus G47Δ, unpaired t test.
MHC Class I Expression in G47Δ-Infected Cells.
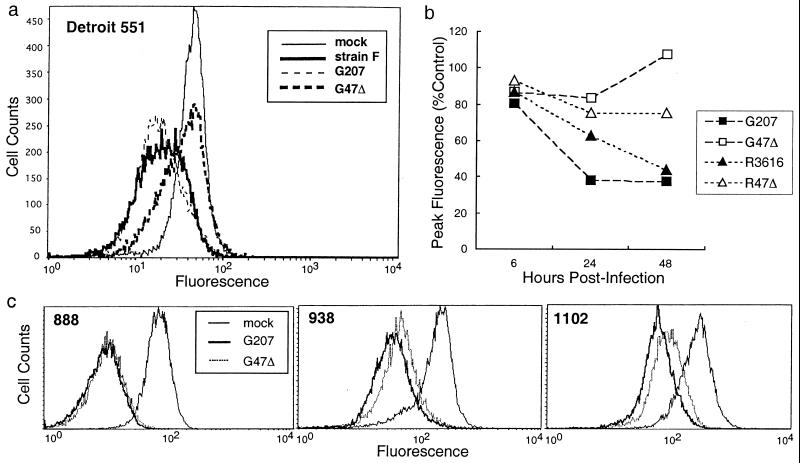
ICP47 inhibits the function of TAP in translocating peptides across the endoplasmic reticulum in human cells but not in mouse or rat cells (23, 33). Because G47Δ lacks ICP47, infected cells should have levels of MHC class I expression typical of uninfected cells. We examined MHC class I down-regulation in Detroit 551 human diploid fibroblasts by using flow cytometric analyses for HLA class I antigen. At 48 h after infection, all cells infected with HSV-1 containing an intact α47 gene (strain F, G207, and R3616) had a decrease in cell surface MHC class I (Fig. 4a). In contrast, there was no down-regulation in G47Δ-infected cells (Fig. 4a). In R47Δ-infected cells, MHC class I expression remained higher than in strain F (data not shown) or in R3616-infected cells, but was reduced compared with G47Δ (≈75% of mock-infected peak levels; Fig. 4b). Studies at different time points (6, 24, and 48 h after infection) revealed that differences in MHC class I down-regulation between ICP47-expressing (G207, R3616) and -nonexpressing (G47Δ, R47Δ) infected cells did not become apparent until passing 6 h after infection (Fig. 4b).
Figure 4.
G47Δ precludes down-regulation of MHC class I expression in infected host cells. (a) Flow cytometric analyses of MHC class I expression in Detroit 551 human fibroblast cells 48 h after infection with HSV-1 (moi = 3). Whereas all HSVs with an intact α47 gene (wild-type strain F and G207) significantly down-regulated MHC class I expression, G47Δ completely precluded the down-regulation. (b) Time course of MHC class I down-regulation in Detroit 551 cells infected with HSV-1. For each virus, the peak value of MHC class I expression at 6, 24, or 48 h after infection, as analyzed by flow cytometry, was expressed as a percentage of the peak value of mock-infected cells (Control) at each time point. MHC class I down-regulation by G207 and R3616 occurred in a time-dependent fashion. Dissociation of MHC class I expression between α47-deleted mutants (G47Δ and R47Δ) and α47-intact viruses became apparent at 24–48 h after infection. (c) Flow cytometric analyses of MHC class I expression in human melanoma cell lines 24 h after infection with G207 and G47Δ. G47Δ caused a partial preclusion of MHC class I down-regulation in melanomas 1102 and 938, resulting in greater MHC class I expression than G207.
Infection of human melanoma cells with G47Δ also resulted in higher levels of MHC class I expression than with G207, although the effect was partial. A greater effect was observed in cell lines with high basal levels of MHC class I (938 and 1102) compared with those with low levels of MHC class I (624, 888, and 1383) (Fig. 4c; data not shown for 624 and 1383).
G47Δ-Infected Human Melanoma Cells Stimulate Human T Cells in Vitro.
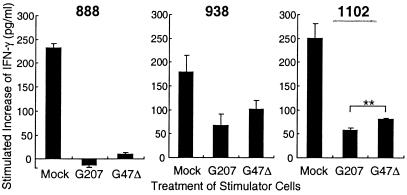
Three human melanoma cell lines were tested for their ability to stimulate the matched TIL lines after G47Δ infection [888 and 1102 with TIL888 (30), and 938 with TIL1413 (31)]. G47Δ-infected 1102 melanoma cells, with the highest level of MHC class I expression, caused a better stimulation of TIL cells compared with G207-infected cells, resulting in 41% more IFN-γ secretion (Fig. 5). There was essentially no stimulation of this same TIL line with G47Δ- or G207-infected 888 melanoma cells, which had very low levels of MHC class I expression. G47Δ-infected 938 melanoma cells stimulated TIL1413 cells, causing an increase in IFN-γ secretion that was not statistically significant. The results demonstrate that the higher MHC class I expression that may ensue in G47Δ- vs. G207-infected cells can enhance T cell stimulation.
Figure 5.
G47Δ-infected tumor cells stimulate T cells to a greater extent than G207-infected tumor cells. Human melanoma cells were mock infected (no virus) or infected with G207 or G47Δ at an moi of 3, and after 3–6 h, were cocultured with an equal number of responding human T cells for 18 h. T cell stimulation was assessed by an increase in IFN-γ release into conditioned media. G47Δ-infected melanoma 1102 cells caused a significantly greater stimulation of TIL888 cells compared with G207-infected 1102 cells (P < 0.01, unpaired t test). G47Δ-infected 938 melanoma cells also stimulated TIL1413 cells, although the improvement was not statistically significant compared with G207-infected 938 cells (P = 0.1, unpaired t test). Neither G207- nor G47Δ-infected melanoma 888 cells caused a significant stimulation of TIL888 cells.
Antitumor Efficacy of G47Δ in Vivo.
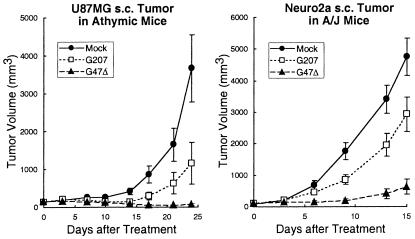
In a human xenograft model, athymic mice harboring established s.c. U87MG glioma tumors (≈6 mm in diameter), intraneoplastic inoculation of G207 or G47Δ (106 pfu) followed by a second inoculation 3 days later caused a significant reduction in U87MG tumor growth (P < 0.05 and P < 0.001 vs. control on day 24, respectively; unpaired t test; Fig. 6). G47Δ treatment was significantly more efficacious than was G207, resulting in reduced average tumor volumes (Fig. 6). This increased inhibition of tumor growth was reflected in the prolonged survival of animals and the number of “cures” (that is, complete tumor regression with no tumor regrowth during a 3-month follow up) (Table 1). Compared with mock-infected controls, survival was significantly prolonged in the G207-treatment group (P < 0.05 vs. mock, Wilcoxon test), and it was prolonged to an even greater extent in the G47Δ-treated animals (P < 0.05 vs. G207, Wilcoxon test).
Figure 6.
G47Δ exhibits greater antitumor efficacy than G207 in vivo. S.c. tumors of U87MG human glioma (Left) or Neuro2a murine neuroblastoma (Right) were generated in 6-week-old female athymic mice or 6-week-old female A/J mice, respectively. Established tumors of ≈6-mm diameter were inoculated with G207 or G47Δ (1 × 106 pfu) or Mock (PBS with 10% glycerol) on days 0 and 3. G47Δ treatment was significantly more efficacious than G207 in both tumor models, resulting in smaller average tumor volumes (P < 0.05 for U87MG on day 24 and P < 0.001 for Neuro2a on day 15; G207 versus G47Δ, unpaired t test).
Table 1.
Subcutaneous tumor therapy by G47Δ
| Tumor (Mouse) | Number cured/total treated
|
||
|---|---|---|---|
| Mock | G207 | G47Δ | |
| U87MG (Athymic) | 0/13 | 3/12 | 8/12*† |
| Neuro2a (A/J) | 0/10 | 1/10 | 3/10 |
*, P < 0.05 vs. G207.
†, P < 0.001 vs. Mock, Fisher's test.
The efficacy of G47Δ was further tested in an immunocompetent mouse tumor model; s.c. and poorly immunogenic Neuro2a neuroblastoma tumors in syngeneic A/J mice. Established tumors ≈6 mm in diameter were inoculated with mock, G207, or G47Δ (106 pfu) on days 0 and 3. Again, whereas both G207 and G47Δ caused a significant reduction in Neuro2a tumor growth (P < 0.05 and P < 0.001 versus control on day 15, respectively; unpaired t test), the efficacy of G47Δ was greater than that of G207 (Fig. 6). Kaplan–Meier analysis demonstrated that, at this dose, G207 did not significantly extend the survival of Neuro2a tumor-bearing A/J mice, whereas G47Δ significantly prolonged survival of the animals compared with mock and G207 (P < 0.01 and P < 0.05, respectively, Wilcoxon test). In a 3.5-month follow-up period, there was an increased number of “cures” amongst the G47Δ-treated mice (not statistically significant, Fisher's exact test; Table 1).
Safety of G47Δ with Intracerebral Inoculation.
To evaluate the toxicity of G47Δ in the brain, A/J mice were inoculated intracerebrally with mock, strain F (2 × 103 pfu), G207 (2 × 106 pfu), or G47Δ (2 × 106 pfu). This dose was the highest dose obtainable by us for G207 in the volume injected. Each mouse was monitored daily for clinical manifestations for 3 weeks. All 8 mock-inoculated mice survived without any abnormal manifestations, whereas all 10 strain F-inoculated mice deteriorated rapidly and became moribund within 7 days of inoculation (data not shown). All 8 G207-inoculated mice and 10 G47Δ-inoculated mice survived. Two of the G207-inoculated mice and one G47Δ-inoculated mouse temporarily manifested (3–6 days after inoculation) slight hunching or a slightly sluggish response to external stimuli. This result indicates that G47Δ is as nontoxic as G207 when inoculated in the brain of A/J mice at this dose.
Discussion
G47Δ was generated from G207 with properties that may enhance its efficacy for cancer therapy in humans. G207 is currently in phase I clinical trial for recurrent glioma; G207 has demonstrated no significant treatment-associated toxicity, and has demonstrated encouraging anecdotal antitumor activity (16). The goal of deleting the α47 gene was to abrogate the down-regulation of MHC class I expression that occurs in HSV-infected human cells, and to increase oncolytic activity in tumor cells. The effect on down-regulation was prominently demonstrated in Detroit 551 fibroblasts, in which G47Δ infection caused no change in the level of MHC class I expression. Infection with other replication-competent HSVs with intact α47 genes (with or without other coexisting mutations) showed a time-dependent down-regulation of MHC class I expression, resulting in an ≈60% decrease at 48 h after infection. It has been shown (34) that a replication-deficient HSV-1 vector with a deletion in the α47 gene does not down-regulate MHC class I expression in human melanoma cells.
The lack of ICP47 binding to rodent TAP (22, 33) makes it difficult to examine the impact of ICP47 on HSV-mediated tumor therapy. We developed a surrogate in vitro assay by using human melanoma cell lines and matched human TILs. The use of this assay led to the following observations: (i) HSV-1 infection inhibits the capability of human tumor cells to stimulate T cells; (ii) increased MHC class I expression after G47Δ infection can lead to enhanced T cell stimulation; and (iii) the ability of particular melanoma cells to stimulate their cognate T cells depends on the level of MHC class I expression. In a peptide vaccine trial for melanoma patients, increased T cell stimulation (assessed by IFN-γ release in vitro) was shown to correlate with prolonged relapse-free survival (35). The partial preclusion of MHC class I down-regulation in G47Δ-infected human melanoma cells may be due in part to the high susceptibility of these cells to HSV-1 cytotoxicity. In addition, many tumors with poor MHC class I expression have TAP deficits or dysfunctions (36, 37). Transferring a normal TAP gene may be useful in such occasions (37).
The major functions of the γ34.5 gene are (i) to prevent protein kinase R (PKR)-mediated phosphorylation of eIF2α, precluding host-cell shutoff of protein synthesis upon viral infection (6, 26), and (ii) to enable HSV to cause disease in the nervous system (5). γ34.5− mutants prematurely terminate protein synthesis, leading to impaired growth (6, 25), and also have a restricted host range in vitro, replicating poorly in certain human (e.g., SK-N-SH and U373MG) and mouse cells (6, 38). Extragenic suppression of the reduced-growth and restricted-host-range phenotypes of γ34.5− mutants is caused by the ectopic early expression of US11 caused by the deletion in the α47 gene (25–27). For G47Δ, this suppression of γ34.5− phenotypes led to higher virus yields than for G207 and improved cell killing in the cells tested in vitro. The ability to generate higher yields of G47Δ in Vero cells may facilitate the manufacturing of high-titer stocks for clinical use. A similar improvement in yield was observed with R47Δ, which contains the same deletion but is derived from R3616. The improved growth properties of G47Δ correlated with significantly enhanced antitumor activity in vivo. Increased inhibition in tumor growth, longer survival, and higher cure rates were seen in two different nervous system tumors (human U87MG and murine Neuro2a) in athymic and immunocompetent mice. It should be noted that the consequence of the lack of ICP47, i.e., improved antitumor immune cell induction, should not be reflected in either of these animal tumor models but might be expected in humans.
Retaining the safety properties of G207 is an important feature of G47Δ. Because ICP47 is used by HSV-1 to escape host immune surveillance, its deletion might attenuate the virus in normal tissue. In support of this idea, an α47-deleted, replication-competent HSV-1 with wild-type US11 and γ34.5 functions was less neurovirulent than the parental virus (strain F) after uniocular corneal infection (105 pfu) in A/J mice, and this attenuation depended on CD8+ T cells (39). Also, mice deficient in MHC class I expression (β2-microglobulin knockouts) are much more susceptible to HSV (40). Despite increased growth in tumor cells, G47Δ was as safe as G207 at the tested dose (2 × 106 pfu) when inoculated in the brains of A/J mice. γ34.5− mutants have been shown to exhibit wild-type neurovirulence in mice lacking protein kinase R (PKR; ref. 41), suggesting that suppression of PKR-mediated eIF2α phosphorylation by the suppressor mutation in α47 could restore neurovirulence to HSV-1. However, with G47Δ we found that suppression of protein synthesis shutoff and impaired growth do not significantly increase neurovirulence, at least with a coexisting ICP6 inactivation. Whether increased immunogenicity of G47Δ-infected tumor cells affects antitumor efficacy awaits testing in clinical trials.
We identified several properties of the biology of γ34.5-deficient HSV-1 vectors—impaired growth, limited host range, and down-regulation of MHC class I expression—that could be altered in a fashion that might improve their efficacy in cancer therapy. The multiple features that make G47Δ an attractive vector for human cancer therapy include potent stimulation of antitumor immune cells, high yields of virus, improved oncolytic activity, and safety in an HSV-1-sensitive rodent model. The safety and increased MHC class I presentation should also make G47Δ useful as a backbone vector for expressing foreign antigens in the context of vaccination.
Acknowledgments
We thank Dr. J. Wunderlich for providing human melanoma and TIL cell lines and for helpful information regarding these cells, Drs. F. Tufaro and J. Ostrove for their critical reading of the manuscript, and Dr. J. Shinoda for technical assistance. This work was supported in part by National Institutes of Health Grant NS32677 (to R.L.M.).
Abbreviations
- HSV-1
herpes simplex virus type 1
- pfu
plaque-forming units
- ICP
infected cell protein
- TAP
transporter associated with antigen presentation
- MHC
major histocompatibility complex
- TIL
tumor-infiltrating lymphocyte
- moi
multiplicity of infection
References
- 1.Kirn D H. J Clin Invest. 2000;105:837–839. doi: 10.1172/JCI9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martuza R L. J Clin Invest. 2000;105:841–846. doi: 10.1172/JCI9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mineta T, Rabkin S D, Yazaki T, Hunter W D, Martuza R L. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein D J, Weller S K. J Virol. 1988;62:196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou J, Kern E R, Whitley R J, Roizman B. Science. 1990;250:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 6.Chou J, Roizman B. Proc Natl Acad Sci USA. 1992;89:3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKie E A, MacLean A R, Lewis A D, Cruickshank G, Rampling R, Barnett S C, Kennedy P G, Brown S M. Br J Cancer. 1996;74:745–752. doi: 10.1038/bjc.1996.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andreansky S, Soroceanu L, Flotte E R, Chou J, Markert J M, Gillespie G Y, Roizman B, Whitley R J. Cancer Res. 1997;57:1502–1509. [PubMed] [Google Scholar]
- 9.Chambers R, Gillespie G Y, Soroceanu L, Adreansky S, Chatterjee S, Chou J, Roizman B, Whitley R J. Proc Natl Acad Sci USA. 1995;92:1411–1415. doi: 10.1073/pnas.92.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todo T, Rabkin S D, Sundaresan P, Wu A, Meehan K R, Herscowitz H B, Martuza R L. Hum Gene Ther. 1999;10:2741–2755. doi: 10.1089/10430349950016483. [DOI] [PubMed] [Google Scholar]
- 11.Toda M, Rabkin S D, Kojima H, Martuza R L. Hum Gene Ther. 1999;10:385–393. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- 12.Todo T, Rabkin S D, Chahlavi A, Martuza R L. Hum Gene Ther. 1999;10:2869–2878. doi: 10.1089/10430349950016591. [DOI] [PubMed] [Google Scholar]
- 13.Hunter W D, Rabkin S D, Feigenbaum F, Todo T, Mineta T, Yazaki T, Toda M, Newsome J T, Platenberg R C, Manz H J, Martuza R L. J Virol. 1999;73:6319–6326. doi: 10.1128/jvi.73.8.6319-6326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sundaresan P, Hunter W D, Martuza R L, Rabkin S D. J Virol. 2000;74:3832–3841. doi: 10.1128/jvi.74.8.3832-3841.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Todo T, Feigenbaum F, Rabkin S D, Lakeman F, Newsome J T, Johnson P A, Mitchell E, Belliveau D, Ostrove J M, Martuza R L. Mol Ther. 2000;2:588–595. doi: 10.1006/mthe.2000.0200. [DOI] [PubMed] [Google Scholar]
- 16.Markert J M, Medlock M D, Rabkin S D, Gillespie Y, Todo T, Hunter W D, Palmer C A, Feigenbaum F, Tornatore C, Tufaro F, Martuza R L. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 17.Jennings S R, Rice P L, Kloszewski E D, Anderson R W, Thompson D L, Tevethia S S. J Virol. 1985;56:757–766. doi: 10.1128/jvi.56.3.757-766.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill A B, Barnett B C, McMichael A J, McGeoch D J. J Immunol. 1994;152:2736–2741. [PubMed] [Google Scholar]
- 19.York I A, Roop C, Andrews D W, Riddell S R, Graham F L, Johnson D C. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 20.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Nature (London) 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 21.Früh K, Ahn K, Djaballah H, Sempe P, van Endert P M, Tampe R, Peterson P A, Yang Y. Nature (London) 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 22.Jugovic P, Hill A M, Tomazin R, Ploegh H, Johnson D C. J Virol. 1998;72:5076–5084. doi: 10.1128/jvi.72.6.5076-5084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahn K, Meyer T H, Uebel S, Sempe P, Djaballah H, Yang Y, Peterson P A, Früh K, Tampe R. EMBO J. 1996;15:3247–3255. [PMC free article] [PubMed] [Google Scholar]
- 24.Mavromara-Nazos P, Ackermann M, Roizman B. J Virol. 1986;60:807–812. doi: 10.1128/jvi.60.2.807-812.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohr I, Gluzman Y. EMBO J. 1996;15:4759–4766. [PMC free article] [PubMed] [Google Scholar]
- 26.He B, Chou J, Brandimarti R, Mohr I, Gluzman Y, Roizman B. J Virol. 1997;71:6049–6054. doi: 10.1128/jvi.71.8.6049-6054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassady K A, Gross M, Roizman B. J Virol. 1998;72:7005–7011. doi: 10.1128/jvi.72.9.7005-7011.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson P A, Wang M J, Friedmann T. J Virol. 1994;68:6347–6362. doi: 10.1128/jvi.68.10.6347-6362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyatake S, Iyer A, Martuza R L, Rabkin S D. J Virol. 1997;71:5124–5132. doi: 10.1128/jvi.71.7.5124-5132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robbins P F, El-Gamil M, Kawakami Y, Rosenberg S A. Cancer Res. 1994;54:3124–3126. [PubMed] [Google Scholar]
- 31.Kang X, Kawakami Y, el-Gamil M, Wang R, Sakaguchi K, Yannelli J R, Appella E, Rosenberg S A, Robbins P F. J Immunol. 1995;155:1343–1348. [PubMed] [Google Scholar]
- 32.Todo T, Martuza R L, Dallman M J, Rabkin S D. Cancer Res. 2001;61:153–161. [PubMed] [Google Scholar]
- 33.Tomazin R, van Schoot N E, Goldsmith K, Jugovic P, Sempe P, Früh K, Johnson D C. J Virol. 1998;72:2560–2563. doi: 10.1128/jvi.72.3.2560-2563.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krisky D M, Marconi P C, Oligino T J, Rouse R J D, Fink D J, Cohen J B, Watkins S C, Glorioso J C. Gene Ther. 1998;5:1517–1530. doi: 10.1038/sj.gt.3300755. [DOI] [PubMed] [Google Scholar]
- 35.Wang F, Bade E, Kuniyoshi C, Spears L, Jeffery G, Marty V, Groshen S, Weber J. Clin Cancer Res. 1999;5:2756–2765. [PubMed] [Google Scholar]
- 36.Seliger B, Maeurer M J, Ferrone S. Immunol Today. 1997;18:292–299. doi: 10.1016/s0167-5699(97)01052-9. [DOI] [PubMed] [Google Scholar]
- 37.Alimonti J, Zhang Q-J, Gabathuler R, Reid G, Chen S S, Jefferies W A. Nat Biotechnol. 2000;18:515–520. doi: 10.1038/75373. [DOI] [PubMed] [Google Scholar]
- 38.Brown S M, Harland J, MacLean A R, Podlech J, Clements J B. J Gen Virol. 1994;75:2367–2377. doi: 10.1099/0022-1317-75-9-2367. [DOI] [PubMed] [Google Scholar]
- 39.Goldsmith K, Chen W, Johnson D C, Hendricks R L. J Exp Med. 1998;187:341–348. doi: 10.1084/jem.187.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holterman A X, Rogers K, Edelmann K, Koelle D M, Corey L, Wilson C B. J Virol. 1999;73:2058–2063. doi: 10.1128/jvi.73.3.2058-2063.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leib D A, Machalek M A, Williams B R, Silverman R H, Virgin H W. Proc Natl Acad Sci USA. 2000;97:6097–6101. doi: 10.1073/pnas.100415697. . (First Published May 9, 2000; 10.1073/pnas.100415697) [DOI] [PMC free article] [PubMed] [Google Scholar]