Abstract
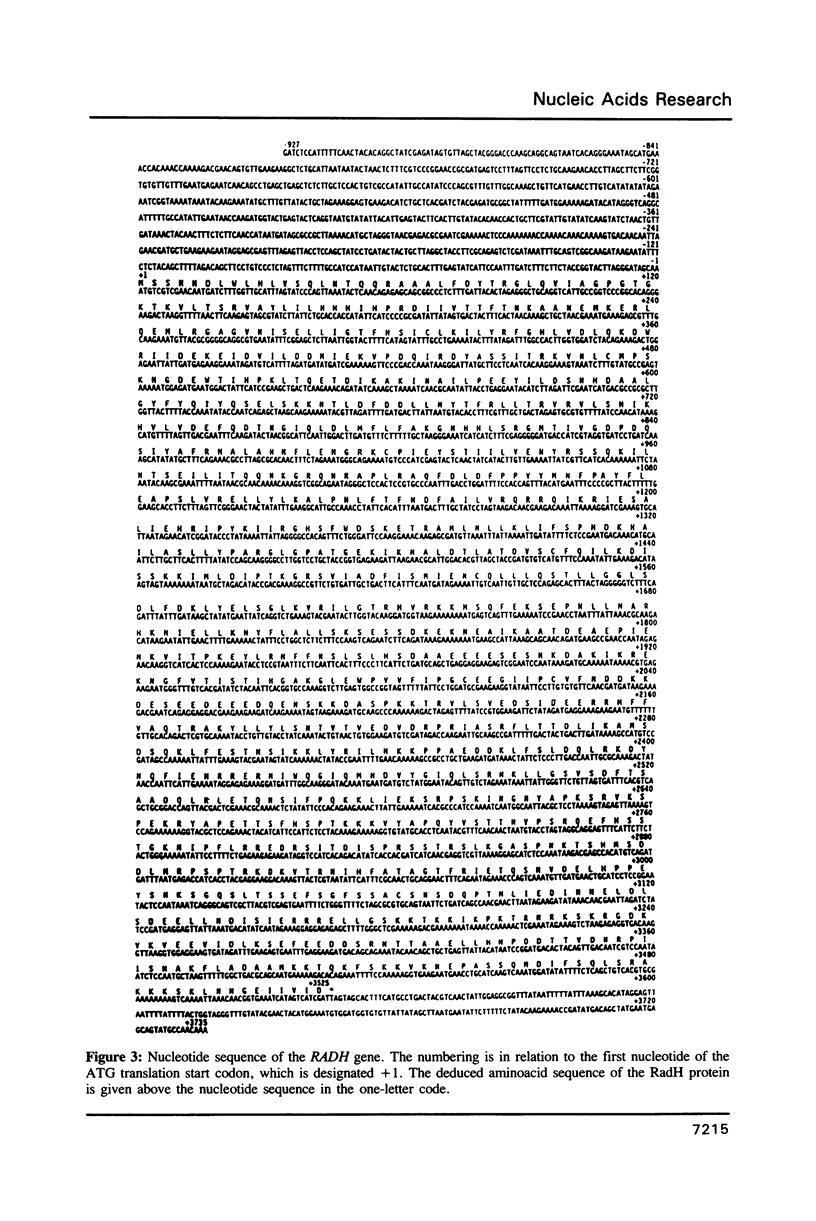
A new type of radiation-sensitive mutant of S. cerevisiae is described. The recessive radH mutation sensitizes to the lethal effect of UV radiations haploids in the G1 but not in the G2 mitotic phase. Homozygous diploids are as sensitive as G1 haploids. The UV-induced mutagenesis is depressed, while the induction of gene conversion is increased. The mutation is believed to channel the repair of lesions engaged in the mutagenic pathway into a recombination process, successful if the events involve sister-chromatids but lethal if they involve homologous chromosomes. The sequence of the RADH gene reveals that it may code for a DNA helicase, with a Mr of 134 kDa. All the consensus domains of known DNA helicases are present. Besides these consensus regions, strong homologies with the Rep and UvrD helicases of E. coli were found. The RadH putative helicase appears to belong to the set of proteins involved in the error-prone repair mechanism, at least for UV-induced lesions, and could act in coordination with the Rev3 error-prone DNA polymerase.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Brunborg G., Williamson D. H. The relevance of the nuclear division cycle to radiosensitivity in yeast. Mol Gen Genet. 1978 Jul 4;162(3):277–286. doi: 10.1007/BF00268853. [DOI] [PubMed] [Google Scholar]
- Finch P. W., Emmerson P. T. The nucleotide sequence of the uvrD gene of E. coli. Nucleic Acids Res. 1984 Jul 25;12(14):5789–5799. doi: 10.1093/nar/12.14.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Foury F., Lahaye A. Cloning and sequencing of the PIF gene involved in repair and recombination of yeast mitochondrial DNA. EMBO J. 1987 May;6(5):1441–1449. doi: 10.1002/j.1460-2075.1987.tb02385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Game J. C., Mortimer R. K. A genetic study of x-ray sensitive mutants in yeast. Mutat Res. 1974 Sep;24(3):281–292. doi: 10.1016/0027-5107(74)90176-6. [DOI] [PubMed] [Google Scholar]
- Gilchrist C. A., Denhardt D. T. Escherichia coli rep gene: sequence of the gene, the encoded helicase, and its homology with uvrD. Nucleic Acids Res. 1987 Jan 26;15(2):465–475. doi: 10.1093/nar/15.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson I. D., Arthur H. M., Bramhill D., Emmerson P. T. The E. coli uvrD gene product is DNA helicase II. Mol Gen Genet. 1983;190(2):265–270. doi: 10.1007/BF00330649. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgman T. C. A new superfamily of replicative proteins. Nature. 1988 May 5;333(6168):22–23. doi: 10.1038/333022b0. [DOI] [PubMed] [Google Scholar]
- Husain I., Van Houten B., Thomas D. C., Abdel-Monem M., Sancar A. Effect of DNA polymerase I and DNA helicase II on the turnover rate of UvrABC excision nuclease. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6774–6778. doi: 10.1073/pnas.82.20.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbey B. J., Brychcy T., Nasim A. Initiation of UV mutagenesis in Saccharomyces cerevisiae. Nature. 1978 Aug 31;274(5674):888–891. doi: 10.1038/274889a0. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Langford C. J., Klinz F. J., Donath C., Gallwitz D. Point mutations identify the conserved, intron-contained TACTAAC box as an essential splicing signal sequence in yeast. Cell. 1984 Mar;36(3):645–653. doi: 10.1016/0092-8674(84)90344-1. [DOI] [PubMed] [Google Scholar]
- Lawrence C. W., Christensen R. B. Metabolic suppressors of trimethoprim and ultraviolet light sensitivities of Saccharomyces cerevisiae rad6 mutants. J Bacteriol. 1979 Sep;139(3):866–876. doi: 10.1128/jb.139.3.866-876.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. C., Lei S. P., Wilcox G. An improved DNA sequencing strategy. Anal Biochem. 1985 May 15;147(1):114–119. doi: 10.1016/0003-2697(85)90016-8. [DOI] [PubMed] [Google Scholar]
- Naumovski L., Chu G., Berg P., Friedberg E. C. RAD3 gene of Saccharomyces cerevisiae: nucleotide sequence of wild-type and mutant alleles, transcript mapping, and aspects of gene regulation. Mol Cell Biol. 1985 Jan;5(1):17–26. doi: 10.1128/mcb.5.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P., Higgins D. R., Prakash L., Prakash S. The nucleotide sequence of the RAD3 gene of Saccharomyces cerevisiae: a potential adenine nucleotide binding amino acid sequence and a nonessential acidic carboxyl terminal region. Nucleic Acids Res. 1985 Apr 11;13(7):2357–2372. doi: 10.1093/nar/13.7.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung P., Higgins D., Prakash L., Prakash S. Mutation of lysine-48 to arginine in the yeast RAD3 protein abolishes its ATPase and DNA helicase activities but not the ability to bind ATP. EMBO J. 1988 Oct;7(10):3263–3269. doi: 10.1002/j.1460-2075.1988.tb03193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung P., Prakash L., Matson S. W., Prakash S. RAD3 protein of Saccharomyces cerevisiae is a DNA helicase. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8951–8955. doi: 10.1073/pnas.84.24.8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A., Smith G. R. Unwinding and rewinding of DNA by the RecBC enzyme. Cell. 1980 Nov;22(2 Pt 2):447–457. doi: 10.1016/0092-8674(80)90355-4. [DOI] [PubMed] [Google Scholar]
- Tschumper G., Carbon J. Sequence of a yeast DNA fragment containing a chromosomal replicator and the TRP1 gene. Gene. 1980 Jul;10(2):157–166. doi: 10.1016/0378-1119(80)90133-x. [DOI] [PubMed] [Google Scholar]
- Williamson D. H. The timing of deoxyribonucleic acid synthesis in the cell cycle of Saccharomyces cerevisiae. J Cell Biol. 1965 Jun;25(3):517–528. doi: 10.1083/jcb.25.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M., Buttin G., Hurwitz J. The isolation and characterization from Escherichia coli of an adenosine triphosphate-dependent deoxyribonuclease directed by rec B, C genes. J Biol Chem. 1971 Nov;246(21):6543–6555. [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982 Mar;28(3):563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]


