Abstract
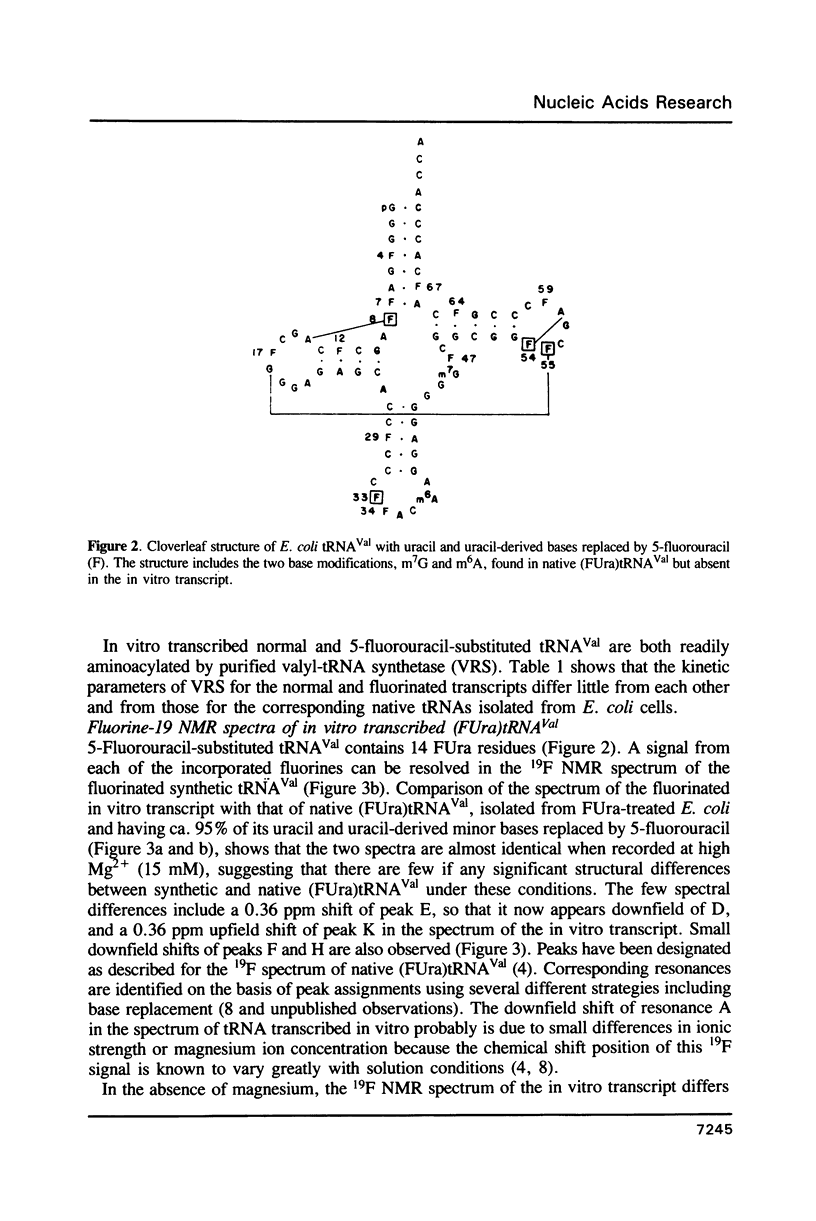
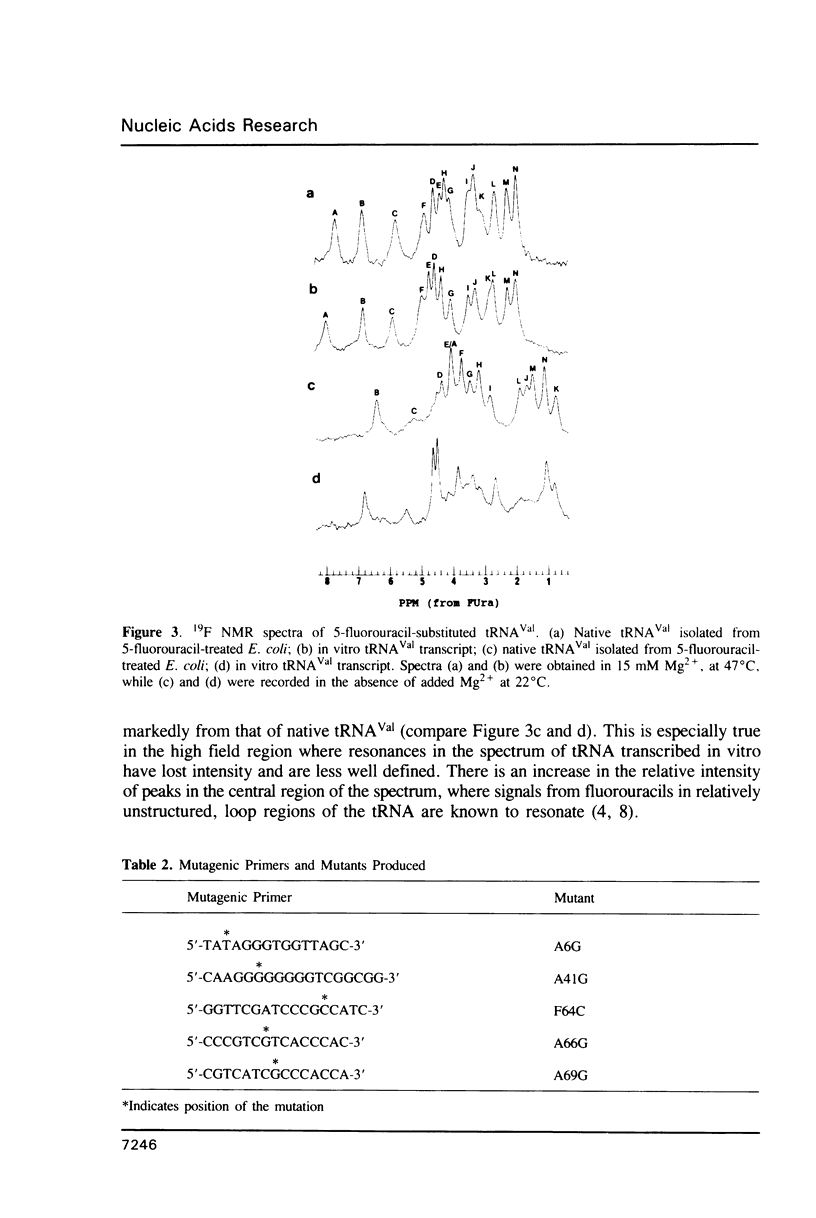
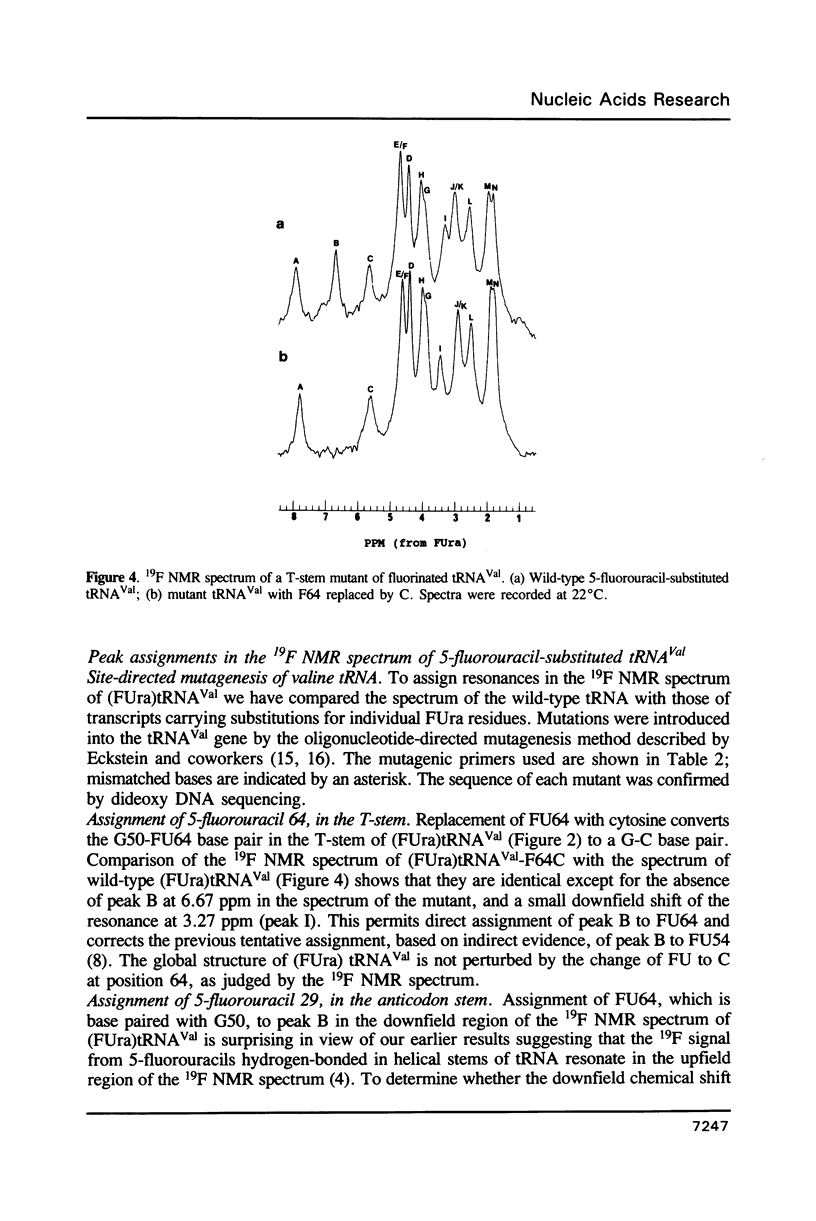
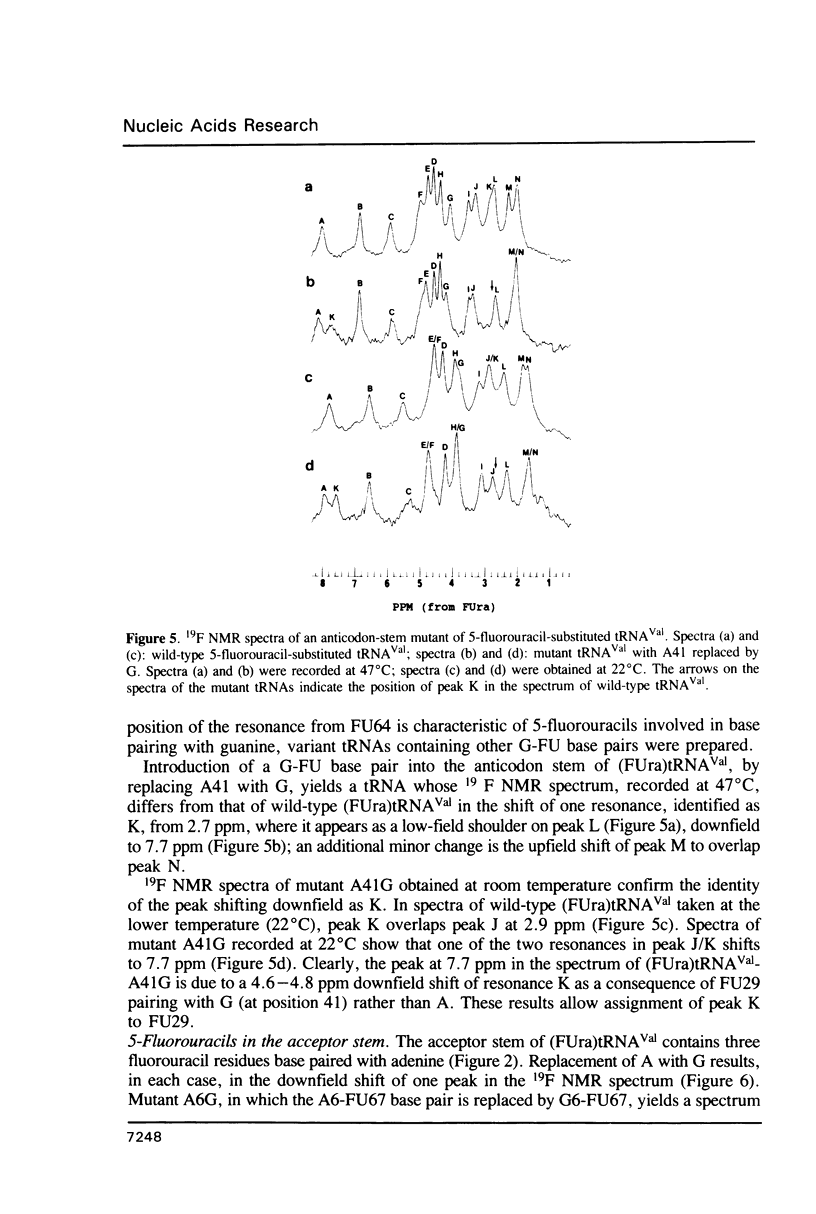
5-Fluorouracil is readily incorporated into active tRNA(Val) transcribed in vitro from a recombinant phagemid containing a synthetic E. coli tRNA(Val) gene. This tRNA has the expected sequence and a secondary and tertiary structure resembling that of native 5-fluorouracil-substituted tRNA(Val), as judged by 19F NMR spectroscopy. To assign resonances in the 19F spectrum, mutant phagemids were constructed having base changes in the tRNA gene. Replacement of fluorouracil in the T-stem with cytosine, converting a FU-G to a C-G base pair, results in the loss of one downfield peak in the 19F NMR spectrum of the mutant tRNA(Val). The spectra of other mutant tRNAs having guanine for adenine substitutions that convert FU-A to FU-G base pairs all have one resonance shifted 4.5 to 5 ppm downfield. These results allow assignment of several 19F resonances and demonstrate that the chemical shift of the 19F signal from base-paired 5-fluorouracil differs considerably between Watson-Crick and wobble geometry.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce A. G., Uhlenbeck O. C. Enzymatic replacement of the anticodon of yeast phenylalanine transfer ribonucleic acid. Biochemistry. 1982 Mar 2;21(5):855–861. doi: 10.1021/bi00534a007. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Coll M., Saal D., Frederick C. A., Aymami J., Rich A., Wang A. H. Effects of 5-fluorouracil/guanine wobble base pairs in Z-DNA: molecular and crystal structure of d(CGCGFG). Nucleic Acids Res. 1989 Feb 11;17(3):911–923. doi: 10.1093/nar/17.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick P., Hardin C. C., Horowitz J. 19F nuclear magnetic resonance as a probe of anticodon structure in 5-fluorouracil-substituted Escherichia coli transfer RNA. J Mol Biol. 1987 Oct 5;197(3):571–584. doi: 10.1016/0022-2836(87)90565-1. [DOI] [PubMed] [Google Scholar]
- Gollnick P., Hardin C. C., Horowitz J. Fluorine-19 nuclear magnetic resonance study of codon-anticodon interaction in 5-fluorouracil-substituted E. coli transfer RNAs. Nucleic Acids Res. 1986 Jun 11;14(11):4659–4672. doi: 10.1093/nar/14.11.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodberg J., Dunn J. J. ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J Bacteriol. 1988 Mar;170(3):1245–1253. doi: 10.1128/jb.170.3.1245-1253.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin C. C., Gollnick P., Horowitz J. Partial assignment of resonances in the 19F nuclear magnetic resonance spectra of 5-fluorouracil-substituted transfer RNAs. Biochemistry. 1988 Jan 12;27(1):487–495. doi: 10.1021/bi00401a070. [DOI] [PubMed] [Google Scholar]
- Hardin C. C., Gollnick P., Kallenbach N. R., Cohn M., Horowitz J. Fluorine-19 nuclear magnetic resonance studies of the structure of 5-fluorouracil-substituted Escherichia coli transfer RNA. Biochemistry. 1986 Sep 23;25(19):5699–5709. doi: 10.1021/bi00367a053. [DOI] [PubMed] [Google Scholar]
- Hardin C. C., Horowitz J. Mobility of individual 5-fluorouridine residues in 5-fluorouracil-substituted Escherichia coli valine transfer RNA. A 19F nuclear magnetic resonance relaxation study. J Mol Biol. 1987 Oct 5;197(3):555–569. doi: 10.1016/0022-2836(87)90564-x. [DOI] [PubMed] [Google Scholar]
- Hills D. C., Cotten M. L., Horowitz J. Isolation and characterization of two 5-fluorouracil-substituted Escherichia coli initiator methionine transfer ribonucleic acids. Biochemistry. 1983 Mar 1;22(5):1113–1122. doi: 10.1021/bi00274a019. [DOI] [PubMed] [Google Scholar]
- Horowitz J., Ofengand J., Daniel W. E., Jr, Cohn M. 19F nuclear magnetic resonance of 5-fluorouridine-substituted tRNA1Val from Escherichia coli. J Biol Chem. 1977 Jun 25;252(12):4418–4420. [PubMed] [Google Scholar]
- Horowitz J., Ou C. N., Ishaq M. Isolation and partial characterization of Escherichia coli valine transfer RNA with uridine-derived residues replaced by 5-fluorouridine. J Mol Biol. 1974 Sep 15;88(2):301–312. doi: 10.1016/0022-2836(74)90483-5. [DOI] [PubMed] [Google Scholar]
- Kremer A. B., Mikita T., Beardsley G. P. Chemical consequences of incorporation of 5-fluorouracil into DNA as studied by NMR. Biochemistry. 1987 Jan 27;26(2):391–397. doi: 10.1021/bi00376a009. [DOI] [PubMed] [Google Scholar]
- Krupp G. RNA synthesis: strategies for the use of bacteriophage RNA polymerases. Gene. 1988 Dec 10;72(1-2):75–89. doi: 10.1016/0378-1119(88)90129-1. [DOI] [PubMed] [Google Scholar]
- Metzler W. J., Lu P. Lambda cro repressor complex with OR3 operator DNA. 19F nuclear magnetic resonance observations. J Mol Biol. 1989 Jan 5;205(1):149–164. doi: 10.1016/0022-2836(89)90372-0. [DOI] [PubMed] [Google Scholar]
- Nakamaye K. L., Eckstein F. Inhibition of restriction endonuclease Nci I cleavage by phosphorothioate groups and its application to oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1986 Dec 22;14(24):9679–9698. doi: 10.1093/nar/14.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofengand J., Bierbaum J. Protein synthetic ability of Escherichia coli valine transfer RNA with pseudouridine, ribothymidine, and other uridine-derived residues replaced by 5-fluorouridine. J Mol Biol. 1974 Sep 15;88(2):313–325. doi: 10.1016/0022-2836(74)90484-7. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Shapiro L., Hare D. DNA and RNA: NMR studies of conformations and dynamics in solution. Q Rev Biophys. 1987 Aug;20(1-2):35–112. doi: 10.1017/s0033583500004224. [DOI] [PubMed] [Google Scholar]
- Ramberg E. S., Ishaq M., Rulf S., Moeller B., Horowitz J. Inhibition of transfer RNA function by replacement of uridine and uridine-derived nucleosides with 5-fluorouridine. Biochemistry. 1978 Sep 19;17(19):3978–3985. doi: 10.1021/bi00612a016. [DOI] [PubMed] [Google Scholar]
- Reyes V. M., Abelson J. A synthetic substrate for tRNA splicing. Anal Biochem. 1987 Oct;166(1):90–106. doi: 10.1016/0003-2697(87)90551-3. [DOI] [PubMed] [Google Scholar]
- Sampson J. R., Uhlenbeck O. C. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1033–1037. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Borén T., Johansen T. I., Lustig F. Properties of a transfer RNA lacking modified nucleosides. J Biol Chem. 1988 Sep 25;263(27):13692–13699. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Skogman S. G., Nilsson J. Molecular cloning and characterization of the gene for Escherichia coli valyl-tRNA synthetase. Gene. 1984 Oct;30(1-3):219–226. doi: 10.1016/0378-1119(84)90123-9. [DOI] [PubMed] [Google Scholar]
- Sowers L. C., Eritja R., Kaplan B. E., Goodman M. F., Fazakerley G. V. Structural and dynamic properties of a fluorouracil-adenine base pair in DNA studied by proton NMR. J Biol Chem. 1987 Nov 15;262(32):15436–15442. [PubMed] [Google Scholar]
- Sowers L. C., Eritja R., Kaplan B., Goodman M. F., Fazakerly G. V. Equilibrium between a wobble and ionized base pair formed between fluorouracil and guanine in DNA as studied by proton and fluorine NMR. J Biol Chem. 1988 Oct 15;263(29):14794–14801. [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]


