Abstract
Objective: Our objective was to construct a recombinant bacillus Calmette-Guérin vaccine (rBCG) that secretes human interferon-alpha 2b (IFNα-2b) and to study its immunogenicity and in vitro antitumor activity against human bladder cancer cell lines T24 and T5637. Methods: The signal sequence BCG Ag85B and the gene IFNα-2b were amplified from the genome of BCG and human peripheral blood, respectively, by polymerase chain reaction (PCR). The two genes were cloned in Escherichia coli-BCG shuttle-vector pMV261 to obtain a new recombinant plasmid pMV261-Ag85B-IFNα-2b. BCG was transformed with the recombinant plasmid by electroporation and designated rBCG-IFNα-2b. Mononuclear cells were isolated from human peripheral blood (PBMCs) and stimulated with rBCG-IFNα-2b or wild type BCG for 3 d, and then cultured with human bladder cancer cell lines T24 and T5637. Their cytotoxicities were measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Results: BCG was successfully transformed with the recombinant plasmid pMV261-Ag85B-IFNα-2b by electroporation and the recombinant BCG (rBCG-IFNα-2b) was capable of synthesizing and secreting cytokine IFNα-2b. PBMC proliferation was enhanced significantly by rBCG-IFNα-2b, and the cytotoxicity of PBMCs stimulated by rBCG-IFNα-2b to T24 and T5627 was significantly stronger in comparison to wild type BCG. Conclusions: A recombinant BCG, secreting human IFNα-2b (rBCG-IFNα-2b), was constructed successfully and was superior to control wild type BCG in inducing immune responses and enhancing cytotoxicity to human bladder cancer cell lines T24 and T5637. This suggests that rBCG-IFNα-2b could be a promising agent for bladder cancer patients in terms of possible reductions in both clinical dosage and side effects of BCG immunotherapy.
Keywords: Bacillus Calmette-Guérin (BCG) vaccine, Bladder neoplasms, Gene recombination, Interferon-alpha 2b
1. Introduction
Bladder transitional cell carcinoma (BTCC), the most common urological malignance in China, is characterized by a strong tendency to recur. Since Morales et al. (1976) first reported intravesical instillation of bacillus Calmette-Guérin (BCG) for the treatment of superficial bladder tumors, BCG has been shown to reduce the number and frequency of post-operational recurrences of BTCC. Compared to many chemotherapeutic agents, BCG is considered the best drug for preventing tumor development in bladder cancer patients, and periodical intravesical BCG administration is one of the most effective supplemental treatments for bladder cancer (Hassen and Droller, 2000; Malmström et al., 2009; Shelley et al., 2010). However, there are still around 30% of BTCC patients for whom BCG treatment does not have satisfactory effects, especially for those with serious illness (Shahin et al., 2003; Shelley et al., 2010). Also, the side effects of BCG have imposed considerable limitations on its clinical applications (Hassen and Droller, 2000; van der Meijden et al., 2001; Shahin et al., 2003; Malmström et al., 2009; Shelley et al., 2010). The extent of BCG side effects is closely related to the dosage, but reduction in dosage would consequently decrease its effectiveness (Yalçinkaya et al., 1998). Much effort has been made to modify BCG strains in order to enhance their effectiveness and reduce the side effects.
It is generally accepted that the mechanism of antitumor effects of BCG is linked to the patients’ immune response to BCG after intravesical treatment (de Boer et al., 2003; Suttmann et al., 2004; Shintani et al., 2007; Alexandroff et al., 2010). Cytokines such as interleukin-2 (IL-2), interferon (IFN), and tumor necrosis factor-alpha (TNF-α) are considered to play important roles in this process (Belldegrun et al., 1998; Mohanty et al., 2002; O′Donnell et al., 2004; Agarwal et al., 2010). It was expected that an increased local expression level of such molecules could enhance the antitumor effects of BCG. To a certain extent, various studies in which BCG was combined with certain immunological molecules have supported this expectation. The combination of intravesical BCG and IL-2 or IFN treatment has shown better short-term effects on BTCC patients who initially did not respond well to BCG alone (Mohanty et al., 2002; O′Donnell et al., 2004). However, long-term effects were not improved. On the other hand, treatment with exogenous cytokines has many unresolved problems such as high dosage, high frequency of side effects, short duration of drug activities, and high cost (Mohanty et al., 2002; O′Donnell et al., 2004). Recent research has focused more on exploring the function of BCG as a new vaccine vehicle, and on building recombinant BCG (rBCG) to increase its immunogenicity (Yamada et al., 2000; Arnold et al., 2004; Lee et al., 2004; Luo et al., 2004; Yu et al., 2007; Chade et al., 2008; Liu et al., 2009; Xu et al., 2009). This technique uses BCG as engineered bacteria that carry inserted exogenous genes so that BCG can replicate in the host and secrete exogenous antigens that induce immune responses. The use of recombinant BCGs, especially those that express cytokines, is considered to be the strategy with the most potential to enhance the antigenicity and antitumor effects of BCG (Yamada et al., 2000; Arnold et al., 2004; Luo et al., 2004; Liu et al., 2009).
Interferon-alpha 2b (IFNα-2b) is a type I IFN that was developed relatively early and has been widely used in clinical practice. It acts directly on tumor cells to inhibit their proliferation and differentiation (Pfeffer et al., 1998; Zella et al., 1999; Moro et al., 2001). Data from large multicenter clinical trials have shown that BCG combined with IFNα-2b can enhance antitumor effects (Belldegrun et al., 1998; Mohanty et al., 2002; O′Donnell et al., 2004). However, this combination is not successful in eliminating negative aspects of the treatment such as the high dosage of IFNα-2b, the frequent occurrence of side effects, and the short duration of therapeutic effects. In the current research, we hypothesized that a recombinant BCG that expressed IFNα-2b may show potential as a new treatment capable of reducing the dosage and side effects of BCG, while improving its antitumor effects against bladder cancer.
2. Materials and methods
2.1. Materials
The Danish strain of BCG was purchased from the Shanghai Institute of Biological Products, China, and the plasmid pMV261 was a generous gift from Dr. Charles K. STOVER (the John Hopkins University School of Medicine, USA). Deoxyribonucleoside triphosphate (dNTP) was purchased from Sangon Biotech Co., Ltd. (Shanghai, China), and restriction enzymes BamHI, EcoRI, HindIII, Taq enzyme, proteinase K, and T4 DNA ligase were purchased from Promega (Madison, WI, USA). DH5α was frozen in the Urology lab at the First Affiliated Hospital, School of Medicine, Zhejiang University (Hangzhou, China). Primers for IFNα-2b and BCGAg85B peptide signal sequences were synthesized at Bioasia Biotech Co., Ltd. (Shanghai, China). BCG media Middlebrook 7H9 broth, Middlebrook 7H10 agar, and additive Middlebrook ADC enrichment were purchased from Difco (Franklin Lakes, NJ, USA). Human IFNα-2b enzyme-linked immunosorbent assay (ELISA) kit was obtained from ADI (Norwood, MA, USA). Human bladder cancer cell lines T24 and T5637 were purchased from the Institute of Biochemistry and Cell Biology (Shanghai, China) and were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) at 37 °C in 5% CO2. IFNα-2b and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma (St. Louis, MO, USA).
2.2. Construction of pMV261-Ag85B-IFNα-2b
Genomic DNA, extracted from human peripheral blood, was purified and then amplified using sense primer 5′-GACGAATTCATGTGTGATCTGCCTCAAACC-3′ and antisense primer 5′-CGCAAGCTTTCATTCCTTACTTCTTAAAC-3′. BCG was cultured and its genomic DNA was extracted and purified. The peptide signal sequence BCGAg85B was amplified by PCR (sense 5′-GATGGATCCAATGACAGACGTGAGCCGAAAG-3′, and antisense 5′-GTAGAATTCCGCGCCCGCGGTTGCCGCTCC-3′). Human IFNα-2b and BCGAg85B signal sequences, digested by EcoRI-HindIII and EcoRI-BamHI respectively, were inserted into pMV261 and amplified in DH5α. The DNA sequence of pMV261-Ag85B-IFNα-2b was verified by DNA sequencing.
2.3. Construction of rBCG-IFNα-2b
BCG was cultured in Middlebrook 7H9 medium in a shaker at 37 °C, until the optical density at 600 nm (OD600) reached 0.6. A total of 200 μl of competent BCG and 10 μl of pMV261-Ag85B-IFNα-2b (~2 μg) were mixed well in a tube and electroporated to generate recombinant BCG as described by Luo et al. (1996), with BCG and BCG containing empty plasmid pMV261 as controls. Positive clones were picked after three to four weeks and verified by acid fast staining. rBCG-IFNα-2b plasmids were extracted and human IFNα-2b was amplified using the same primer pairs. The size of the insert was confirmed by electrophoresis. rBCG-IFNα-2b was induced by temperature-shifted induction and hydrogen peroxide. The bacteria and supernatant were obtained and subjected to Western blotting analysis by using a previously reported protocol (Varaldo et al., 2004). The supernatant IFNα-2b protein level was determined by ELISA assay according to the manufacturer’s instructions.
2.4. Measurement of the effects of rBCG-IFNα-2b on proliferation of peripheral blood mononuclear cells (PBMCs)
PBMCs were prepared by glucan-diatrizoate meglumin density gradient centrifugation as described by Luo et al. (2001) and cultured in RPMI-1640 (10% FBS) at a density of 106 cells/ml. A total of 100 μl of cells were added in triplicates into a 96-well plate. rBCG-IFNα-2b was added at a concentration gradient of 0, 1×104, 2×104, 4×104, and 8×104 CFU/ml. Treated cells were cultured at 37 °C with 5% CO2 for 1, 2, 3, or 5 d. The medium was removed and replaced with 100 μl of fresh medium. Then 20 μl of 5 mg/ml MTT was added to each well for 4 h. The medium was again removed and 120 μl of dimethyl sulphoxide (DMSO) was added. Plates were shaken at 600 r/min for 10 min and measured at 570 nm.
2.5. Measurement of cytotoxicity of rBCG-IFNα-2b activated PBMCs on bladder cancer cells
Human bladder cancer cell strains T24 and T5637 were cultured as target cells. Recombinant BCG-activated killer (RAK) effector cells were prepared by adding fresh PBMCs to 6-well plates at a cell density of 1×106 ml−1 (1 ml/well) and activated by 8×104 CFU/ml recombinant BCG. BIAK (PBMC activated by a combination of 8×104 CFU/ml wild type BCG and 50 U/ml IFNα-2b), BAK (PBMC activated by 8×104 CFU/ml wild type BCG), IAK (PBMC activated by 50 U/ml IFNα-2b), and PAK (PBMC activated by phosphate buffer saline) were included as controls. All cells were cultured for 3 d at 37 °C with 5% CO2. For MTT assays, 100 μl of effector cells were added to 96-well plates, followed by 100 μl target cells at an effector/target ratio of 10:1, 20:1, or 40:1. Target cells or effector cells, as appropriate, served as controls. All conditions were triplicated and 20 μl of 5 mg/ml MTT was added to each well after 12 h of culture at 37 °C with 5% CO2.
2.6. Statistical analysis
All results are shown as mean±standard deviation (SD). Multivariate analysis of variance (MANOVA) and one-way ANOVA were performed with differences considered significant at P<0.05.
3. Results
3.1. Verification of rBCG-IFNα-2b and detection of IFNα-2b
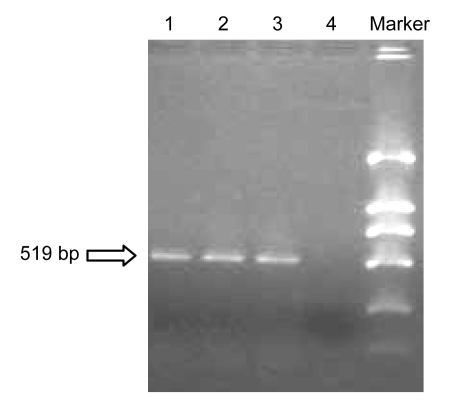
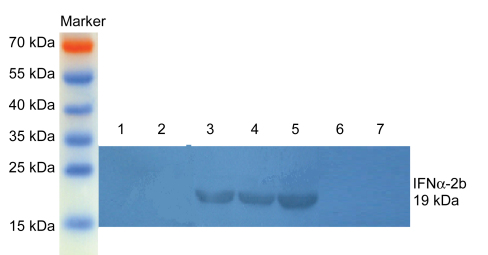
A positive acid fast staining indicated that the constructed rBCG-IFNα-2b was anti-acid bacteria rather than random contamination. rBCG-IFNα-2b plasmid DNA was extracted and amplified using IFNα-2b primers. We obtained a DNA segment of 519 bp, corresponding to the size of IFNα-2b gene (Fig. 1). Western blotting showed the protein expression of IFNα-2b in rBCG-IFNα-2b cultured supernatant and bacteria, while no such expression was found in recombinant BCG containing empty vector (pBCG), BCG culture media supernatant, or BCG bacteria (Fig. 2). Quantification of the protein expressed by IFNα-2b was achieved using ELISA and showed a concentration of 301.45 pg/ml in rBCG-IFNα-2b cultured supernatant, but not in the pBCG or BCG supernatants. Thus, we concluded that rBCG-IFNα-2b is capable of secreting human IFNα-2b protein.
Fig. 1.
PCR product of recombinant BCG plasmid
Lanes 1–4 are the PCR product: 1–3, recombinant BCG that secretes human IFNα-2b (rBCG-IFNα-2b); 4, recombinant BCG containing empty vector (pBCG). The size of the PCR product was about 519 kb, which is the same size as IFNα-2b
Fig. 2.
Western blotting of rBCG-IFNα-2b bacteria and supernatant
1, 2: pBCG transformed bacteria and culture supernatant, respectively (no protein was observed); 3: positive control (standard IFNα-2b protein); 4, 5: rBCG-IFNα-2b transformed bacteria and culture supernatant, respectively (the IFNα-2b protein had a size of 19 kDa); 6, 7: BCG transformed bacteria and supernatant, respectively (no expression of IFNα-2b protein was observed)
3.2. Effect of rBCG-IFNα-2b on PBMC proliferation
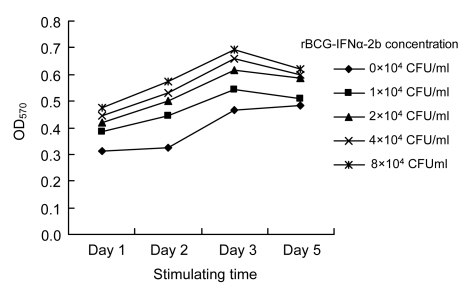
rBCG-IFNα-2b significantly enhanced proliferation of PBMCs on the first day, and proliferation peaked on the third day. PBMC proliferation increased with increasing concentrations of rBCG-IFNα-2b (Fig. 3).
Fig. 3.
Effects of various concentrations of rBCG-IFNα-2b on PBMC proliferation
OD570 was measured for relative survival. PBMC proliferation increased with increasing rBCG-IFNα-2b concentration. The rBCG-IFNα-2b concentration of 8×104 CFU/ml exerted the most proliferative effect. PBMC proliferation started 24 h after treatment with rBCG-IFNα-2b and peaked on Day 3
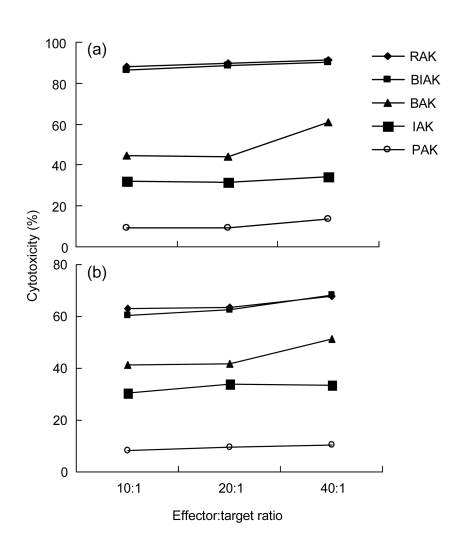
3.3. Cytotoxicity of RAK on human bladder cancer cells
The effector cells, identified according to their method of activation, were cultured for 3 d and then applied to target cells (T24 and T5637) at effector/target ratios of 10:1, 20:1, and 40:1. MTT assays showed significant cytotoxicities of RAK and BIAK on PBMC (P=0.0002, P<0.001, respectively) compared to BAK. There was no significant difference between the RAK and BIAK groups. Their cytotoxicities peaked at the effector/target ratio of 40:1 (Fig. 4).
Fig. 4.
Cytotoxicity of effector cells on human bladder cancer cells
(a) T24 cell line; (b) T5637 cell line
4. Discussion
We first successfully constructed the shuttle plasmid pMV261-Ag85B-IFNα-2b and obtained the recombinant BCG strain which expressed IFNα-2b as confirmed by Western blotting and ELISA. The recombinant IFNα-2b could be expressed and secreted extracellularly, which is a precondition for its proper biological function.
PBMCs activated by rBCG-IFNα-2b or BCG plus exogenous IFNα-2b exhibited more significant inhibition of cell proliferation on human bladder cancer cells (T24 and T5637) than those activated by BCG or IFNα-2b alone (Fig. 4). However, the combination of BCG and exogenous IFNα-2b is not clinically desirable due to the short half life of IFNα-2b, which leads to repeated intravesical administration. Moreover, intravesical IFNα-2b can be lost quickly through urination, and thus fail to remain inside the bladder for a prolonged period of time (Belldegrun et al., 1998; Mohanty et al., 2002; O′Donnell et al., 2004).
These shortcomings could be overcome by rBCG-IFNα-2b. BCG can bind to bladder wall for up to several months through fibronectin and is consequently taken up by bladder mucosa epithelial cells and macrophages through phagocytosis (Ratliff, 1992; Bowyer et al., 1995; Durek et al., 2001). During its attachment to the bladder wall, rBCG-IFNα-2b continues to express IFNα-2b to a level high enough to induce immune responses to BCG. Although this IFNα-2b level is lower than the high dosage of exogenous IFNα-2b, a continuous low level of IFNα-2b is expected to suffice to function as a tumor suppressor (Luo et al., 2001). It has been reported that an IFNα-2b level as low as 100 U/ml is adequate for stimulating PBMC proliferation and the antitumor effects (Luo et al., 2001). Our research corroborates that rBCG-IFNα-2b is able to enhance PBMC proliferation, which increases in proportion to the concentration of rBCG-IFNα-2b (Fig. 3). Effector cells activated by rBCG-IFNα-2b showed a significantly higher cytotoxicity on T24 and T5637 in comparison to effector cells activated by wild type BCG (Fig. 4) (P<0.001). Therefore, rBCG-IFNα-2b may be more promising clinically than the traditional intravesical solution (BCG plus IFNα-2b), since a one-time intravesical treatment may induce a strong and prolonged immune activity against cancer. IFNα-2b secreted by rBCG-IFNα-2b can directly inhibit tumor cell proliferation, whereas wild type BCG exhibits its cytotoxicity through an immunological mechanism. In addition to its synergic effects with BCG, rBCG-IFNα-2b may be used on patients who are insensitive to wild type BCG intravesical treatment (Luo et al., 2001; Mohanty et al., 2002; O′Donnell et al., 2004). Furthermore, rBCG-IFNα-2b has a stronger effect on stimulating proliferation of PBMCs and IFN-γ relative to wild type IFNα-2b. For example, IFN-γ can be detected in PBMCs treated with rBCG-IFNα-2b for 4 h and peaks at 24 h after treatment, while wild type BCG treatment leads to very limited secretion of IFN-γ (Luo et al., 2001). We also observed PBMC proliferation 24 h after rBCG-IFNα-2b treatment (Fig. 3), which indicates that rBCG-IFNα-2b is able to activate the immune system within a short time and thus may impose considerable pressure on bladder cancer cell division and proliferation. This property also increases the cytotoxic efficiency of the killer cells activated by rBCG-IFNα-2b.
Systemic inflammation is gaining increasing recognition as a diagnostic and therapeutic option even in patients with invasive bladder cancer. A recent article has demonstrated that preoperative serum C-reactive protein (CRP), which is produced by the liver after IL-6 secretion, is an independent prognostic factor in patients with high-risk bladder cancer (Gakis et al., 2011). Our rBCG-IFNα-2b might have more powerful effects in vivo. In a further study, we will evaluate the efficacy of rBCG-IFNα-2b in xenograft mice and later in patients. The pre- and postinterventional serum CRP levels could help to select and monitor these high risk patients.
In conclusion, we successfully constructed rBCG-IFNα-2b and validated its immunological activities and antitumor effects in two bladder cancer cell lines. Our data showed that rBCG-IFNα-2b significantly increased the antitumor effects of BCG against bladder cancer and has advantages compared with the traditional treatment of the combination of wild type BCG and exogenous IFNα-2b. However, future research is required on clinical applications of rBCG-IFNα-2b, with the aim of reducing the BCG dosage, side effects, BTCC post-operational recurrence, and medical costs for patients.
Footnotes
Project (No. 2006C30011) supported by the Science and Technology Department of Zhejiang Province of China
References
- 1.Agarwal A, Agrawal U, Verma S, Mohanty NK, Saxena S. Serum Th1 and Th2 cytokine balance in patients of superficial transitional cell carcinoma of bladder pre- and post-intravesical combination immunotherapy. Immunopharmacol Immunotoxicol. 2010;32(2):348–356. doi: 10.3109/08923970903300151. [DOI] [PubMed] [Google Scholar]
- 2.Alexandroff AB, Nicholson S, Patel PM, Jackson AM. Recent advances in bacillus Calmette-Guerin immunotherapy in bladder cancer. Immunotherapy. 2010;2(4):551–560. doi: 10.2217/imt.10.32. [DOI] [PubMed] [Google Scholar]
- 3.Arnold J, de Boer EC, O′Donnell MA, Böhle A, Brandau S. Immunotherapy of experimental bladder cancer with recombinant BCG expressing interferon-gamma. J Immunother. 2004;27(2):116–123. doi: 10.1097/00002371-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Belldegrun AS, Franklin JR, O′Donnell MA, Gomella LG, Klein E, Neri R, Nseyo UO, Ratliff TL, Williams RD. Superficial bladder cancer: the role of interferon-alpha. J Urol. 1998;159(6):1793–1801. doi: 10.1016/S0022-5347(01)63160-4. [DOI] [PubMed] [Google Scholar]
- 5.Bowyer L, Hall RR, Reading J, Marsh MM. The persistence of bacille Calmette-Guerin in the bladder after intravesical treatment for bladder cancer. Br J Urol. 1995;75(2):188–192. doi: 10.1111/j.1464-410X.1995.tb07309.x. [DOI] [PubMed] [Google Scholar]
- 6.Chade DC, Borra RC, Nascimento IP, Villanova FE, Leite LC, Andrade E, Srougi M, Ramos KL, Andrade PM. Immunomodulatory effects of recombinant BCG expressing pertussis toxin on TNF-alpha and IL-10 in a bladder cancer model. J Exp Clin Cancer Res. 2008;27(1):78. doi: 10.1186/1756-9966-27-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Boer EC, Rooijakkers SJ, Schamhart DH, Kurth KH. Cytokine gene expression in a mouse model: the first instillations with viable bacillus Calmette-Guerin determine the succeeding Th1 response. J Urol. 2003;170(5):2004–2008. doi: 10.1097/01.ju.0000091826.83705.79. [DOI] [PubMed] [Google Scholar]
- 8.Durek C, Richter E, Basteck A, Rüsch-Gerdes S, Gerdes J, Jocham D, Böhle A. The fate of bacillus Calmette-Guerin after intravesical instillation. J Urol. 2001;165(5):1765–1768. doi: 10.1016/S0022-5347(05)66410-5. [DOI] [PubMed] [Google Scholar]
- 9.Gakis G, Todenhofer T, Renninger M, Schilling D, Sievert KD, Schwentner C, Stenzl A. Development of a new outcome prediction model in carcinoma invading the bladder based on preoperative serum C-reactive protein and standard pathological risk factors: the TNR-C score. BJU Int. 2011;108(11):1800–1805. doi: 10.1111/j.1464-410X.2011.10234.x. [DOI] [PubMed] [Google Scholar]
- 10.Hassen W, Droller MJ. Current concepts in assessment and treatment of bladder cancer. Curr Opin Urol. 2000;10(4):291–299. doi: 10.1097/00042307-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Lee CF, Chang SY, Hsieh DS, Yu DS. Immunotherapy for bladder cancer using recombinant bacillus Calmette-Guerin DNA vaccines and interleukin-12 DNA vaccine. J Urol. 2004;171(3):1343–1347. doi: 10.1097/01.ju.0000103924.93206.93. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, O′Donnell MA, Chen X, Han R, Luo Y. Recombinant bacillus Calmette-Guérin (BCG) expressing interferon-alpha 2b enhances human mononuclear cell cytotoxicity against bladder cancer cell lines in vitro. Cancer Immunol Immunother. 2009;58(10):1647–1655. doi: 10.1007/s00262-009-0673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo Y, Szilvasi A, Chen X, deWolf WC, O′Donnell MA. A novel method for monitoring Mycobacterium bovis BCG trafficking with recombinant BCG expressing green fluorescent protein. Clin Diagn Lab Immunol. 1996;3(6):761–768. doi: 10.1128/cdli.3.6.761-768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo Y, Chen X, Han R, O′Donnell MA. Recombinant bacille Calmette-Guérin (BCG) expressing human interferon-alpha 2b demonstrates enhanced immunogenicity. Clin Exp Immunol. 2001;123(2):264–270. doi: 10.1046/j.1365-2249.2001.01428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo Y, Yamada H, Chen X, Ryan AA, Evanoff DP, Triccas JA, O′Donnell MA. Recombinant Mycobacterium bovis bacillus Calmette-Guérin (BCG) expressing mouse IL-18 augments Th1 immunity and macrophage cytotoxicity. Clin Exp Immunol. 2004;137(1):24–34. doi: 10.1111/j.1365-2249.2004.02522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malmström PU, Sylvester RJ, Crawford DE, Friedrich M, Krege S, Rintala E, Solsona E, di Stasi SM, Witjes JA. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56(2):247–256. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 17.Mohanty NK, Malhotra V, Nayak RL, Arora RP. Combined low-dose intravesical immunotherapy (BCG+ interferon alpha-2b) in the management of superficial transitional cell carcinoma of the urinary bladder: a five-year follow-up. J Chemother. 2002;14(2):194–197. doi: 10.1179/joc.2002.14.2.194. [DOI] [PubMed] [Google Scholar]
- 18.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 19.Moro A, Perea SE, Pantoja C, Santos A, Araña MD, Serrano M. IFNalpha 2b induces apoptosis and proteasome-mediated degradation of p27Kip1 in a human lung cancer cell line. Oncol Rep. 2001;8(2):425–429. [PubMed] [Google Scholar]
- 20.O′Donnell MA, Lilli K, Leopold C. Interim results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alfa-2b for superficial bladder cancer. J Urol. 2004;172(3):888–893. doi: 10.1097/01.ju.0000136446.37840.0a. [DOI] [PubMed] [Google Scholar]
- 21.Pfeffer LM, Dinarello CA, Herberman RB, Williams BR, Borden EC, Bordens R, Walter MR, Nagabhushan TL, Trotta PP, Pestka S. Biological properties of recombinant α-interferons: 40th anniversary of the discovery of interferons. Cancer Res. 1998;58(12):2489–2499. [PubMed] [Google Scholar]
- 22.Ratliff TL. Role of the immune response in BCG for bladder cancer. Eur Urol. 1992;21(S2):17–21. doi: 10.1159/000474916. [DOI] [PubMed] [Google Scholar]
- 23.Shahin O, Thalmann GN, Rentsch C, Mazzucchelli L, Studer UE. A retrospective analysis of 153 patients treated with or without intravesical bacillus Calmette-Guerin for primary stage T1 grade 3 bladder cancer: recurrence, progression and survival. J Urol. 2003;169(1):96–100. doi: 10.1016/S0022-5347(05)64044-X. [DOI] [PubMed] [Google Scholar]
- 24.Shelley MD, Mason MD, Kynaston H. Intravesical therapy for superficial bladder cancer: a systematic review of randomised trials and meta-analyses. Cancer Treat Rev. 2010;36(3):195–205. doi: 10.1016/j.ctrv.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Shintani Y, Sawada Y, Inagaki T, Kohjimoto Y, Uekado Y, Shinka T. Intravesical instillation therapy with bacillus Calmette-Guérin for superficial bladder cancer: study of the mechanism of bacillus Calmette-Guérin immunotherapy. Int J Urol. 2007;14(2):140–146. doi: 10.1111/j.1442-2042.2007.01696.x. [DOI] [PubMed] [Google Scholar]
- 26.Suttmann H, Jacobsen M, Reiss K, Jocham D, Bohle A, Brandau S. Mechanisms of bacillus Calmette-Guerin mediated natural killer cell activation. J Urol. 2004;172(4):1490–1495. doi: 10.1097/01.ju.0000131944.52354.63. [DOI] [PubMed] [Google Scholar]
- 27.van der Meijden AP, Brausi M, Zambon V, Kirkels W, de Balincourt C, Sylvester R. Intravesical instillation of epirubicin, bacillus Calmette-Guerin and bacillus Calmette-Guerin plus isoniazid for intermediate and high risk Ta, T1 papillary carcinoma of the bladder: a European Organization for Research and Treatment of Cancer genito-urinary group randomized phase III trial. J Urol. 2001;166(2):476–481. doi: 10.1016/S0022-5347(05)65966-6. [DOI] [PubMed] [Google Scholar]
- 28.Varaldo PB, Leite LC, Dias WO, Miyaji EN, Torres FI, Gebara VC, Armôa GR, Campos AS, Matos DC, Winter N, et al. Recombinant Mycobacterium bovis BCG expressing the Sm14 antigen of Schistosoma mansoni protects mice from cercarial challenge. Infect Immun. 2004;72(6):3336–3343. doi: 10.1128/IAI.72.6.3336-3343.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y, Liu W, Shen H, Yan J, Qu D, Wang H. Recombinant Mycobacterium bovis BCG expressing the chimeric protein of antigen 85B and ESAT-6 enhances the Th1 cell-mediated response. Clin Vaccine Immunol. 2009;16(8):1121–1126. doi: 10.1128/CVI.00112-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yalçinkaya F, Kamis L, Ozteke O, Gunlusoy B, Yigitbasi O, Unal S. Prospective randomized comparison of intravesical BCG therapy with standard dose versus low doses in superficial bladder cancer. Int Urol Nephrol. 1998;30(1):41–44. doi: 10.1007/BF02550276. [DOI] [PubMed] [Google Scholar]
- 31.Yamada H, Matsumoto S, Matsumoto T, Yamada T, Yamashita U. Murine IL-2 secreting recombinant Bacillus Calmette-Guerin augments macrophage-mediated cytotoxicity against murine bladder cancer MBT-2. J Urol. 2000;164(2):526–531. doi: 10.1016/S0022-5347(05)67417-4. [DOI] [PubMed] [Google Scholar]
- 32.Yu DS, Lee CF, Chang SY. Immunotherapy for orthotopic murine bladder cancer using bacillus Calmette-Guerin recombinant protein Mpt-64. J Urol. 2007;177(2):738–742. doi: 10.1016/j.juro.2006.09.074. [DOI] [PubMed] [Google Scholar]
- 33.Zella D, Barabitskaja O, Casareto L, Romerio F, Secchiero P, Reitz MS, Jr, Gallo RC, Weichold FF. Recombinant IFN-alpha (2b) increases the expression of apoptosis receptor CD95 and chemokine receptors CCR1 and CCR3 in monocytoid cells. J Immunol. 1999;163(6):3169–3175. [PubMed] [Google Scholar]