Abstract
We examined the role of α7- and β2-containing nicotinic acetylcholine receptors (nAChRs) in the induction of long-term potentiation (LTP). Theta-burst stimulation (TBS), mimicking the brain's naturally occurring theta rhythm, induced robust LTP in hippocampal slices from α7 and β2 knockout mice. This suggests TBS is capable of inducing LTP without activation of α7- or β2-containing nAChRs. However, when weak TBS was applied, the modulatory effects of nicotinic receptors on LTP induction became visible. We showed that during weak TBS, activation of α7 nAChRs occurs by the release of ACh, contributing to LTP induction. Additionally, bath-application of nicotine activated β2-containing nAChRs to promote LTP induction. Despite predicted nicotine-induced desensitization, synaptically mediated activation of α7 nAChRs still occurs in the presence of nicotine and contributed to LTP induction. Optical recording of single-stimulation-evoked excitatory activity with a voltage-sensitive dye revealed enhanced excitatory activity in the presence of nicotine. This effect of nicotine was robust during high frequency stimulation, and was accompanied by enhanced burst excitatory postsynaptic potentials. Nicotine-induced enhancement of excitatory activity was observed in slices from α7 knockout mice, but was absent in β2 knockout mice. These results suggest that the nicotine-induced enhancement of excitatory activity is mediated by β2-containing nAChRs, and is related to the nicotine-induced facilitation of LTP induction. Thus, our study demonstrates that the activation of α7-and β2-containing nAChRs differentially facilitates LTP induction via endogenously released ACh and exogenous nicotine, respectively, in the hippocampal CA1 region of mice.
Keywords: nicotinic acetylcholine receptors, α7 knockout mice, β2 knockout mice, optical recording, voltage-sensitive dye, synaptic plasticity
Introduction
Nicotine enhances cognitive function (Jones et al., 1992; Levin & Rezvani, 2002; Picciotto & Zoli, 2002; Newhouse et al., 2004), however, the cellular mechanisms underlying this effect remain largely unknown. Long-term potentiation (LTP) of excitatory synaptic transmission is an essential component of the cellular substrates of memory (Bliss & Collingridge, 1993; Malenka & Nicoll, 1999; Malenka & Bear, 2004), and nicotine facilitates the induction of LTP (Hunter et al., 1994; Hamid et al., 1997; Fujii et al., 1999; Fujii et al., 2000a; Fujii et al., 2000b; Mansvelder & McGehee, 2000; Matsuyama et al., 2000; Mann & Greenfield, 2003; Welsby et al., 2006; Welsby et al., 2009). This effect of nicotine is most likely related to its influence on cognitive function. Nicotine acts upon nicotinic acetylcholine receptors (nAChRs), and understanding the roles of the different nAChR subtypes in nicotine-induced facilitation of LTP induction may help identify which subtypes are involved in nicotine-induced cognitive enhancement.
There are nine unique nAChR subunits known to be widely expressed in the brain: α2–α7, and β2–β4 (Heinemann et al., 1990; Lindstrom et al., 1991; McGehee & Role, 1996; Role & Berg, 1996; Lukas et al., 1999). α2–α6 subunits assemble with β2–β4 subunits to produce functional hetero-oligomers, whereas α7 subunits form functional homo-oligomers. There are at least four nAChR subtypes in the hippocampus, a brain region critical for the formation of certain types of memory: α2*, α3β4* and α4β2* nAChRs (non-α7), and α7 nAChRs (Alkondon et al., 1998; Frazier et al., 1998a; Frazier et al., 1998b; McQuiston & Madison, 1999; Sudweeks & Yakel, 2000; Alkondon & Albuquerque, 2001; Ji et al., 2001; Yamazaki et al., 2005; Nakauchi et al., 2007; Rozsa et al., 2008; Jia et al., 2009). Among them, α7 nAChRs are the most abundant functional receptors. Several studies suggest that the activation of α7* nAChRs by endogenously released acetylcholine (ACh) plays a role in cognitive function (Levin & Simon, 1998; Levin et al., 2002; Curzon et al., 2006; Kenney & Gould, 2008), and timing-dependently facilitates LTP induction (Ji et al., 2001; Ge & Dani, 2005; Gu & Yakel, 2011). α7* nAChRs also appear to play a role in nicotine-induced facilitation of LTP at the Schaffer collateral (SC) pathway (Mann & Greenfield, 2003; Kenney & Gould, 2008). However, there are contradictory reports that both activation and desensitization of α7* nAChRs by nicotine mediate this effect (Fujii et al., 2000a; Mann & Greenfield, 2003; Chen et al., 2006; Kenney & Gould, 2008), thus further study is required. Furthermore, behavioral studies that utilized nAChR subtype-selective antagonists or nAChR subunit knockout mice indicate that it is β2* nAChRs, and not α7 nAChRs, that are critically involved in the enhancing effect of nicotine on hippocampal-dependent memory (Paylor et al., 1998; Davis & Gould, 2007; Davis et al., 2007). These results suggest not only that the less abundant non-α7 nAChR subtypes mediate nicotine-induced memory enhancement, but also that nicotine can enhance cognitive function without α7* nAChR activation.
The α4β2* nAChR subtype has the highest affinity for nicotine (Whiting et al., 1991; Flores et al., 1992) and is, therefore, the primary candidate for mediating nicotine's effect. However, the expression of α4 subunit mRNA is weak throughout the hippocampus (Wada et al., 1989; Rogers et al., 1998; Son & Winzer-Serhan, 2008). Studies have shown that β2* is involved in cognitive enhancement (Levin et al., 2002; Davis & Gould, 2007; Davis et al., 2007; Kenney & Gould, 2008). However, it is unknown whether the β2-containing nAChRs that are involved in nicotine-induced cognitive enhancement are solely α4β2* nAChRs. In the hippocampal CA1 region, α2* nAChRs are located in GABAergic interneurons in the alveus and oriens layers (Wada et al., 1989; Ishii et al., 2005; Son & Winzer-Serhan, 2008; Sudweeks & Yakel, 2000; Jia et al., 2009). Nicotine-induced facilitation of LTP induction appears to be mediated by a circuitry-dependent mechanism that is absent in α2 knockout mice (Nakauchi et al., 2007; Jia et al., 2009). Because previous research has indicated that α2* nAChRs contain the β2 subunit (Sudweeks & Yakel, 2000), the α2β2* nAChR is a potential candidate for mediating nicotine's effect. However, it largely remains to be determined whether β2-containing nAChR subtypes are involved in the nicotine-induced facilitation of LTP induction.
Additionally, some evidence suggests that α7 and β2 subunits can assemble to form heteromeric receptors (Khiroug et al., 2002; Liu et al., 2009). In the hippocampus, α7 and β2 subunit mRNAs are abundantly expressed (Wada et al., 1989; Son & Winzer-Serhan, 2008), whereas other nAChR subunit mRNAs have only scattered expression (Wada et al., 1989; Ishii et al., 2005; Son & Winzer-Serhan, 2008). The limited expression of other α subunits suggests the possibility that β2 could coassemble with α7. Interestingly, previous studies have suggested a connection between α7 subunits and Alzheimer s disease (AD) (Wang et al., 2000; Dziewczapolski et al., 2009), and such α7β2 nAChRs could be a potential target of the pathogenic peptide amyloid β1–;42 (Aβ1–;42) (Liu et al., 2001; Liu et al., 2009). However, it is still unknown how such receptors would contribute to the effect of nicotine in the hippocampus. Furthermore, it is unknown whether endogenously released ACh and exogenous nicotine influence LTP induction via distinct nAChR subtypes.
In the present study, we investigated the nicotinic modulation of LTP induction in the hippocampal CA1 region of wild type, α7-, and β2-null mutant mice using electrophysiological and optical recordings. The data obtained demonstrate that β2-containing nAChRs are essential for nicotine-induced hippocampal synaptic plasticity, whereas α7 nAChRs mediate the effect of endogenously released ACh on LTP induction.
Materials and methods
Animals
All animal procedures were conducted in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and with protocols approved by the Institutional Animal Care and Use Committee of the University of California at Irvine. Most of the experiments were carried out using littermate wild-type, α7 knockout, and β2 knockout mice. These mutant mice were from established colonies of heterozygous breeders in the C57BL/6J strain, originally obtained from Dr. Marina Picciotto (Yale University). However, in initial experiments some wild-type C57BL/6J mice were also used.
Slice preparation
Transverse hippocampal slices (375–400 μm) were prepared from mice (4–6 weeks) anaesthetized with urethane. For optical recordings, slices were placed onto a membrane filter (SVLP01300; Millipore, Billerica, MA, USA) and maintained at 30°C for at least 30 min in oxygenated ACSF to recover before staining for 20 min with voltage sensitive dye (VSD) staining solution (0.2 mM Di-4-ANEPPS, Molecular Probes, Carlsbad, CA, USA) oxygenated with 95% O2 and 5% CO2. Slices were maintained at 30°C for at least 1 h before recordings in artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl, 124; KCl, 5; NaH2PO4, 1.25; MgSO4, 2; CaCl2, 2.5; NaHCO3, 22; and glucose, 10, and oxygenated with 95% O2 and 5% CO2.
Extracellular field recordings
Slices were placed in a recording chamber, submerged, and continuously superfused at 2–3 ml/min with oxygenated ACSF at 30°C. A bipolar stimulating electrode (Rhodes Medical Instruments, Summerland, CA, USA) was placed in the stratum radiatum (SR) of CA1 region to stimulate the SC path. The field excitatory postsynaptic potentials (fEPSPs) were recorded from the SR of the CA1 region, using glass electrodes filled with ACSF (3–8 MΩ). Stimuli were short current pulses (200 μs duration) delivered every 20 s. At the beginning of each experiment, a stimulus-response curve was established by measuring the slope of fEPSPs. The strength of the stimulus was adjusted to elicit fEPSPs that were ~30–50% of the maximum response, which fell within stimulus intensities of 40–80 μA in the SC path. To avoid high variance in LTP magnitude, slices failing to meet these criteria were not used for experiments. The intensity and duration of each stimulus pulse remained invariant thereafter for each experiment. Following delivery of test stimuli via the stimulating electrode, baseline responses were recorded to establish the stability of the preparation. LTP was induced by theta burst stimulation (TBS; 10 bursts delivered at 5 Hz of four pulses at 100 Hz at 200 ms apart) or weak TBS (two theta bursts of four pulses at 100 Hz) as indicated. To evaluate the magnitudes of LTP, the mean value for the slopes of fEPSPs recorded 40–50 min after LTP-inducing stimulation was calculated and expressed as a ratio of the mean value of the initial baseline slope of fEPSPs. Recorded signals were amplified (A-M Systems, Sequim, WA, USA), digitized, stored on a computer and analyzed using NAC 2.0 software (Theta Burst Corp., Irvine, CA, USA).
Whole-cell recordings
Neurons were visualized using a 40× water immersion objective and differential interference contrast system under infrared light (Axioskop, Zeiss, Germany). Patch electrodes had resistance of 3–8 MΩ after being filled with pipette solution containing (in mM) Cs-methansulphonate, 117; HEPES, 10; EGTA, 0.5; TEA-Cl, 5; NaCl, 2.8; Mg-ATP, 2.5; Na-GTP, 0.3; and QX-314, 5; (pH 7.3 with CsOH). For recording inhibitory postsynaptic currents (IPSCs), pyramidal cells were clamped at −60 mV. Synaptic responses were evoked in cells by a single stimulation in the stratum radiatum in the presence of antagonists of glutamate receptors. All current recordings were amplified (Axopatch-200B amplifier; Axon Instruments, Inc., Union City, CA, USA), filtered (2 kHz), digitized at 10 kHz, stored on a computer and analyzed using custom software.
Optical recordings with VSD
Slices were transferred to a submerged recording chamber mounted on the stage of a fluorescence microscope (BX51WI; Olympus, Tokyo, Japan), and continuously superfused at 2–3 ml/min with oxygenated ACSF at 30°C. A monopolar glass electrode was placed in the SR to stimulate the SC path. A 4× objective lens (0.28 NA; Olympus) focused the excitation light on the CA1 region of the hippocampus. VSD imaging was performed with a high resolution CCD camera (MiCAM02; BrainVision, Tokyo, Japan). Optical recordings were acquired at a sampling rate of 4.0 ms per a frame. The trials were conducted every 20 s (0.05 Hz) and the recording period was 256 frames (1024.0 ms) for test stimulation. To avoid bleaching of the VSD, an electronically controlled shutter was opened 100 ms before the start of the recordings. Eight or 16 trials were averaged to improve the signal-to-noise ratio of the image. The fractional change in fluorescence intensity (ΔF/F) was used to normalize the differences of recordings. The level of neural activities was illustrated with pseudocolor. Activated areas were smoothed by averaging images with spatial and cubic filters. The signal gain and threshold levels were adjusted to optimize the signal-to-noise ratio of the response relative to background. In a series of recordings as control experiments, ΔF/F did not change significantly, indicating that bleaching of the VSD and photodynamic toxicity were negligible under conditions used. In some experiments, the extracellular potential recordings were simultaneously performed with optical recordings to ensure that the optical response was consistent with the electric response. Images were displayed and analysed using acquisition and analysis software (BV-Ana; BrainVision). Further details of the VSD imaging and recording techniques have been described previously (Tominaga et al., 2000; Nakauchi et al., 2007).
Drug application
Nicotine and dihydro-β-erythroidine (DHβE) were obtained from Sigma (St Louis, MO, USA). PNU120596 was purchased from Tocris Bioscience (Ellisville, MO, USA). Methyllycaconitine (MLA) was obtained from RBI (Natrick, MA, USA). 6,7-Dinitroquinoxaline-2,3-dione and DL-(−)-2-Amino-5-phosphonopentanoic acid were obtained from Ascent Scientific (New Jersey, USA). Nicotine was dissolved in ACSF and bath-applied for 10 min before delivery of LTP-inducing stimulation. Other drugs were dissolved in ACSF and bath-applied throughout recordings.
Statistical analysis
Statistical analyses using one- and two-way ANOVAs were applied. The overall ANOVA was followed by post hoc Tukey HSD tests to identify which groups were significantly different. The integrated area was measured from a trace of TBS-induced EPSP. Physiological data were plotted and analyzed using OriginPro 8.1 (OriginLab, Northampton, MA, USA). Data were normalized and expressed as means ± SEM.
Results
Normal LTP is induced in hippocampi of α7 knockout and β2 knockout mice
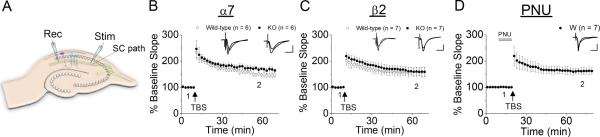
Because the α7 nAChR is highly Ca2+ permeable (Seguela et al., 1993), it has been thought to be involved in developmental and synaptic plasticity (Le Magueresse et al., 2006; Liu et al., 2006; Campbell et al., 2010). Evidence also indicates that β2-containing nAChRs also have distinct roles in the hippocampal development (Harrist et al., 2004; Mechawar et al., 2004; Le Magueresse et al., 2006). Thus, we initially measured the global impact of genetic inactivation of α7* nAChRs and β2-containing nAChRs on LTP induction in the hippocampal CA1 region by applying the theta-burst pattern of stimulation to the SC projections in non-littermate wild-type, littermate wild-type, α7 knockout and β2 knockout mice. This stimulation protocol, which is designed to mimic the burst firing of CA1 pyramidal cells in vivo generated during spatial exploration, induced robust LTP in all groups (Fig. 1). There was no significant difference in the magnitude of LTP between non-littermate and littermate wild-type controls and, thus, data obtained from these groups were combined for statistical analyses. The magnitude of LTP induced in wild-type, α7 and β2 knockout mice were very similar (Fig. 1). Furthermore, LTP induced in β2 knockout mice (159.4 ± 13.8%, n=7) were not significantly different from β2 wild-type control (159.2 ± 17.4%, n=7; one-way ANOVA, F1,12=0.02, P=0.88; Fig. 1). While LTP induced in α7 knockout mice (168.8 ± 7.3%, n=6) was slightly larger than that induced in α7 wild-type control (147.9 ± 10.3%, n=6, one-way ANOVA, F1,10=6.23, P<0.05; Fig. 1). These results suggest not only that the absence of either α7- or β2-containing nAChRs does not affect the induction of LTP in the SC pathway, but also that hippocampi formed in the absence of α7- or β2-containing nAChRs still develop cellular and molecular components required for LTP induction. To further test the possible involvement of α7 nAChR activation in LTP induction, we delivered TBS in the presence of PNU 120596, a drug that acts as a potent and selective positive allosteric modulator for the α7 nAChR (Hurst et al., 2005). We found that PNU 120596 had no significant effect on the magnitude of LTP (160 ± 2.1%, n=7; one-way ANOVA, F1,18=0.37, P=0.55; Fig.1), suggeting that the contribution of α7 nAChR activation to LTP is negligible under the conditions used.
Fig. 1.
LTP induced by conventional TBS was normal in α7 knockout and β2 knockout mice (A) Scheme of recording setup showing the position of stimulating and recording electrodes. (B) Conventional LTP in littermate wild-type and α7 knockout (α7 KO) mice. (C) Conventional LTP in littermate wild-type and β2 knockout (β2 KO) mice. (D) LTP induced in the presence of PNU 120596 (PNU) in wild-type mice. (B, C, and D) Changes in the slope of fEPSPs are plotted as the percentage change from initial baseline responses. Each trace above the graph in B, C, and D, and the following figures, was recorded at the time indicated. In this figure and the following figures, LTP-inducing stimulation was delivered at the time indicated by the arrow. Numbers in parentheses in this figure and the following figures indicate the numbers of experiments. Scale bars; 1 mV and 10 msec.
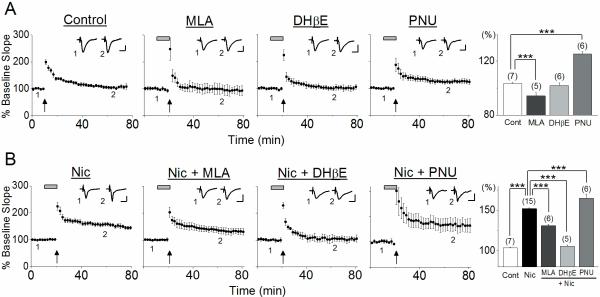
Activation of α7 nAChRs occurs during weak theta-burst stimulation and contributes to LTP induction
Theta burst stimulation is optimal for the induction of LTP because it reduces feedforward inhibition via activation of GABAB autoreceptors, allowing greater activation of the N-methyl-D-aspartate (NMDA) receptors (Bliss & Collingridge, 1993). However, the contribution of α7- and β2-containing nAChRs are difficult to observe under these conventional stimulus conditions. Therefore, to examine the contribution of these nAChRs to LTP induction, we next used the weak theta-burst pattern of stimulation, which is subthreshold for the induction of LTP (Overall ANOVA F3,804=45.13, P<0.001; Fig. 2A). This stimulation protocol produced very slight potentiation in hippocampi of wild-type mice (103.4 ± 0.6%, n=7), which was significantly reduced in the presence of the α7 nAChR antagonist MLA (94.3 ± 2.4%, n=5, post hoc Tukey test P<0.001) but not the antagonist DHβE, which blocks β2 subunit-containing nAChRs (101.8 ± 1.9%, n=6, post hoc Tukey test P=0.91; Fig. 2A). To confirm the activation of α7 nAChRs during weak TBS and their contribution to LTP induction, the stimulation was applied in the presence of PNU 120596. The resultant LTP observed was significantly larger (125.3 ± 1.8%, n=6, post hoc Tukey test P<0.001) than that induced in the absence of the drug (Fig. 2A). These observations confirm that ACh is released during weak TBS, which in turn activates α7 nAChRs, contributing to LTP induction. The lack of effect of DHβE on LTP induction suggests either that there is no sufficient activation of non-α7 nAChRs during the stimulation, or that non-α7 nAChR activation does not influence LTP induction. The possible mechanisms underlying α7 nAChR-mediated facilitation of LTP induction are the enhancement of NMDAR-mediated responses via increasing depolarization of pyramidal cells and additional influx of Ca2+ through α7 nAChR channels. These modulatory effects are required for the induction of LTP, when weak TBS induces only low-level NMDAR activation. However, when conventional TBS is delivered, high-level NMDAR activation occurs that is sufficient to induce LTP by itself. This is most likely reason why the effect of α7 nAChR activation on LTP induction was negligible when conventional TBS was used.
Fig. 2.
Endogenously released ACh and exogenous nicotine differentially facilitated LTP induction (A) In the absence of nicotine, weak TBS induced very small potentiation, which was blocked by the α7 nAChR antagonist MLA, but not β2-containing nAChR antagonist DHβE. The positive α7 nAChR allosteric modulator PNU 120596 enhanced LTP. (B) In the presence of nicotine, weak TBS induced large LTP, which was completely blocked by DHβE. MLA reduced LTP, while PNU 120596 increased LTP. (A), (B) Changes in the slope of fEPSPs were plotted as the percent change from initial baseline responses. Administration of nicotine in this figure and the following figure is indicated by the horizontal bar. Histograms show the percent change (mean ± SEM) in the slope of fEPSPs measured 40–50 min after delivery of weak TBS. Scale bars; 1 mV and 10 msec. *** P <0.001
The observed effect of MLA on LTP induction contradicts our previous finding that LTP was induced in the CA1 region of rats when a weak tetanus, which alone is not sufficient for LTP induction, was given in the presence of MLA (Fujii et al., 2000a). To examine whether opposing effects of MLA on LTP induction in rats and mice are due to different stimulation protocols used, we investigated the effect of MLA on LTP induction using a weak tetanus. We found that MLA blocked small LTP induced by a weak tetanus in mice (control: 108.1 ± 1.7%, n=6 vs. MLA: 99.7 ± 0.9%, n=7, one-way ANOVA, F1,416=20.26, P <0.001) as in the case of weak TBS. Thus, opposing effects of MLA on LTP induction in rats and mice are not due to different stimulation protocols used. We currently do not know why MLA elicits the opposing effects on LTP induction in rats and mice, but it is most likely that the different effects of MLA arise from differences in numbers of α7 nAChRs at various cellular and subcellular locations in the CA1 region of rats and mice.
Nicotine facilitates LTP induction via activation of non-α7 nAChRs
We have previously reported that weak TBS induces robust LTP at the SC pathway of mice in the presence of 1 μM nicotine (Nakauchi et al., 2007). In the study, the stimulation was delivered following 8 minute bath application of nicotine, during which different nAChR subtypes are differentially activated or desensitized, making it difficult to distinguish whether an agonistic or antagonistic effect of nicotine facilitates LTP induction. Bath application of 1 μM nicotine is known to reduce α7 nAChR-mediated responses due to desensitization, but does not inactivate the receptors completely (Frazier et al., 1998b; McQuiston & Madison, 1999; Yamazaki et al., 2005; Yamazaki et al., 2006). It should be noted that an antagonistic effect of nicotine on α7 nAChR-mediated responses is only observed if α7 nAChRs are activated by ACh released during stimulation. We found that the magnitude of LTP induced in the presence of nicotine (152.1 ± 1.0%, n=15, Overall ANOVA F4,1519=258.56, p<0.001; Fig. 2B) was significantly reduced by MLA (131.0 ± 1.6%, n=6, post hoc Tukey test P<0.001) and increased by PNU 120596 (165.0 ± 5.2%, n=6, post hoc Tukey test P<0.001; Fig. 2B). We have previously found that bath application of nicotine desensitizes α7 nAChRs without producing detectable activation, although a rapid pressure-ejected nicotine application induces α7 nAChR-mediated responses (Yamazaki et al., 2006). Thus, the suppressive effect of MLA on nicotine-induced facilitation of LTP induction is most likely due to blocking the facilitative effect of endogenous ACh released during weak TBS on LTP induction. These observations indicate that some α7 nAChRs remain able to be activated by endogenous ACh in the presence of nicotine, and that their activation still contributes to LTP induction, even though α7 nAChRs are at least partially inactivated via desensitization under the conditions (Frazier et al., 1998b; McQuiston & Madison, 1999; Yamazaki et al., 2005; Yamazaki et al., 2006). Nicotine's effect was completely blocked by DHβE (105.5 ± 1.7%, n=5, post hoc Tukey test P<0.001; Fig. 2B). This suggests that nicotine facilitates LTP induction via activation of non-α7 nAChRs. Our results also indicate that the non-α7 nAChRs involved in the nicotine's effect are not activated by ACh released during the weak TBS, and that their activation requires the presence of nicotine. Thus, they may be located at a distance from the stimulation site at the SC pathway where ACh is most likely released. The presence of DHβE apparently masks the effect of α7 nAChR activation (Fig. 2B). Because heteromeric α7β2 nAChRs are more sensitive to DHβE (Liu et al., 2009), activation of α7β2 nAChRs might also be involved in LTP induction.
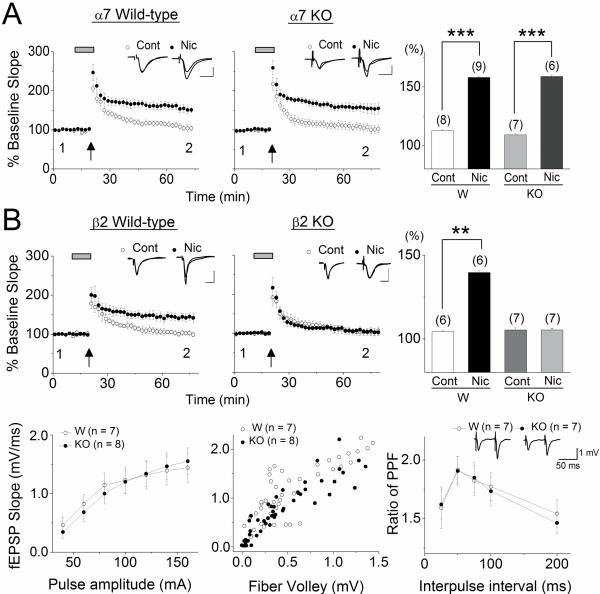
Nicotine-induced facilitation of LTP induction is absent in β2 knockout mice, but not α7 knockout mice
To further investigate the contribution of α7- and non-α7 nAChRs to the nicotine-induced facilitation of LTP induction, we utilized α7 knockout and β2 knockout mice. We confirmed that in the presence of 1 μM nicotine, weak TBS induced robust LTP in littermate wild-type controls from either α7- (control: 112.6 ± 1.0%, n=8 vs. nicotine: 158.1 ± 1.2%, n=9, one-way ANOVA F1,726=816.7, p<0.001; Fig. 3A) and β2-mutant mice (control: 104.3 ± 1.0%, n=6 vs. nicotine: 140.0 ± 1.4%, n=6, one-way ANOVA F1,459=426.52, p<0.01; Fig. 3B). When the stimulation was delivered to the SC pathway in α7 knockout mice in the absence of nicotine, small potentiation comparable to that observed in wild-type controls was measured (109.1 ± 1.1%, n=7; Fig. 3A). In contrast, in the presence of nicotine, the same stimulation at the SC pathway in α7 knockout mice induced robust LTP (158.9 ± 1.2%, n=6; Fig. 3A) with a very similar magnitude to that observed in the presence of nicotine in wild-type controls (α7 knockout control vs. nicotine, one-way ANOVA, F1,796=805.36, p<0.001;Fig.3A). Thus, α7 nAChRs are not required for nicotine-induced facilitation of LTP induction, suggesting that activation of α7 and α7β2 nAChRs is not involved in nicotine's effect. The results also suggest that the cellular mechanisms activated by nicotine appears to be intact in hippocampi developed in the absence of α7 nAChRs. When the stimulation was applied to SC pathway in β2 knockout mice in the absence of nicotine, very small potentiation was induced (105.3 ± 1.3%, n=7) that was similar in the magnitude to that induced wild-type controls (Fig. 3B). In contrast to α7 nAChR knockout mice, however, the potentiating effect of nicotine on LTP was absent (105.3 ± 1.1%, n=7; β2 knockout control vs. nicotine, one-way ANOVA, F1,582=0.12, p=0.73; Fig. 3B). To gain insight into the global effect of β2-null mutation on hippocampal development, we then examined whether basal synaptic transmission and paired-pulse facilitation (PPF, a presynaptic form of short-term plasticity) at the SC pathway are altered in the β2 knockout mice. First, we measured the input-output relationships by plotting the stimulus amplitude against the slope of the fEPSP, and by plotting the size of the fiber volley, which is related to the number of presynaptic neurons recruited by stimulation, against the slope of the fEPSP (Fig. 3B). We found that there were no significant differences between wild-type and β2 nAChR knockout mice in these measures (two-way ANOVAs; input-output curve F1,97=0.09, P=0.76; fiber volley F1,217=0.12, P=0.73; Fig. 3B). We next examined PPF, a measure of neurotransmitter release in both wild-type and β2 nAChR knockout mice, and found that there were no significant differences at any interpulse intervals (two-way ANOVA F1,64=0.04, P=0.84; Fig. 3B). These results suggest that there are no gross differences in basal synaptic transmission and glutamate release in the SC pathway of hippocampi developed without β2* nAChRs. Thus, our evidence suggests that the lack of nicotine's effect on LTP induction in β2 knockout mice is due to absence of β2* nAChRs in the local circuit. However, it remains possible that the absence of β2* nAChRs affected the development of local circuits, and prevented the nicotine's effect that is mediated by other non- α7 nAChR subtypes.
Fig. 3.
Nicotine-induced facilitation of LTP induction was present in α7 knockout mice, but absent in β2 knockout mice A weak TBS was delivered in the absence and presence of nicotine in (A) littermate wild-type and α7 knockout mice (α7 KO), and in (B) littermate wild-type and β2 knockout mice (β2 KO). (A) A weak TBS induced LTP in the presence of nicotine in wild-type and α7 KO mice. (B, top) A weak TBS induced LTP in the presence of nicotine in wild-type, but not β2 KO mice. (A and B, top) Histograms show the percentage change (mean ± SEM) in the slope of fEPSPs measured 40–50 min after delivery of weak TBS. (B, bottom) The lack of β2* nAChRs has no significant effects on synaptic transmission and short-term synaptic plasticity. Input-output relationships of fEPSPs in wild-type and β2 KO mice (left). Initial slopes of fEPSPs as a function of presynaptic fiber volley amplitudes in wild-type and β2 KO mice (middle). Paired-pulse ratio of fEPSPs in wild-type and β2 KO mice (right). The ratio of the second fEPSP slope to the first fEPSP slope was calculated and is shown at interpulse intervals ranging from 25 to 200 msec. Representative traces for 50 msec interpulse interval are shown. There were no significant differences in all measures between wild-type and β2 KO mice. Scale bars; 1 mV and 10 msec. **P < 0.01, ***P <0.001
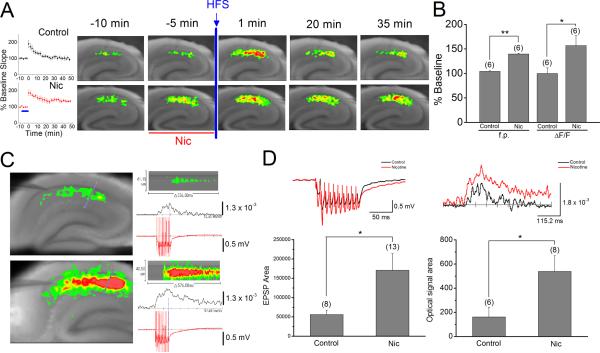
Nicotine-induced increases in excitatory activity underlie nicotine-mediated facilitation of LTP induction
Because electrophysiological recordings failed to detect a change in the slope of fEPSPs during bath application of nicotine (Figs. 2 and 3), we next used an optical imaging technique with VSD to simultaneously monitor the effect of nicotine on the excitatory activity during a LTP induction protocol. As previously reported (Nakauchi et al., 2007), optical signals recorded in response to a single stimulation at the SC pathway became stronger during bath application of nicotine, the effect of which could not be detected by extracellular field recording (Fig. 4A). This indicates that optical recordings are detecting slight nicotine-induced changes in the excitation/inhibition balance at the SC pathway. Optical signals, which were recorded 1 minute after weak high frequency stimulation in the absence and presence of nicotine, were significantly stronger than the corresponding responses recorded before delivery of weak high frequency stimulation (Fig. 4A). This most likely reflects post-tetanic potentiation. At this time point, there was no clear difference between optical signals obtained in the absence and presence of nicotine. However, at later time points, optical signals revealed significantly stronger membrane depolarization in slices exposed to nicotine than unexposed (control: 100.1 ± 11.4%, n=6 vs. nicotine: 156.8 ± 20.6, n=6; one-way ANOVA F1,10=5.80; P <0.05; Fig. 4A,B). This enhancement was well correlated to the increase in fEPSP slope (control: 104.3 ± 1.0%, n=6 vs. nicotine: 139.6 ± 1.4%, n=6, one-way ANOVA F1,459=426.52, P <0.01; Fig. 4A,B), and therefore, most likely reflects the nicotine-induced facilitation of LTP induction.
Fig. 4.
Nicotine enhanced optical signal and EPSPs during weak high frequency stimulation (A) Field EPSPs (left) and optical signal (right) were simultaneously recorded in the absence (Control, top) and presence of nicotine (Nic, bottom) during a LTP induction protocol. Pseudocolor representations of the voltage changes show in the response to a single stimulation in the absence (right, top) and presence of 1 μM nicotine (right, bottom) at different time points. (B) Histograms show the percent change (mean ± SEM) in the slope of fEPSPs and the amplitude of optical signals measured 35 min after delivery of high frequency stimulation. (C) Optical signal and EPSPs were simultaneously recorded during weak high frequency stimulation in the absence and presence of nicotine. Stimulation intensity was adjusted so that a single stimulation evoked similar sizes of fEPSPs in different slices. Pseudocolor representations of the voltage changes show in the response to weak high frequency stimulation in the absence (left, top) and presence of 1 μM nicotine (left, bottom). Pseudocolor representations of the line scanning across various anatomical layers, indicated in blue with a red dot (in left panels), over time in the absence (right, top) and presence (right, bottom) of nicotine. Comparisons of burst EPSPs and optical signal (ΔF/F) obtained in control (top traces) and nicotine (bottom traces) conditions are also shown. (D) Waveform comparison of burst EPSPs (left) and optical signals (right) evoked in the absence (black line) and presence (red line) of nicotine. Histograms show EPSP and optical signal areas recorded in the absence (Control) and presence of nicotine (Nic). *P < 0.05, **P < 0.01
The optical signal evoked by a single stimulation, but not the slope of fEPSPs, was enhanced during bath application of nicotine (Fig. 4A). However, it remains to be further tested whether this enhancement represents the mechanism for the nicotine-induced facilitation of LTP induction. To gain further insight into the enhanced optical signal during the nicotine-induced facilitation of LTP induction, we simultaneously recorded optical signals and fEPSPs during weak high frequency stimulation in the absence and presence of nicotine (Fig. 4C). As expected from the enhancing effect of nicotine on single stimulation-evoked optical signal, we found that the optical signal elicited in the presence of nicotine during weak high frequency stimulation was significantly stronger than that generated in the absence of nicotine (one-way ANOVA F1,12=5.12, P <0.05; Fig. 4C,D). Furthermore, we were able to detect that burst EPSPs evoked in the presence of nicotine are significantly larger than those triggered in the absence of nicotine (one-way ANOVA F1,19=4.59, P <0.05; Fig. 4C,D). The observations suggest that nicotine-induced changes in the excitation/inhibition balance are amplified during weak high frequency stimulation, resulting in the ability to detect enhancement of EPSPs by electrophysiological recordings. The implication of these observations is that the nicotine-induced enhancement of single stimulation-evoked optical signal represents the mechanism underlying the nicotine-induced facilitation of LTP induction.
Nicotine increases the excitatory neural activity at the SC path without involving α7 nAChRs
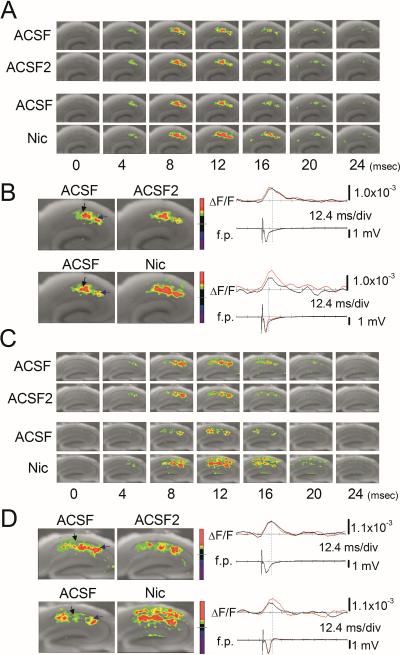
Nicotine-induced facilitation of LTP induction is intact in α7 knockout mice. The effect of nicotine appears to be mediated by a circuitry-dependent change in excitation/inhibition balance (Nakauchi et al., 2007; Jia et al., 2009). Thus, we next examined whether the lack of α7 nAChRs affects the circuitry-dependent mechanism by monitoring circuit activity in littermate wild-type and α7 knockout mice. Optical responses were triggered by stimulation of the SC pathway and measured as fractional changes of fluorescence (ΔF/F). As shown in Fig. 5A, the evoked optical signal was first detected 4 ms after stimulation, peaked within 12 ms, and gradually disappeared by 24 ms after stimulation. When we evoked optical signals twice in the same slice, we found that the propagation patterns of excitatory neural activity were almost identical between the first (ACSF) and second (ACSF2) recordings (Fig. 5A, upper two sequences). This confirms our previous report that an evoked optical signal is highly reproducible in the same slice (Nakauchi et al., 2007). We then measured the effect of nicotine on the optical signal in wild-type controls by recording it in the absence and presence of nicotine. A comparison of optical signals clearly shows that the optical signal evoked in the presence of nicotine is stronger, spreads further along and across the different anatomical layers, and lasts 4–8 ms longer (Fig. 5A, lower two sequences). Simultaneous field recordings show that nicotine application caused no detectable change in the amplitude of fEPSPs, despite the effect of nicotine on optical signal (Fig. 5B). Thus, electrophysiological recordings failed to reveal the nicotine-induced change in circuit activity as described above. We then carried out similar experiments with hippocampal slices from α7 knockout mice. After confirming that the optical signal evoked in the same slice is highly reproducible (Fig. 5C), we measured the effect of nicotine on optical signal. We found that, as in the case of hippocampal slices from wild-type controls, nicotine increased and facilitated the spread of optical signal (Fig. 5C, D) and that it caused no detectable change in the fEPSP amplitude (Fig. 5D).
Fig. 5.
Nicotine promoted excitatory neural activity in wild-type and α7 knockout mice (A–D) Pseudocolor representations of the voltage changes in response to a single stimulus in (A and B) wild-type and (C and D) α7 knockout (KO) mice. (A, C) Time series of optical signal recordings. (A and C, upper two panels) Comparison between the first (ACSF) and the second (ACSF2) optical signal recording. (A and C, lower two panels) Comparison between the first optical signal recording (ACSF) and the second optical signal recording in the presence of nicotine (Nic). (B and D) The maximum magnitude patterns of optical responses obtained in the first (ACSF, left panels) and the second (ACSF2 or Nic, right panels) recordings in control (upper panels) and nicotine (lower panels) conditions. The positions of stimulation (blue arrow) and recording (black arrow) electrodes in each experimental condition are indicated (B and D, left panels). (B and D, right traces) Comparisons of fEPSP (f.p.) and optical recording (ΔF/F) obtained in control (black line) and nicotine (red line) conditions in (B) wild-type and (D) α7 KO mice.
Nicotine promotes the spread of excitatory activity in both wild-type and α7 knockout mice
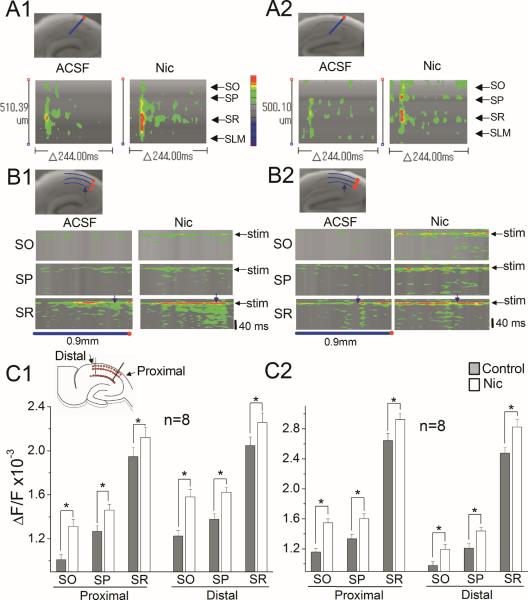
To further investigate the nicotine-induced change in the intricate spatial patterning of circuit activity in wild-type and α7 knockout mice, we compared line scans of optical responses across and along the various anatomical layers. These optical recordings showed that stimulation of the SC pathway triggered short-lasting depolarization in the SR, and that this initial response became stronger in the presence of nicotine in both wild-type and α7 knockout mice (Fig. 6A1, A2, B1, B2). Furthermore, nicotine facilitated the spread of excitatory activity spatially and temporally from the initial stimulation site in both wild-type and α7 knockout mice (Fig. 6A1, A2, B1, B2). For comparisons across hippocampal slices, the maximum optical responses to a single stimulus were sampled with a 3 × 21 grid that included several anatomical layers (Fig. 6C1, insert). The 21 points of each layer were divided into two groups (proximal and distal to the site of stimulation), and the average optical response in each group was calculated (one-way ANOVAs; proximal SO F1,174=16.04, SP F1,174=6.87, SR F1,174=1.97; distal SO F1,158=17.92, SP F1,158=12.13, SR F1,158=3.19; Fig. 6C1; proximal SO F1,174=30.64, SP F1,174=9.06, SR F1,174=5.06, distal SO F1,158=7.23, SP F1,158=9.49, SR F1,158=7.57; Fig. 6C2). At both proximal and distal sites in all anatomical layers in wild-type mice, nicotine clearly increased the optical signal. Similar increases in optical signal were also observed at all sites in α7 knockout mice. These results suggest that nicotine facilitates the excitatory neural activity without involving α7 nAChRs and, thus, the circuitry-dependent mechanism for nicotine s effect on LTP still operates in hippocampi developed in the absence of α7 nAChRs.
Fig. 6.
Nicotine promoted the spread of excitatory activity in both wild-type and α7 knockout mice (A1–C2) Nicotine increased the excitatory neural activity spatiotemporally in wild-type and α7 KO mice. (A1 and A2) Pseudocolor representations of the line scanning across various anatomical layers (blue with a red dot, top left in A1 and A2) over time in the absence (ACSF) and presence (Nic) of nicotine in (A1) wild-type and (A2) α7 KO mice. The scanning started 52 ms before stimulation and the fast depolarization peaked at 8 ms after stimulation. (B1 and B2) Pseudocolor representation of the line scanning along each anatomical layer over time in the absence (ACSF) and presence (Nic) of nicotine in (B1) wild-type and (B2) α7 KO mice. The curved line (blue with a red dot, 0.9 mm in length) was set along each layer. The blue arrow shows the place of stimulating electrode, and the black arrow indicates the time point of stimulation delivery. The blue line with a red dot under the image corresponds to that set along each layer (top left in B1 and B2). (C1 and C2) For comparisons across slices, the maximum optical responses were sampled with a 3 × 21 sampling grid (top left), anchored to the proximal site of the stimulating electrode and covering various anatomical layers. Average responses in the absence (control) and presence of nicotine (Nic) along various anatomical layers in (C1) wild-type and (C2) α7 KO mice were plotted. Data are means ± SEM. *P < 0.05
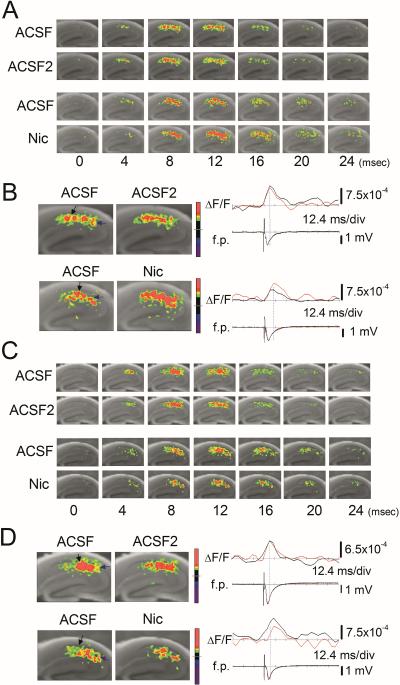
Nicotine decreases the excitatory neural activity in β2 knockout mice
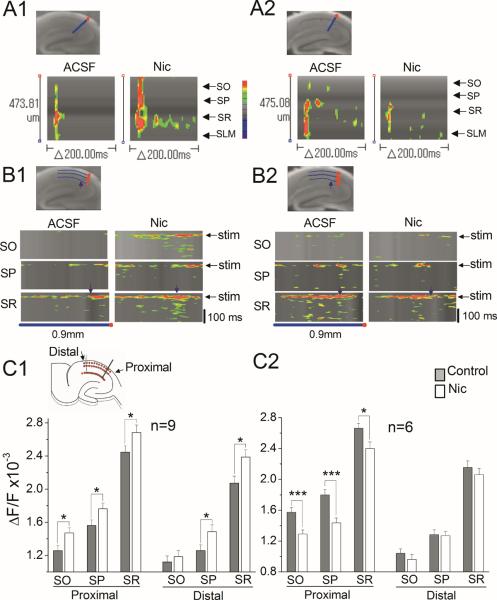
Nicotine-induced facilitation of LTP induction is absent in β2 knockout mice. Therefore, we predicted that nicotine would have no effect on the spread of excitatory neural activity in β2 knockout mice. This prediction was tested in littermate wild-type and β2 knockout mice as described above for α7 knockout mice. We first confirmed that nicotine increases the excitatory neural activity originating from the SC pathway in littermate wild-type controls without significantly altering fEPSPs (Fig. 7A, B). We then examined the effect of nicotine in β2 knockout mice and found that nicotine suppressed the excitatory neural activity triggered from the SC pathway without obvious changes in fEPSPs (Fig. 7C, D). We then performed two-way line scanning in littermate wild-type and β2 knockout mouse hippocampal slices (Fig. 8A1, A2, B1, B2). Line scanning across the anatomical layers in wild-type animals showed that nicotine facilitated initial depolarization in all layers, and that spread of excitation occurred in the SR (Fig. 8A1). However, this nicotine-induced facilitation of excitatory activity was not observed in β2 knockout mice (Fig. 8A2). Instead, the initial response became weaker in the presence of nicotine, and suppressed the spread of excitatory activity from the SR (Fig. 8A2). In the line scanning along the anatomical layers, we found that nicotine facilitated and spread the neural activity in all layers in littermate wild-type, but not β2 knockout mice (Fig. 8B1, B2). For statistical analysis, the maximum optical responses were obtained with three grid lines, covering various anatomical layers: stratum oriens (SO), stratum pyramidale (SP) and SR (Fig. 6G1, insert) as above. The graphs show that nicotine significantly increases the excitatory neural activity in both proximal and distal sites of all layers of littermate wild-type mice, and decreases the neural activity in the proximal, but not distal sites, of all layers of β2 knockout mice (one-way ANOVAs; proximal SO F1,196=5.99, SP F1,196=4.41, SR F1,196=3.99; distal SO F1,177=0.36, P=0.54, SP F1,178=4.54, SR F1,178=6.54 ; Fig. 8C1; proximal SO F1,130=12.11, SP F1,130=14.81, SR F1,130=6.09; distal SO F1,118=0.85, P=0.35, SP F1,118=0.027, P=0.87, SR F1,118=0.61, P=0.43; Fig. 8C2). These results suggest that the activation of β2* nAChRs is involved in the nicotine-induced increase in excitatory activity, or that hippocampi developed in the absence of β2* nAChRs lack the circuitry-dependent mechanism for the nicotine-induced enhancement of excitatory activity and, thus, LTP induction.
Fig. 7.
Nicotine decreased the excitatory neural activity in β2 knockout mice (A–D) Pseudocolor representations of the voltage changes in response to a single stimulus in (A and B) wild-type and (C and D) β2 knockout (KO) mice. Each recording was obtained and presented as in Fig. 5. (A, C) Time series of optical signal recordings. (B and D) The maximum magnitude patterns of optical responses. (B and D, right traces) Comparisons of fEPSP (f.p.) and optical recording (ΔF/F) obtained in control and nicotine conditions in (B) wild-type and (D) β2 KO mice as in Fig. 5.
Fig. 8.
Nicotine suppressed the spread of excitatory activity in β2 knockout mice (A1–C2) Nicotine suppressed the excitatory neural activity in β2 KO mice. (A1 and A2) Pseudocolor representations of the line scanning across various anatomical layers over time in the absence (ACSF) and presence of nicotine (Nic) in (A1) wild-type and (A2) β2 KOmice. (B1 and B2) Pseudocolor representation of the line scanning along each anatomical layer over time in the absence (ACSF) and presence of nicotine (Nic) in (B1) wild-type and (B2) β2 KO mice. Experiments were carried out as in Fig. 6. (C1 and C2) For comparisons across slices, the maximum optical responses were sampled as in Fig. 6. Average responses in the absence (control) and presence of nicotine (Nic) along various anatomical layers in (C1) wild-type and (C2) β2 KO mice were plotted. Data are means ± SEM. *P < 0.05, *** P < 0.001.
Nicotine depresses evoked IPSCs by activating β2-containing nAChRs
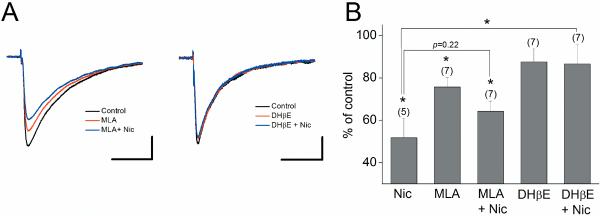
Nicotine increases optical signal without causing detectable changes in the amplitude of fEPSPs. Because the extracellular field potential recording is insensitive to small changes in GABAergic activity, this indicates that optical recordings are detecting slight changes in the excitation/inhibition balance due to nicotine-induced suppression of inhibition at the SC pathway. To confirm this prediction, we performed whole-cell recordings from CA1 pyramidal cells and examined the effect of nicotine on evoked monosynaptic IPSCs (Overall ANOVA F4,28=4.47, P=0.006; Fig. 9). We found that nicotine (1 μM) suppresses the amplitude of IPSCs (51.8 ± 9.2% of control, n=5, P <0.05; Fig. 9). This effect of nicotine was significantly blocked by DHβE (86.6 ± 9.3% of control, n=7; P <0.05; Fig. 9A,B), but not by MLA (64.2 ± 4.8% of control, n=7; P=0.22; Fig. 9A,B). DHβE alone had no significant effect on the amplitude of IPSCs (87.6 ± 6.1% of control, n=7; Fig. 9A,B). MLA alone slightly decreases the amplitude of IPSCs (75.7 ± 4.5% of control, n=7, P <0.05; Fig. 9A,B), suggesting that activation of α7 nAChRs occurs during stimulation and in turn modulates GABAergic synaptic transmission. These results demonstrate that nicotine suppresses GABAergic inhibition in large part by activating β2-containing nAChRs. Thus, nicotine-induced increases in optical signal in α7 knockout mice is at least in part attributed to activation of β2* nAChRs, which suppresses inhibition. This also explains why nicotine-induced increases in optical signal are absent in β2 knockout mice.
Fig. 9.
Nicotine depressed evoked IPSCs recorded in CA1 pyramidal cells via activation of β2-containing nAChRs Voltage-clamped cells (at −60 mV) were stimulated at 300 μA, at which nicotine causes a clear effect, in the stratum radiatum in the presence of 6,7-Dinitroquinoxaline-2,3-dione (20 μM) and DL-(−)-2-Amino-5-phosphonopentanoic acid (50 μM). A stimulating electrode was placed in the stratum radiatum within 500 μm of recorded pyramidal cells. (A) Sample traces triggered in the absence (control) and presence of nicotinic drugs. Each trace was an average of three sequential responses. (B) Histograms show the effects of different nicotinic drugs on IPSCs, expressed as a percentage of control amplitude. Numbers in parentheses in this and the following figures indicate the numbers of experiments. *P < 0.05. Scale bars: 50 ms and 100 pA.
Discussion
Our current study demonstrates that for naturalistic theta burst patterns of stimulation applied to the SC projections in the hippocampus, α7 nAChRs facilitate LTP induction via the release of ACh. While this ACh release appears to be insufficient to modulate LTP induction via DHβE-sensitive non-α7 nAChRs, the contribution of non-α7 nAChRs to LTP induction becomes apparent when they are activated by bath application of nicotine beforehand. This effect of nicotine is absent in β2 knockout mice, suggesting that the non-α7 nAChRs involved in nicotine's effect contain the β2 subunit. Thus, our study revealed differential roles of endogenous ACh and exogenous nicotine in the nicotinic modulation of LTP induction, each involving a different nAChR subtype. Furthermore, because our results show that β2- and α7-nAChRs independently modulate LTP induction, α7β2 nAChRs are not a major player in the nicotinic modulation of LTP induction in the hippocampal CA1 region.
α7 nAChRs are localized on glutamatergic and GABAergic nerve terminals (Gray et al., 1996; Alkondon et al., 1997; Radcliffe & Dani, 1998). LTP induction at the SC pathway is NMDAR-dependent (Bliss & Collingridge, 1993; Malenka & Nicoll, 1999; Malenka & Bear, 2004), and α7 nAChRs occur on the dendrites of pyramidal cells (Ji et al., 2001). Therefore, increased glutamate release via presynaptic α7 nAChRs and/or coactivation of postsynaptic α7 nAChRs contributes to voltage-dependent relief of the Mg2+ block of NMDARs. Furthermore, because α7 nAChR channels are highly permeable to Ca2+ (Seguela et al., 1993), activation of postsynaptic α7 nAChRs provides an additional source of Ca2+. Thus, pre- and postsynaptic activation of α7 nAChRs by endogenous ACh allows simple mechanisms of α7 nAChR-mediated facilitation of LTP induction. Indeed, it has been demonstrated that precisely timed activation of pre- or postsynaptic α7 nAChRs at glutamatergic synapses by exogenous ACh promotes LTP induction, with the postsynaptic mechanism having a more dominant effect (Ji et al., 2001). More recent study also indicates that synaptically released ACh can activate both pre- and postsynaptic α7 nAChRs at glutamatergic synapses, and timing-dependent activation of postsynaptic α7 nAChRs has a strong influence on LTP induction (Gu & Yakel, 2011). However, the study failed to detect α7 nAChR-mediated postsynaptic currents and Ca2+ transients in pyramidal cells, although it found α7 nAChR activation-induced prolongation of the NMDAR-mediated Ca2+ transients in pyramidal cell spines (Gu & Yakel, 2011). As demonstrated by the study, it has been difficult to directly detect α7 nAChR-mediated synaptic responses in pyramidal cells (Alkondon et al., 1998; Frazier et al., 1998b). However, we have previously found that weak tetanic stimulation evoked appreciable α7 nAChR-mediated responses in six out of 39 pyramidal cells of rats (Yamazaki et al., 2005), suggesting that activation of postsynaptic α7 nAChRs occurs during weak TBS and contributes to LTP induction. Thus, activation of postsynaptic α7 nAChRs below the limit of experimental detection appears to be a potential mechanism for the α7 nAChR-mediated LTP induction observed in the current study.
In the present study, the α7 nAChR-dependent LTP induction appears to occur even after 10 min of nicotine exposure, during which the majority of α7 nAChRs would become desensitized (Frazier et al., 1998b; McQuiston & Madison, 1999; Yamazaki et al., 2005; Yamazaki et al., 2006). It is therefore unclear how these receptors still contribute to LTP induction. However, it is possible that bath application of nicotine briefly activates α7 nAChRs before they desensitize, and triggers intracellular signaling sufficient to contribute to LTP induction, even if the α7 nAChRs remain desensitized during weak TBS.
α7 nAChRs are abundantly expressed on GABAergic interneurons in the CA1 region of the hippocampus (Jones et al., 1992; Alkondon et al., 1998; Frazier et al., 1998a; Frazier et al., 1998b; McQuiston & Madison, 1999; Sudweeks & Yakel, 2000; Alkondon & Albuquerque, 2001), where they mediate rapidly desensitizing responses and can be activated by endogenously released ACh (Alkondon et al., 1998; Frazier et al., 1998b; Yamazaki et al., 2005). However, it is unlikely that they contribute to α7 nAChR-dependent LTP induction. It has been shown that precisely timed activation of SR interneurons by exogenous ACh blocks LTP induction (Ji et al., 2001). Also, we have previously observed that LTP-inducing stimulation effectively activates α7 nAChRs on SR interneurons (Yamazaki et al., 2005). These interneurons are likely feedforward interneurons and, thus, their activation negatively contributes to LTP induction. Furthermore, it has been demonstrated that activation of α7 nAChRs on interneurons causes heterosynaptic depression of GABAergic IPSCs (Wanaverbecq et al., 2007; Zhang & Berg, 2007). This in turn may further increase inhibition of pyramidal cells. Because theta burst stimulation suppresses GABA release from the terminals of feedforward interneurons via activation of GABAB receptors (Bliss & Collingridge, 1993), the negative contribution of α7 nAChRs on feedforward interneurons to LTP induction is likely canceled out.
β2 subunit mRNAs are expressed in CA1 pyramidal cells and many interneurons (Wada et al., 1989; Son & Winzer-Serhan, 2008). Recent study shows that stimulation of cholinergic fibers elicits DHβE-sensitive nAChR-mediated responses in about 20% of mouse pyramidal cells (Gu & Yakel, 2011). We have also found that weak tetanic stimulation induced DHβE-sensitive nAChR-mediated responses in small numbers of rat pyramidal cells (Yamazaki et al., 2005). Thus, these non-α7 nAChRs, which most likely contain the β2 subunit, are activated during weak TBS. However, because DHβE has no obvious effect on endogenous ACh-induced LTP induction, the contribution of these non-α7 nAChRs to LTP induction appears to be very limited, if at all.
Rapid application of ACh onto GABAergic interneurons elicits non-α7 nAChR mediated slow responses that can be blocked by low concentrations of nicotine due to desensitization (Frazier et al., 1998a; McQuiston & Madison, 1999). These interneurons are located primarily in the stratum oriens where α4β2 and α2* nAChRs are predominantly expressed (Wada et al., 1989; Rogers et al., 1998; Ishii et al., 2005; Son & Winzer-Serhan, 2008). There are relatively few neurons expressing α2 mRNA in the mouse brain, but GABAergic interneurons in the stratum oriens/alveus do contain α2 mRNA (Ishii et al., 2005). These interneurons are continuously activated in the presence of nicotine (Jia et al., 2009), and appear to be an important component in hippocampal circuitry because the effects of nicotine on LTP induction and excitatory neural activity are absent in α2 knockout mice (Nakauchi et al., 2007). In the present study, β2* nAChR-dependent LTP was blocked by DHβE in the presence of nicotine, which was bath-applied for 10 min before delivery of the LTP-inducing stimulation. This suggests that β2* nAChRs, like α2* nAChRs, are non-desensitizing receptors. Because the previous study indicates that the α2 subunit is coexpressed with the β2 subunit in GABAergic interneurons in the oriens/alveus (Sudweeks & Yakel, 2000), the β2* and α2* nAChRs are very likely the same receptor. Thus, the lack of nicotine-induced facilitation of LTP induction and spread of excitatory activity in β2 and α2 knockout mice could be due to the absence of α2β2* nAChRs on GABAergic interneurons in the oriens/alveus. Because α4β2* nAChRs on GABAergic interneurons are known to desensitize during nicotine application (Alkondon et al., 2000), the contribution of α4β2* nAChRs on GABAergic interneurons to the effects of nicotine may not be significant.
The nicotine-induced facilitation of LTP induction appears to be mediated by at least two separate mechanisms; circuitry-dependent disinhibition of pyramidal cells, and indirect activation of muscarinic receptors by nicotine-induced ACh release, both of which result in the enhancement of NMDAR-mediated responses in CA1 pyramidal cells (Yamazaki et al., 2005; Yamazaki et al., 2006). Optical recordings with VSD reveal that the nicotine-induced enhancement of excitatory activity at the SC pathway is absent in β2 knockout mice, as with α2 knockout mice (Nakauchi et al., 2007), but is still observed in α7 knockout mice. This effect of nicotine is most likely due to circuitry-dependent disinhibition of pyramidal cells and is related to enhancement of burst EPSPs, and thus LTP induction. However, although several potential mechanisms were previously proposed (Yamazaki et al., 2005; Yamazaki et al., 2006; Nakauchi et al., 2007; Jia et al., 2009), it largely remains to be determined how the activation of α2β2* nAChRs on interneurons in the stratum oriens/alveus causes disinhibition of pyramidal cells. Furthermore, nicotine decreases the neural activity in the proximal sites of all layers in β2 knockout mice, but not in α2 knockout mice (Nakauchi et al., 2007). The involvement of β2* nAChRs other than α2β2* nAChRs in the nicotine's effect remains to be examined.
Evidence increasingly suggests that activation of α7* nAChRs by endogenously released ACh contributes to cognitive function (Levin & Simon, 1998; Levin et al., 2002; Curzon et al., 2006; Kenney & Gould, 2008). The α7 nAChR-dependent LTP induction observed in the present study is most likely correlated to α7* nAChR-mediated cognitive enhancement. In addition, nicotine administration enhances hippocampal-dependent fear conditioning (Davis & Gould, 2007; Davis et al., 2007). This effect of nicotine is absent in β2 knockout mice, but not α7 knockout mice, suggesting that it is mediated by β2* nAChRs (Davis & Gould, 2007; Davis et al., 2007). However, the identity of the β2* nAChR remains to be determined. Our studies demonstrate that nicotine facilitates the induction of LTP at the SC pathway in wild-type and α7 knockout mice, but not α2 (Nakauchi et al., 2007) and β2 knockout mice. This suggests that α2β2* nAChR is most likely the β2* nAChR involved in nicotine-induced enhancement of hippocampus-dependent fear conditioning.
Moreover, α7 nAChR activation appears to mediate Aβ-induced pathology (Wang et al., 2003; Hu et al., 2008), whereas deletion of the α7 subunit improves cognitive and synaptic plasticity deficits in the hippocampus of a mouse model of AD (Dziewczapolski et al., 2009). Timing-dependent α7 nAChR-mediated LTP induction appears to be highly sensitive to blockade by Aβ (Gu & Yakel, 2011), suggesting that Aβ impairs cognitive function by disrupting α7 nAChR-dependent control of LTP induction. Because in basal forebrain cholinergic neurons, α7 nAChRs show low sensitivity to Aβ1–42 in β2 subunit knockout mice (Liu et al., 2009), heteromeric α7β2 nAChRs rather than homomeric α7 nAChRs might be targets of Aβ1–42. Thus, it is possible that the LTP induced by synaptically released ACh in the current study involves the activation of not only homomeric α7 nAChRs, but also heteromeric α7β2 nAChRs. However, the timing-dependent, α7 nAChR-mediated LTP induction is insensitive to DHβE (Gu & Yakel, 2011), suggesting that homomeric α7 nAChRs rather than heteromeric α7β2 nAChRs are a major contributor to the effect. Our current study demonstrates that exogenous nicotine facilitates LTP induction via activation of non-α7 nAChRs, perhaps α2β2* nAChRs. This effect of nicotine does not require ACh release and may still be exerted in AD patients, in which basal forebrain cholinergic neurons are deteriorated. Thus, co-administration of selective inhibitors for α7/α7β2 nAChRs with selective activators for α2β2* nAChRs may have therapeutic benefits in the treatment of AD.
Acknowledgements
We would like to thank Dr. Marina Picciotto (Yale University) for heterozygous α7 and β2 knockout mice. The authors gratefully acknowledge Elise Kleeman for helpful comments on the manuscript. This research was supported by the National Institute on Drug Abuse (DA14542, DA025269, DA026458, DA25676).
Abbreviations
- AD
Alzheimer's disease
- ACSF
artificial cerebrospinal fluid
- DHβE
dihydro-beta-erythroidine
- fEPSPs
field excitatory postsynaptic potentials
- GABA
γ-aminobutyric acid
- IPSC
inhibitory postsynaptic current
- LTP
long-term potentiation
- MLA
Methyllycaconitine
- nAChR
nicotinic acetylcholine receptor
- NMDAR
N-methyl-D-aspartate receptor
- PPF
paired-pulse facilitation
- SC
Schaffer collateral
- SLM
stratum lacunosum moleculare
- SO
stratum oriens
- SP
stratum pyramidale
- SR
stratum radiatum
- TBS
theta burst stimulation
- VSD
voltage-sensitive dye
References
- Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–3055. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Albuquerque EX. alpha-bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 1998;810:257–263. doi: 10.1016/s0006-8993(98)00880-4. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Almeida LE, Randall WR, Albuquerque EX. Nicotine at concentrations found in cigarette smokers activates and desensitizes nicotinic acetylcholine receptors in CA1 interneurons of rat hippocampus. Neuropharmacology. 2000;39:2726–2739. doi: 10.1016/s0028-3908(00)00156-8. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther. 1997;283:1396–1411. [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Campbell NR, Fernandes CC, Halff AW, Berg DK. Endogenous signaling through alpha7-containing nicotinic receptors promotes maturation and integration of adult-born neurons in the hippocampus. J Neurosci. 2010;30:8734–8744. doi: 10.1523/JNEUROSCI.0931-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yamada K, Nabeshima T, Sokabe M. alpha7 Nicotinic acetylcholine receptor as a target to rescue deficit in hippocampal LTP induction in beta-amyloid infused rats. Neuropharmacology. 2006;50:254–268. doi: 10.1016/j.neuropharm.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Curzon P, Anderson DJ, Nikkel AL, Fox GB, Gopalakrishnan M, Decker MW, Bitner RS. Antisense knockdown of the rat alpha7 nicotinic acetylcholine receptor produces spatial memory impairment. Neurosci Lett. 2006;410:15–19. doi: 10.1016/j.neulet.2006.09.061. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. beta2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 2007;190:343–352. doi: 10.1007/s00213-006-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci. 2007;27:10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziewczapolski G, Glogowski CM, Masliah E, Heinemann SF. Deletion of the alpha 7 nicotinic acetylcholine receptor gene improves cognitive deficits and synaptic pathology in a mouse model of Alzheimer's disease. J Neurosci. 2009;29:8805–8815. doi: 10.1523/JNEUROSCI.6159-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- Frazier CJ, Buhler AV, Weiner JL, Dunwiddie TV. Synaptic potentials mediated via alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. J Neurosci. 1998a;18:8228–8235. doi: 10.1523/JNEUROSCI.18-20-08228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998b;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Ji Z, Morita N, Sumikawa K. Acute and chronic nicotine exposure differentially facilitate the induction of LTP. Brain Res. 1999;846:137–143. doi: 10.1016/s0006-8993(99)01982-4. [DOI] [PubMed] [Google Scholar]
- Fujii S, Ji Z, Sumikawa K. Inactivation of alpha7 ACh receptors and activation of non-alpha7 ACh receptors both contribute to long term potentiation induction in the hippocampal CA1 region. Neurosci Lett. 2000a;286:134–138. doi: 10.1016/s0304-3940(00)01076-4. [DOI] [PubMed] [Google Scholar]
- Fujii S, Jia Y, Yang A, Sumikawa K. Nicotine reverses GABAergic inhibition of long-term potentiation induction in the hippocampal CA1 region. Brain Res. 2000b;863:259–265. doi: 10.1016/s0006-8993(00)02119-3. [DOI] [PubMed] [Google Scholar]
- Ge S, Dani JA. Nicotinic acetylcholine receptors at glutamate synapses facilitate long-term depression or potentiation. J Neurosci. 2005;25:6084–6091. doi: 10.1523/JNEUROSCI.0542-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Gu Z, Yakel JL. Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron. 2011;71:155–165. doi: 10.1016/j.neuron.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid S, Dawe GS, Gray JA, Stephenson JD. Nicotine induces long-lasting potentiation in the dentate gyrus of nicotine-primed rats. Neurosci Res. 1997;29:81–85. doi: 10.1016/s0168-0102(97)00074-6. [DOI] [PubMed] [Google Scholar]
- Harrist A, Beech RD, King SL, Zanardi A, Cleary MA, Caldarone BJ, Eisch A, Zoli M, Picciotto MR. Alteration of hippocampal cell proliferation in mice lacking the beta 2 subunit of the neuronal nicotinic acetylcholine receptor. Synapse. 2004;54:200–206. doi: 10.1002/syn.20081. [DOI] [PubMed] [Google Scholar]
- Heinemann S, Boulter J, Deneris E, Conolly J, Duvoisin R, Papke R, Patrick J. The brain nicotinic acetylcholine receptor gene family. Prog Brain Res. 1990;86:195–203. doi: 10.1016/s0079-6123(08)63177-5. [DOI] [PubMed] [Google Scholar]
- Hu M, Waring JF, Gopalakrishnan M, Li J. Role of GSK-3beta activation and alpha7 nAChRs in Abeta(1–42)-induced tau phosphorylation in PC12 cells. J Neurochem. 2008;106:1371–1377. doi: 10.1111/j.1471-4159.2008.05483.x. [DOI] [PubMed] [Google Scholar]
- Hunter BE, de Fiebre CM, Papke RL, Kem WR, Meyer EM. A novel nicotinic agonist facilitates induction of long-term potentiation in the rat hippocampus. Neurosci Lett. 1994;168:130–134. doi: 10.1016/0304-3940(94)90433-2. [DOI] [PubMed] [Google Scholar]
- Hurst RS, Hajos M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, Groppi VE, Allaman G, Ogier R, Bertrand S, Bertrand D, Arneric SP. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Wong JK, Sumikawa K. Comparison of alpha2 nicotinic acetylcholine receptor subunit mRNA expression in the central nervous system of rats and mice. J Comp Neurol. 2005;493:241–260. doi: 10.1002/cne.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Jia Y, Yamazaki Y, Nakauchi S, Sumikawa K. Alpha2 nicotine receptors function as a molecular switch to continuously excite a subset of interneurons in rat hippocampal circuits. Eur J Neurosci. 2009;29:1588–1603. doi: 10.1111/j.1460-9568.2009.06706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GM, Sahakian BJ, Levy R, Warburton DM, Gray JA. Effects of acute subcutaneous nicotine on attention, information processing and short-term memory in Alzheimer's disease. Psychopharmacology (Berl) 1992;108:485–494. doi: 10.1007/BF02247426. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol. 2008;38:101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiroug SS, Harkness PC, Lamb PW, Sudweeks SN, Khiroug L, Millar NS, Yakel JL. Rat nicotinic ACh receptor alpha7 and beta2 subunits co-assemble to form functional heteromeric nicotinic receptor channels. J Physiol. 2002;540:425–434. doi: 10.1113/jphysiol.2001.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Magueresse C, Safiulina V, Changeux JP, Cherubini E. Nicotinic modulation of network and synaptic transmission in the immature hippocampus investigated with genetically modified mice. J Physiol. 2006;576:533–546. doi: 10.1113/jphysiol.2006.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Bradley A, Addy N, Sigurani N. Hippocampal alpha 7 and alpha 4 beta 2 nicotinic receptors and working memory. Neuroscience. 2002;109:757–765. doi: 10.1016/s0306-4522(01)00538-3. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Nicotinic treatment for cognitive dysfunction. Curr Drug Targets CNS Neurol Disord. 2002;1:423–431. doi: 10.2174/1568007023339102. [DOI] [PubMed] [Google Scholar]
- Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology (Berl) 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- Lindstrom J, Schoepfer R, Conroy W, Whiting P, Das M, Saedi M, Anand R. The nicotinic acetylcholine receptor gene family: structure of nicotinic receptors from muscle and neurons and neuronal alpha-bungarotoxin-binding proteins. Adv Exp Med Biol. 1991;287:255–278. doi: 10.1007/978-1-4684-5907-4_22. [DOI] [PubMed] [Google Scholar]
- Liu Q, Huang Y, Xue F, Simard A, DeChon J, Li G, Zhang J, Lucero L, Wang M, Sierks M, Hu G, Chang Y, Lukas RJ, Wu J. A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. J Neurosci. 2009;29:918–929. doi: 10.1523/JNEUROSCI.3952-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kawai H, Berg DK. beta -Amyloid peptide blocks the response of alpha 7-containing nicotinic receptors on hippocampal neurons. Proc Natl Acad Sci U S A. 2001;98:4734–4739. doi: 10.1073/pnas.081553598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- Lukas RJ, Changeux JP, Le Novere N, Albuquerque EX, Balfour DJ, Berg DK, Bertrand D, Chiappinelli VA, Clarke PB, Collins AC, Dani JA, Grady SR, Kellar KJ, Lindstrom JM, Marks MJ, Quik M, Taylor PW, Wonnacott S. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev. 1999;51:397–401. [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Mann EO, Greenfield SA. Novel modulatory mechanisms revealed by the sustained application of nicotine in the guinea-pig hippocampus in vitro. J Physiol. 2003;551:539–550. doi: 10.1113/jphysiol.2003.045492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Matsumoto A, Enomoto T, Nishizaki T. Activation of nicotinic acetylcholine receptors induces long-term potentiation in vivo in the intact mouse dentate gyrus. Eur J Neurosci. 2000;12:3741–3747. doi: 10.1046/j.1460-9568.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Presynaptic ionotropic receptors. Curr Opin Neurobiol. 1996;6:342–349. doi: 10.1016/s0959-4388(96)80118-8. [DOI] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. J Neurosci. 1999;19:2887–2896. doi: 10.1523/JNEUROSCI.19-08-02887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechawar N, Saghatelyan A, Grailhe R, Scoriels L, Gheusi G, Gabellec MM, Lledo PM, Changeux JP. Nicotinic receptors regulate the survival of newborn neurons in the adult olfactory bulb. Proc Natl Acad Sci U S A. 2004;101:9822–9826. doi: 10.1073/pnas.0403361101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakauchi S, Brennan RJ, Boulter J, Sumikawa K. Nicotine gates long-term potentiation in the hippocampal CA1 region via the activation of alpha2* nicotinic ACh receptors. Eur J Neurosci. 2007;25:2666–2681. doi: 10.1111/j.1460-9568.2007.05513.x. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learn Mem. 1998;5:302–316. [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M. Nicotinic receptors in aging and dementia. J Neurobiol. 2002;53:641–655. doi: 10.1002/neu.10102. [DOI] [PubMed] [Google Scholar]
- Radcliffe KA, Dani JA. Nicotinic stimulation produces multiple forms of increased glutamatergic synaptic transmission. J Neurosci. 1998;18:7075–7083. doi: 10.1523/JNEUROSCI.18-18-07075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SW, Gahring LC, Collins AC, Marks M. Age-related changes in neuronal nicotinic acetylcholine receptor subunit alpha4 expression are modified by long-term nicotine administration. J Neurosci. 1998;18:4825–4832. doi: 10.1523/JNEUROSCI.18-13-04825.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Rozsa B, Katona G, Kaszas A, Szipocs R, Vizi ES. Dendritic nicotinic receptors modulate backpropagating action potentials and long-term plasticity of interneurons. Eur J Neurosci. 2008;27:364–377. doi: 10.1111/j.1460-9568.2007.05999.x. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JH, Winzer-Serhan UH. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs in rat hippocampal GABAergic interneurons. J Comp Neurol. 2008;511:286–299. doi: 10.1002/cne.21828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J Physiol. 2000;527(Pt 3):515–528. doi: 10.1111/j.1469-7793.2000.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T, Tominaga Y, Yamada H, Matsumoto G, Ichikawa M. Quantification of optical signals with electrophysiological signals in neural activities of Di-4-ANEPPS stained rat hippocampal slices. J Neurosci Methods. 2000;102:11–23. doi: 10.1016/s0165-0270(00)00270-3. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wanaverbecq N, Semyanov A, Pavlov I, Walker MC, Kullmann DM. Cholinergic axons modulate GABAergic signaling among hippocampal interneurons via postsynaptic alpha 7 nicotinic receptors. J Neurosci. 2007;27:5683–5693. doi: 10.1523/JNEUROSCI.1732-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Lee DH, Davis CB, Shank RP. Amyloid peptide Abeta(1–42) binds selectively and with picomolar affinity to alpha7 nicotinic acetylcholine receptors. J Neurochem. 2000;75:1155–1161. doi: 10.1046/j.1471-4159.2000.0751155.x. [DOI] [PubMed] [Google Scholar]
- Wang HY, Li W, Benedetti NJ, Lee DH. Alpha 7 nicotinic acetylcholine receptors mediate beta-amyloid peptide-induced tau protein phosphorylation. J Biol Chem. 2003;278:31547–31553. doi: 10.1074/jbc.M212532200. [DOI] [PubMed] [Google Scholar]
- Welsby P, Rowan M, Anwyl R. Nicotinic receptor-mediated enhancement of long-term potentiation involves activation of metabotropic glutamate receptors and ryanodine-sensitive calcium stores in the dentate gyrus. Eur J Neurosci. 2006;24:3109–3118. doi: 10.1111/j.1460-9568.2006.05187.x. [DOI] [PubMed] [Google Scholar]
- Welsby PJ, Rowan MJ, Anwyl R. Intracellular mechanisms underlying the nicotinic enhancement of LTP in the rat dentate gyrus. Eur J Neurosci. 2009;29:65–75. doi: 10.1111/j.1460-9568.2008.06562.x. [DOI] [PubMed] [Google Scholar]
- Whiting P, Schoepfer R, Lindstrom J, Priestley T. Structural and pharmacological characterization of the major brain nicotinic acetylcholine receptor subtype stably expressed in mouse fibroblasts. Mol Pharmacol. 1991;40:463–472. [PubMed] [Google Scholar]
- Yamazaki Y, Fujii S, Jia Y, Sumikawa K. Nicotine withdrawal suppresses nicotinic modulation of long-term potentiation induction in the hippocampal CA1 region. Eur J Neurosci. 2006;24:2903–2916. doi: 10.1111/j.1460-9568.2006.05160.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Jia Y, Hamaue N, Sumikawa K. Nicotine-induced switch in the nicotinic cholinergic mechanisms of facilitation of long-term potentiation induction. Eur J Neurosci. 2005;22:845–860. doi: 10.1111/j.1460-9568.2005.04259.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Berg DK. Reversible inhibition of GABAA receptors by alpha7-containing nicotinic receptors on the vertebrate postsynaptic neurons. J Physiol. 2007;579:753–763. doi: 10.1113/jphysiol.2006.124578. [DOI] [PMC free article] [PubMed] [Google Scholar]